- *Corresponding Author:
- Binny Mehta
Department of Pharmaceutical Chemistry and Analysis, Nootan Pharmacy College, Sankalchand Patel University, Visnagar, Gujarat 384315, India
E-mail: binnymehta.ph@gmail.com
| Date of Submission | 23 February 2021 |
| Date of Revision | 24 August 2021 |
| Date of Acceptance | 29 May 2022 |
| Indian J Pharm Sci 2022;84(3):669-675 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
In this work, a numerical method, based on the use of spectrophotometric data coupled to partial least squares, multivariate calibration is evaluated for the simultaneous determination of olmesartan medoxomil and hydrochlorothiazide in bulk and tablet dosage form. Tablet Olmetor-H (HTZ 12.5 mg and OLM 20.0 mg) was used in this study. The equipment used was a Ultraviolet-Visible double beam spectrophotometer with a matching pair of 1 cm quartz cell and electronic balance. Spectra of olmesartan medoxomil and hydrochlorothiazide were recorded at concentrations within their linear ranges 2.5-20 μg/ml and 4-32 μg/ ml, respectively and were used to compute a total of 25 synthetic mixtures involving 16 calibration and 9 validation sets between the wavelength range of 200 nm and 350 nm with the wavelengths intervals, lambda=3 nm in methanol. The analytical performances of these chemometric methods were characterized by relative prediction errors and recovery studies (%), and were compared with each other. The proposed method is simple, rapid and can be easily used as an alternative analysis tool in the quality control of drugs and formulation. PLS was applied successfully for the simultaneous determination of OLM and HTZ in laboratory mixtures and pharmaceutical formulation.
Keywords
Hydrochlorothiazide, olmesartan medoxomil, partial least squares, chemometrics, ultravioletvisible spectrophotometry
Olmesartan Medoxomil (OLM), chemically 2,3-dihydroxy-2-butenyl 4-(1-hydroxy-1-methylethyl)- 2-propyl-1-[p-(O-1H-tetrazol-5-yl phenyl) benzyl] imidazole-5-carboxylate, cyclic 2,3-carbonate is a prodrug and it is hydrolysed to olmesartan during absorption from the gastrointestinal tract (fig. 1A). It is a selective Angiotensin 1 (AT1) subtype angiotensin II receptor antagonist. Hydrochlorothiazide (HTZ), chemically 6-chloro-3,4-dihydro-2,4-1,2,4- benzothiadiazine-7-sulfonamide-1,1-dioxide (fig. 1B), is a widely used thiazide diuretic[1-3]. Olmesartan and HTZ are available in the market as a combined dosage form for the treatment of hypertension. An extensive literature survey revealed the determination of OLM in dosage form by Ultraviolet (UV)-visible spectrophotometry[4,5], High Performance Liquid Chromatography (HPLC)- UV[6,7] and capillary electrophoresis[8] and in biological fluids by HPLC[9] and Liquid Chromatography- Mass Spectrometry (LC-MS)[10,11]. Determination methods of HTZ in pharmaceutical dosage form and biological fluids include chemiluminescence[12], HPLC[13] and electrochemical study[14]. Determination methods of OLM and HTZ combination include UVspectrophotometry[ 15-18], Reverse Phase (RP)-HPLC and High-Performance Thin Layer Chromatography (HPTLC)[19,20]. In this work, a simple, accurate, precise and inexpensive quantitative method has been developed for the simultaneous determination of the coexisting two drugs in the tablet dosage form. The method is based on a Partial Least Square (PLS) multivariate calibration chemometric procedure. Moreover, being simple and inexpensive, it is more appealing to use for routine assay of OLM and HTZ combination in a tablet than HPLC which is demanding in terms of running cost and sophistication.
Materials and Methods
The reference standard of HTZ and OLM was obtained as a gratis sample from Sidmak Labs Pvt. Ltd., Valsad, Gujarat. Methanol was procured from Chemdyes Corporation, Vadodara, Gujarat. Tablet Olmetor-H (HTZ 12.5 mg and OLM 20.0 mg) was provided by Torrent Pharmaceuticals. The equipment used was a UV-Visible double beam spectrophotometer with a matching pair of 1 cm quartz cell (Shimadzu UV-1800, Shimadzu Corporation, Kyoto, Japan) and electronic balance (Mettler Toledo). Data was acquired and processed using XLSTAT software and it was used for PLS model development and data analysis.
Preparation of standard stock solution:
Accurately weighed and transferred OLM (10 mg) and HTZ (10 mg) into two different 100 ml volumetric flask respectively and volume was made up to 100 ml with methanol up to the mark. The final concentration of OLM and HTZ were 100 μg/ml.
Preparation of working stock solution:
The standard stock solution of OLM and HTZ 100 μg/ ml was used as a working solution.
Construction of calibration and validation set:
Two sets of standard solutions, a calibration set and a validation set were prepared. The multivariate calibration requires a suitable experimental design of the standard composition of the calibration set to provide the best prediction. The factorial design method was used to construct the calibration set. The application of two factorial designs led to the construction and optimization of the PLS model. Two binary sets of the drug present in the random ratio were prepared, one set with 5 samples so that a total of 25 samples were employed for optimization by PLS method by mixing appropriate volumes of the working standard solutions of OLM and HTZ, and diluting to volume with methanol. Eight validation standard mixtures were prepared. The combination of OLM and HTZ is illustrated in Table 1. The absorption spectra of the prepared solutions were measured from 200- 400 nm with 3 nm intervals. The absorbance data of the calibration set were then subjected to the XLSTAT program for the PLS model. For validation of the PLS model, the concentrations of OLM and HTZ in the validation set were predicted by using the proposed PLS model. The validation of all the methods was performed by International Council for Harmonisation (ICH) Q2 (R1) and International Union of Pure and Applied Chemistry (IUPAC) guidelines for calibration in analytical chemistry.
| Sample Sr. no. |
Concentration (µg/ml) | |
|---|---|---|
| OLM | HTZ | |
| 1 | 2.5 | 4 |
| 2 | 2.5 | 8 |
| 3 | 2.5 | 16 |
| 4 | 2.5 | 24 |
| 5 | 2.5 | 32 |
| 6 | 5 | 4 |
| 7 | 5 | 8 |
| 8 | 5 | 16 |
| 9 | 5 | 24 |
| 10 | 5 | 32 |
| 11 | 10 | 4 |
| 12 | 10 | 8 |
| 13 | 10 | 16 |
| 14 | 10 | 24 |
| 15 | 10 | 32 |
| 16 | 15 | 4 |
| 17 | 15 | 8 |
| 18 | 15 | 16 |
| 19 | 15 | 24 |
| 20 | 15 | 32 |
| 21 | 20 | 4 |
| 22 | 20 | 8 |
| 23 | 20 | 16 |
| 24 | 20 | 24 |
| 25 | 20 | 32 |
Note: OLM: Olmesartan Medoxomil and HTZ: Hydrochlorothiazide. The calibration set and validation set were randomly prepared with a mixture of OLM and HTZ in methanol
Table 1: Composition of Calibration Sample of Olm and Htz
Assay of marketed formulation:
Twenty tablets were weighed and the average weight was calculated. The tablets were triturated thoroughly and mixed. Tablet powder equivalent to 12.5 mg of HTZ and 20.0 mg of OLM, based on label claim was transferred to a 50.0 ml volumetric flask, dissolved by sonication for 15 min with enough methanol, and volume was made up to mark with methanol. The content was filtered through Whatman filter paper (No. 41). A 10.0 ml portion of the above filtrate was further diluted to 50.0 ml with distilled water. A 10.0 ml portion of this solution was further diluted to 50.0 ml with distilled water. The analysis procedure was repeated six times for tablet formulation and the results were shown below.
Results and Discussion
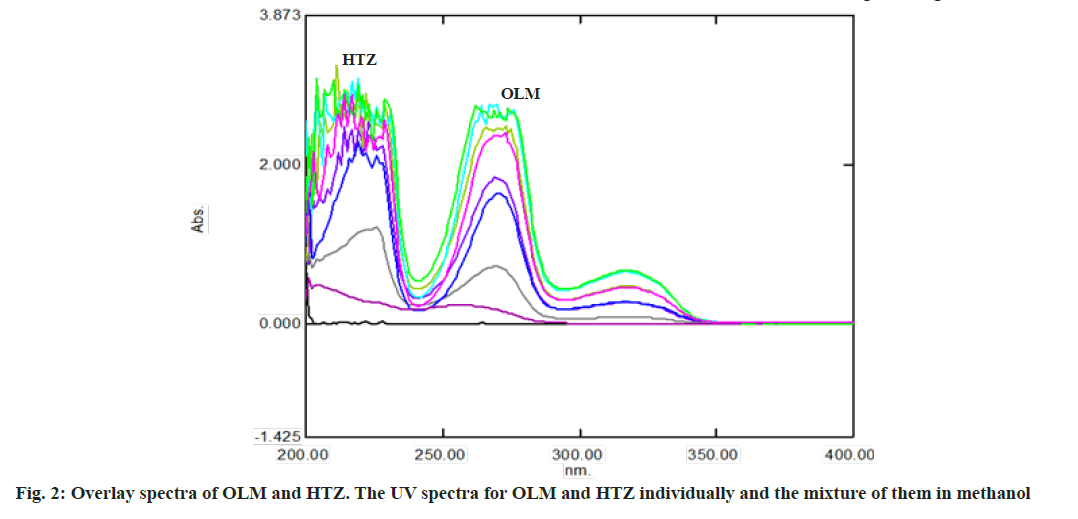
Calibration matrix and selection of spectral zones for analysis by PLS was shown here. Fig. 2 shows the UV spectra for OLM and HTZ individual and the mixture of them in methanol. As shown, there is clear overlapping between them. The spectral overlapping of these drugs prevents the resolution of the mixtures by direct spectrophotometric measurements. OLM exhibits absorption maxima at 257 nm and HTZ exhibits absorption maxima at 225 nm. The OLM and HTZ spectra are overlapped in the absorption maxima.
The first step in multivariate methods involved constructing the calibration matrix. The wavelength range used was 200-350 nm. 52 spectral points with 3 nm intervals were selected within this range. The compositions of the calibration mixtures were randomly designed to collect maximum information from the spectra of these mixtures.
The quality of multicomponent analysis is dependent on the wavelength range and spectral mode used. The UV absorption spectra of HTZ, OLM at their nominal concentrations are shown in fig. 2. The calibration set and validation set were randomly prepared with a mixture of OLM and HTZ in methanol (Table 1). The UV spectra were observed and the absorbances were measured at 52 wavelength points in the region between 200-350 nm with 3 nm intervals. The calibration data obtained from the experimental were gathered in a matrix data by Microsoft Office Excel (Version-1811) (all the data were transposed in Microsoft Office Excel). All these data are subjected to PLS treatment by XLSTAT (Ver. 2019). The predicted concentrations of the components in each sample were compared with the actual concentrations of the components in each of the validation samples and Standard Deviation (SD), mean and Relative Standard Deviation (RSD) was calculated (Table 2 and Table 3).
| Observation | Weight | Actual value | Predicted value | Residual matrix | Standard residual matrix | SD | Mean | RSD |
|---|---|---|---|---|---|---|---|---|
| Obs1 | 1 | 2.5 | 1.089 | 1.411 | 0.239 | |||
| Obs2 | 1 | 2.5 | 1.519 | 0.981 | 0.166 | 1.0208 | 2.313 | 50.963 |
| Obs3 | 1 | 2.5 | 3.278 | -0.778 | -0.132 | |||
| Obs4 | 1 | 2.5 | 3.366 | -0.866 | -0.147 | |||
| Obs5 | 1 | 5 | 3.854 | 1.146 | 0.194 | 1.25645 | 5.6434 | 22.264 |
| Obs6 | 1 | 5 | 4.838 | 0.162 | 0.027 | |||
| Obs7 | 1 | 5 | 6.487 | -1.487 | -0.252 | |||
| Obs8 | 1 | 5 | 6.85 | -1.85 | -0.313 | |||
| Obs9 | 1 | 5 | 6.188 | -1.188 | -0.201 | |||
| Obs10 | 1 | 10 | 6.188 | 3.812 | 0.645 | 2.2574 | 9.9088 | 22.781 |
| Obs11 | 1 | 10 | 9.395 | 0.605 | 0.102 | |||
| Obs12 | 1 | 10 | 11.383 | -1.383 | -0.234 | |||
| Obs13 | 1 | 10 | 11.672 | -1.672 | -0.283 | |||
| Obs14 | 1 | 10 | 10.906 | -0.906 | -0.153 | |||
| Obs15 | 1 | 15 | 16.623 | -1.623 | -0.275 | 0.98631 | 15.697 | 6.283 |
| Obs16 | 1 | 15 | 16.687 | -1.687 | -0.285 | |||
| Obs17 | 1 | 15 | 15.415 | -0.415 | -0.07 | |||
| Obs18 | 1 | 15 | 15.449 | -0.449 | -0.076 | |||
| Obs19 | 1 | 15 | 14.314 | 0.686 | 0.116 | |||
| Obs20 | 1 | 20 | 18.72 | 1.28 | 0.217 | 0.7998 | 18.8998 | 4.231 |
| Obs21 | 1 | 20 | 19.662 | 0.338 | 0.057 | |||
| Obs22 | 1 | 20 | 19.517 | 0.483 | 0.082 | |||
| Obs23 | 1 | 20 | 18.95 | 1.05 | 0.178 | |||
| Obs24 | 1 | 20 | 17.65 | 2.35 | 0.398 |
Note: PLSR: Partial Least Squares Regression, SD, mean and RSD are mentioned for each observation
Table 2: Statistical Analysis of Olm By Pls Method
| Observation | Weight | Actual value | Pred (4) | Residual matrix | Standard residual matrix | SD | Mean | RSD |
|---|---|---|---|---|---|---|---|---|
| Obs1 | 1 | 8.000 | 2.781 | 5.219 | 0.552 | 12.350 | 22.64 | 54.54 |
| Obs2 | 1 | 16.000 | 16.536 | -0.536 | -0.057 | |||
| Obs3 | 1 | 24.000 | 22.765 | 1.235 | 0.131 | |||
| Obs4 | 1 | 32.000 | 28.626 | 3.374 | 0.357 | |||
| Obs5 | 1 | 4.000 | 3.520 | 0.480 | 0.051 | 10.342 | 15.610 | 66.25 |
| Obs6 | 1 | 8.000 | 7.638 | 0.362 | 0.038 | |||
| Obs7 | 1 | 16.000 | 15.568 | 0.432 | 0.046 | |||
| Obs8 | 1 | 24.000 | 22.838 | 1.162 | 0.123 | |||
| Obs9 | 1 | 32.000 | 28.489 | 3.511 | 0.371 | |||
| Obs10 | 1 | 4.000 | 28.489 | -24.489 | -2.589 | 7.954 | 20.22 | 39.33 |
| Obs11 | 1 | 8.000 | 9.489 | -1.489 | -0.157 | |||
| Obs12 | 1 | 16.000 | 15.410 | 0.590 | 0.062 | |||
| Obs13 | 1 | 24.000 | 20.720 | 3.280 | 0.347 | |||
| Obs14 | 1 | 32.000 | 27.014 | 4.986 | 0.527 | |||
| Obs15 | 1 | 4.000 | 6.681 | -2.681 | -0.283 | 8.389 | 16.40 | 51.15 |
| Obs16 | 1 | 8.000 | 9.779 | -1.779 | -0.188 | |||
| Obs17 | 1 | 16.000 | 16.491 | -0.491 | -0.052 | |||
| Obs18 | 1 | 24.000 | 22.235 | 1.765 | 0.187 | |||
| Obs19 | 1 | 32.000 | 26.850 | 5.150 | 0.545 | |||
| Obs20 | 1 | 4.000 | 6.541 | -2.541 | -0.269 | 8.4955 | 16.81 | 50.53 |
| Obs21 | 1 | 8.000 | 10.636 | -2.636 | -0.279 | |||
| Obs22 | 1 | 16.000 | 16.915 | -0.915 | -0.097 | |||
| Obs23 | 1 | 24.000 | 22.634 | 1.366 | 0.144 | |||
| Obs24 | 1 | 32.000 | 27.355 | 4.645 | 0.491 |
Note: PLSR is Partial Least Squares Regression, SD, mean and RSD are mentioned for each observation
Table 3: Statistical Analysis of Htz By Pls Method
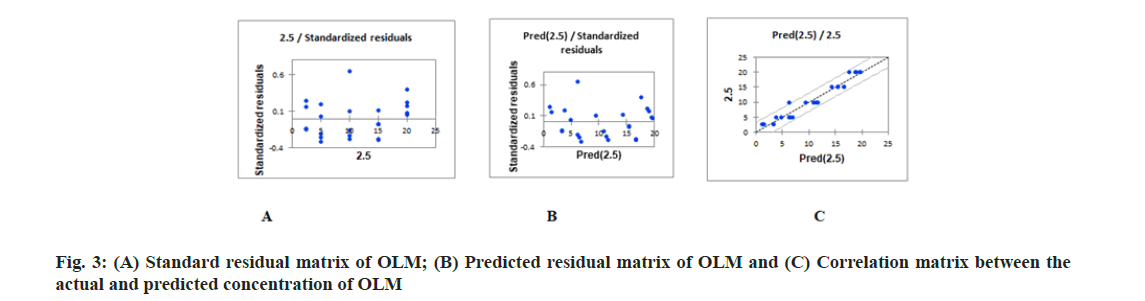
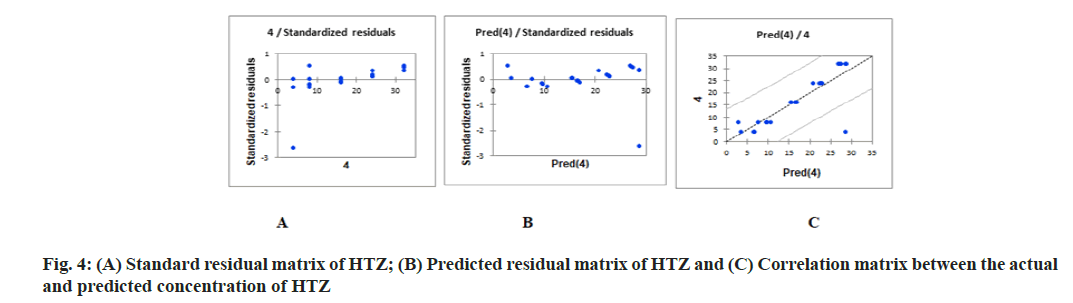
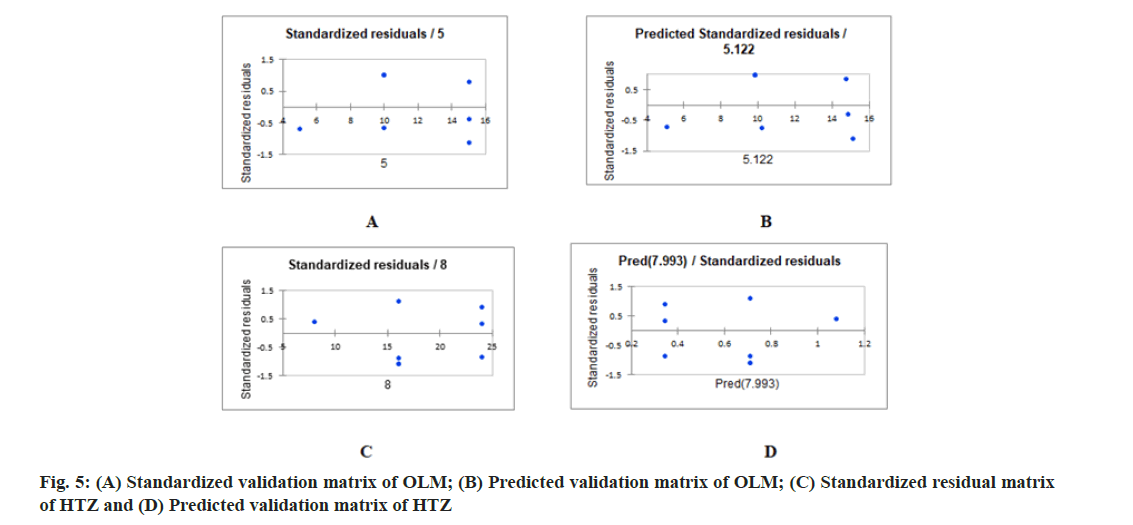
PLS regression, uses the two-block predictive PLS model to model the relationship between two matrices, X and Y. Also, Partial Least Squares Regression (PLSR) models the structure of X and of Y, which gives richer results than the traditional multiple regression approach. PLSR and similar approaches provide quantitative multivariate modeling methods, with inferential possibilities similar to multiple regression, t-tests and Analysis of Variance (ANOVA). The evaluation of the predictive abilities of the models was performed by plotting the actual known concentrations against the predicted concentrations and the plot of the actual known concentrations against the predicted concentrations are mentioned in fig. 3A-fig. 3C for OLM and fig. 4A-fig. 4C for HTZ. Standardized validation matrix and predicted validation matrix of OLM and HTZ are mentioned in fig. 5A-fig. 5.
The accuracy of the method was carried out at three levels 80 %, 100 % and 120 % of the working concentration of the sample. A calculated amount of standard solution of OLM and HTZ were spiked with added sample solution to prepare level 80 %, 100 % and 120 % of the working concentration. The analysis procedure was repeated three times. The result was shown in Table 4. The statistical parameters of the validation set and calibration set were illustrated in Table 5 and Table 6.
| Level of spiking | Blank | Amount of standard drug added | Amount of drug recovery | % Recovery | |||
|---|---|---|---|---|---|---|---|
| OLM | HTZ | OLM | HTZ | OLM | HTZ | ||
| Unspiked | |||||||
| 80 | 0 | 4 | 7.6 | 3.97±0.02 | 7.58±0.56 | 98.93±0.87 | 99.47±1.54 |
| 100 | 0 | 5 | 8 | 4.98±0.02 | 7.96±0.39 | 99.85±0.20 | 99.65±0.25 |
| 120 | 0 | 6 | 9.6 | 5.59±0.02 | 9.57±0.20 | 99.63±0.37 | 99.80±1.68 |
Note: Percent recovery is expressed at 3 different levels
Table 4: Accuracy Data of Olm And Htz
| Observation | Weight | Actual value | Predicted value | Residual matrix | Standard residual matrix | Mean | SD |
|---|---|---|---|---|---|---|---|
| 1 | 1 | 5 | 5.122 | -0.122 | -5.7547 | -0.015 | 0.0212 |
| 2 | 1 | 5 | 5.092 | -0.092 | -4.3396 | ||
| 3 | 1 | 10 | 9.841 | 0.159 | 0.76849 | 0.038 | 0.2069 |
| 4 | 1 | 10 | 9.844 | 0.156 | 0.75399 | ||
| 5 | 1 | 10 | 10.201 | -0.201 | -0.9715 | ||
| 6 | 1 | 15 | 15.122 | 0.122 | 1.88854 | 0.18 | 0.0646 |
| 7 | 1 | 15 | 14.83 | 0.17 | 2.63158 | ||
| 8 | 1 | 15 | 14.75 | 0.25 | 3.86997 |
Note: Statistical parameters of the validation set and calibration set were illustrated
Table 5: Validation Set Analysis By Pls Method for Olm
| Observation | Weight | Actual value | Predicted value | Residual matrix | Standard residual matrix | Mean | SD |
|---|---|---|---|---|---|---|---|
| 1 | 1 | 8 | 7.993 | 0.007 | 0.12374 | 0.047 | 0.05657 |
| 2 | 1 | 8 | 7.913 | 0.087 | 1.53982 | ||
| 3 | 1 | 16 | 16.024 | -0.024 | -0.4932 | -0.0116 | 0.04866 |
| 4 | 1 | 16 | 16.012 | -0.012 | -0.2466 | ||
| 5 | 1 | 16 | 15.905 | 0.095 | 1.95232 | ||
| 6 | 1 | 24 | 23.954 | 0.046 | 1.36905 | 0.01666 | 0.03361 |
| 7 | 1 | 24 | 23.976 | 0.024 | 0.71416 | ||
| 8 | 1 | 24 | 24.02 | -0.02 | -0.5951 |
Note: PLSR is Partial Least Squares Regression, statistical parameters of the validation set and calibration set were illustrated
Table 6: Validation Set Analysis By Pls Method for Htz
The validated chemometrics-assisted UV spectrophotometric methods were used in the analysis of the marketed formulation Olmetor-H with a label claim of 12.5 mg of HTZ and 20.0 mg of OLM per tablets. The results for drug assays show good agreement with the label claims (Table 7).
| Label claim | % Label claim | ||
|---|---|---|---|
| HTZ | OLM | HTZ | OLM |
| 12.5 | 20 | 101.2 | 99.8 |
| 99.8 | 102 | ||
| 100.9 | 100.9 | ||
| 101.2 | 101.2 | ||
| 101.6 | 101.6 | ||
| Mean | 100.98 | 100.66 | |
| SD± | 0.61 | 0.82 | |
| RSD | 0.6 | 0.81 | |
Note: Marketed formulation Olmetor-H with a label claim of 12.5 mg of HTZ and 20.0 mg of OLM per tablets
Table 7: Assay Results of Olm and Htz
PLS chemometric method was applied successfully to the simultaneous determination of OLM and HTZ in the pharmaceutical dosage form. The summary parameters of the chemometric method were shown in Table 8.
| Parameters | OLM | HTZ |
|---|---|---|
| PLS | PLS | |
| Range (μg/ml) | 2.5-20 | 4-32 |
| Wavelength (nm) | 200-350 | |
| Δλ (nm) | 3 | 3 |
| Factor | 7 | 7 |
| % recovery | 98.93±99.85 | 99.47±99.80 |
| Assay | 100.66 | 100.98 |
Note: The summary of the UV method is expressed
Table 8: Summary of Uv Spectrophotometric Method Using Pls Method
Conventional multi-component UV spectroscopic methods are not suitable for combination drugs having a narrow difference in absorption maxima lambda (λmax). In such cases, chemometric method serves as an alternative to other sophisticated methods like HPLC. When once the calibration matrix is built and stored in the data computation device, the samples can simply be prepared, diluted and absorbance was measured and the concentration of the sample was read from the stored matrix. PLS was applied successfully for the simultaneous determination of OLM and HTZ in laboratory mixtures and pharmaceutical formulation.
Acknowledgements:
The author(s) would like to thank Sidmak Laboratories Pvt. Ltd. for providing gift samples of OLM and HTZ and Nootan College of Pharmacy, Visnagar for providing the necessary infrastructure to carry out the research.
Conflict of interests:
The authors declared no conflicts of interest.
References
- Koike H. Olmesartan medoxomil, a novel potent angiotensin II blocker. Annu Rep Sankyo Res Lab 2003;55:1-91.
- Mire DE, Silfani TN, Pugsley MK. A review of the structural and functional features of olmesartan medoxomil, an angiotensin receptor blocker. J Cardiovasc Pharmacol 2005;46(5):585-93.
[Crossref] [Google Scholar] [PubMed]
- Koechel DA, Block JH, Beak JM. Textbook of organic medicinal and pharmaceutical chemistry. Philadelphia: Lippincott Williams and Wilkins; 2004.
- Celebier M, Altinoz S. Determination of olmesartan medoxomil in tablets by UV-Vis spectrophotometry. Pharmazie 2007;62(6):419-22.
[Crossref] [Google Scholar] [PubMed]
- Ça?lar S, Önal A. Two simple and rapid spectrophotometric methods for the determination of a new antihypertensive drug olmesartan in tablets. J Anal Chem 2010;65(3):239-43.
- Sharma RN, Pancholi SS. RP-HPLC-DAD method for determination of olmesartan medoxomil in bulk and tablets exposed to forced conditions. Acta Pharm 2010;60(1):13-24.
[Crossref] [Google Scholar] [PubMed]
- Bajerski L, Rossi RC, Dias CL, Bergold AM, Fröehlich PE. Stability-indicating LC determination of a new antihypertensive, olmesartan medoxomil in tablets. Chromatographia 2008;68(11):991-6.
- Çelebier M, Altinöz S. Development of a CZE method for the determination of olmesartan medoxomil in tablets. Chromatographia 2007;66(11):929-33.
- Farthing D, Fakhry I, Ripley EB, Sica D. Simple method for determination of hydrochlorothiazide in human urine by high performance liquid chromatography utilizing narrowbore chromatography. J Pharm Biomed Anal 1998;17(8):1455-9.
[Crossref] [Google Scholar] [PubMed]
- Vaidya VV, Roy S, Yetal SM, Joshi SS, Parekh SA. LC-MS-MS determination of olmesartan in human plasma. Chromatographia 2008;67(1):147-50.
- Liu D, Hu P, Matsushima N, Li X, Li L, Jiang J. Quantitative determination of olmesartan in human plasma and urine by liquid chromatography coupled to tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2007;856(1-2):190-7.
[Crossref] [Google Scholar] [PubMed]
- Ouyang J, Baeyens WR, Delanghe J, Calokerinos AC. Cerium (IV)-based chemiluminescence analysis of hydrochlorothiazide. Talanta 1998;46(5):961-8.
[Crossref] [Google Scholar] [PubMed]
- Zendelovska D, Sta?lov T, Miloševski P. Development of solid?phase extraction method and its application for determination of hydrochlorothiazide in human plasma using HPLC. Biomed Chromatogr 2004;18(2):71-6.
[Crossref] [Google Scholar] [PubMed]
- Razak OA. Electrochemical study of hydrochlorothiazide and its determination in urine and tablets. J Pharm Biomed Anal 2004;34(2):433-40.
[Crossref] [Google Scholar] [PubMed]
- Rote AR, Bari PD. Spectrophotometric estimation of olmesartan medoxomil and hydrochlorothiazide in tablet. Indian J Pharm Sci 2010;72(1):111-3.
[Crossref] [Google Scholar] [PubMed]
- Bhusari KP, Khedekar PB, Dhole S, Banode VS. Derivative and Q-analysis spectrophotometric methods for estimation of hydrochlorothiazide and olmesartan medoxomil in tablets. Indian J Pharm Sci 2009;71(5):505-8.
[Crossref] [Google Scholar] [PubMed]
- Rote AR, Bari PD. Ratio spectra derivative and zero-crossing difference spectrophotometric determination of olmesartan medoxomil and hydrochlorothiazide in combined pharmaceutical dosage form. AAPS PharmSciTech 2009;10(4):1200-5.
[Crossref] [Google Scholar] [PubMed]
- Kumari B, Burande MD, Choudhari PK. Spectrophotometric method for simultaneous estimation of olmesartan medoxomil and hydrochlorothiazide in tablets. Res J Pharm Biol Chem Sci 2013;4:104-9.
- Bari PD, Rote AR. RP-LC and HPTLC methods for the determination of olmesartan medoxomil and hydrochlorothiazide in combined tablet dosage forms. Chromatographia 2009;69(11):1469-72.
[Crossref] [Google Scholar] [PubMed]
- Kadukar S, Gandhi S, Ranjane P, Ranher S. HPTLC analysis of olmesartan medoxomil and hydrochlorothiazide in combination tablet dosage forms. J Planar Chromatogr 2009;22(6):425-8.