- *Corresponding Author:
- S. B. Kalidhar
Department of Chemistry, CCS Haryana Agricultural University, Hisar-125 004, India.
E-mail: kalidhar@hau.ernet.in
| Date of Submission | 14 October 2005 |
| Date of Revision | 06 March 2006 |
| Date of Acceptance | 23 December 2006 |
| Indian J. Pharm. Sci., 2006, 68 (6): 804-806 |
Abstract
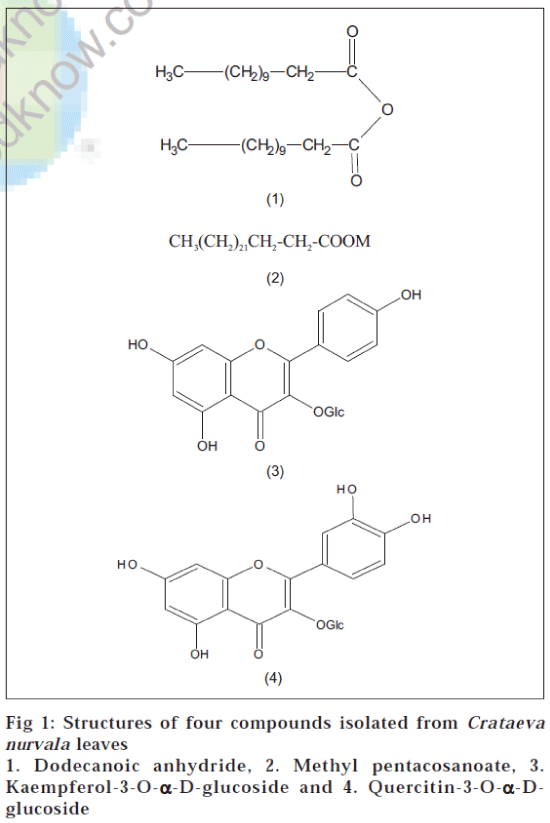
Chemical investigation of Crataeva nurvala leaves resulted in the isolation of four compounds, which are dodecanoic anhydride, methyl pentacosanoate, kaemferol-3-O-α-D-glucoside and quercitin-3-O-α-D-glucoside. Dodecanoic anhydride and methyl pentacosanoate are being reported for the first time from this plant. Kaemferol-3-O-α-D-glucoside and quercitin-3-O-α-D-glucoside have already been reported from this plant.
The genus Crataeva (family: Capparidaceae) is named in honour of the Greek botanist Crataevas. Crataeva nurvala is commonly known as barna and varuna [1] and distributed, throughout India and tropical regions of the world: wild or cultivated [2]. It is often found along streams and also in dry, deep boulder formations in sub Himalayan tract [3]. It is useful as a laxative, antipyretic, antilithic, antihelminthic, diuretic, demulcent, stomachic, alterative tonic in chest and blood diseases and is reported to cure disorders of urinary organs [4]. It is very useful as antiinflammatory drug and acts as a good contraceptive for women. This plant is known to possess immense pharmacological activity and antilithic properties [5]. The major component isolated from this plant is lupeol, which is used to treat hypercrystalluria, hyperoxaluria and hypercalciuria [6]. The compound is also widely used to treat urinary disorders like urolithiasis, and it decreases elevated concentration of oxalate, phosphorous and magnesium in renal tissue [7]. Lupeol also possesses antipyretic, analgesic, antiinflammatory activity [8]. Since there is scanty data on the chemical components of its leaves, we have undertaken the present study.
The melting points were determined on Ganson electrical melting point apparatus. 1H NMR spectra were recorded on Bruker AC-300F 300 MHz NMR spectrometer in CDCl3 using TMS as internal standard. Chemical shifts are given in δ (ppm), and CDCl3 was used as solvent. IR spectra were recorded on Hitachi 570 infrared spectrophotometer. Mass spectra were recorded on VG70S 11-250J GC-MS-DS mass spectrometer.
Leaves of Crataeva nurvala (5 kg) were collected from Landscape, CCS H.A.U., Hisar. These were crushed, dried and extracted with hot methanol, each time refluxing for 6 h. The methanolic extract was concentrated over water bath under reduced pressure. The extractives were then subjected to silica gel (60-120 mesh) column chromatography. The column chromatography of Crataeva nurvala leaves afforded four compounds (A-D) using petroleum ether, benzene, ethyl acetate, methanol and their mixtures as eluents, as shown in the Table 1.
| Compound | Solvent system | Volume (ml) |
|---|---|---|
| - | Petroleum ether | 124×500 |
| Compound A | Benzene-Petroleum ether (1:9) | 39×500 |
| Compound B | Benzene-Petroleum ether (1:3) | 34×500 |
| - | Benzene-Petroleum ether (1:1) | 30×500 |
| - | Benzene | 27×500 |
| Ethyl acetate-Benzene (1:19) | 30×500 | |
| - | Ethyl acetate-Benzene (1:9) | 30×500 |
| - | Ethyl acetate-Benzene (1:3) | 31×500 |
| - | Ethyl acetate-Benzene (1:1) | 24×500 |
| - | Ethyl acetate | 25×500 |
| Compound C | Methanol-Ethyl acetate (1:49) | 25×500 |
| - | Methanol-Ethyl acetate (1:19) | 30×500 |
| - | Methanol-Ethyl acetate (1:9) | 20×500 |
| Compound D | Methanol-Ethyl acetate (1:3) | 40×500 |
Table 1: Compounds isolated from the leaves of crataeva nurvala
Compound A (dodecanoic anhydride, 1, fig. 1) was obtained on elution with benzene-petroleum ether (1:9) and it crystallized from benzene, 10 mg, mp: 45° [9]. IR (K Br, νmax in cm-1): 721, 798, 1023, 1163, 1257, 1463, 1731, 2848, 2914; 1H NMR (CDCl3) δ, ppm: 2.25 (4H, t, J 7.0 Hz, 2 x -CH2COO-), 1.60 (4H, m, 2 x -CH2CH2COO-), 1.25 (32H, br, 16 x -CH2-), 0.88 (6H, t, J 7.5 Hz, 2 x CH3); GC-MS (m/z), 382 (M+ ), 354, 204, 176, 161, 133, 91.
Compound B (methyl pentacosanoate, 2) was obtained on elution with benzene-petroleum ether (1:3), 10 mg, mp: 62° [10]. IR (K Br, νmax in cm-1): 668, 758, 1215, 1710, 2401, 2917, 3020. 1H NMR (CDCl3) δ, ppm: 3.64 (3H, s, -OMe), 2.17 (2H, t, J 7.0 Hz, -CH2COOMe), 1.54 (2H, m, CH2CH2COOMe), 1.25 (42H, br, 21x -CH2- ), 0.88 (3H, t,.medknow.com). J 7.05 Hz, -CH3); GC-MS (m/z): 396 (M+ ), 380, 281, 261, 254, 207, 157,148, 135, 122, 118, 105, 98, 95.
Compound C (kaempferol-3-O-α-D-glucoside, 3) was obtained on elution with methanol-ethyl acetate (1:49), 35 mg, mp: 178°[11]. It gave positive Mg/HCl test. IR (K Br, νmax in cm-1): 671, 801, 907, 1220, 1371, 1439, 1634, 2364,2931, 3484. The compound was acetylated with Ac2O/Py:mp: 178°11 and its 1H NMR (CDCl3) δ, ppm: 7.92 (2H, d, J 7.0 Hz, H-2’, H-6’), 7.26 (2H, d, J 7.0 Hz, H-3’, H-5’), 7.08 (1H, d, J 2.0 Hz, H-8), 6.78 (1H, d, J 2.0 Hz, H-6), 3.94 5.64 (7H, m, 7H of sugar), 2.33 (3H, s, -OAc), 2.17(6H, s, 2 x -OAc), 2.12 (3H, s, -OAc), 2.06 (3H, s, -OAc), 2.04 (3H, s, -OAc), 2.00 (3H, s, -OAc), 1.98 (3H, s, -OAc); GC-MS (m/z), 448 (M+), 279, 207, 167, 149, 132, 104, 83.
Compound D (quercitin-3-O-α-D-glucoside, 4) was obtained on elution with methanol-ethyl acetate (1:3), 40 mg, mp: 238°[12]. It responded to colour reaction with Mg/ HCl. IR (K Br, νmax in cm-1): 671, 801, 907, 1220, 1371, 1439, 1634, 2364, 2931, 3484. The compound was acetylated with Ac2O/Py: mp: 238°12 and its 1H NMR (CDCl3) δ, ppm: 7.89 (2H, m, J 7.0 Hz, H-2’), 7.98 (2H,dd, J 2.0 Hz, J 7.0 Hz, H-6’), 7.26 (1H, m, H-5’), 7.09 (1H, d, J 2.0 Hz, H-8), 6.78 (1 H, d, J 2.0 Hz, H-6), 3.92-5.60 (7H, m, 7H of sugar), 2.40 (3H, s, -OAc), 2.33(3H, s, -OAc), 2.17(3H, s, -OAc), 2.12 (3H, s, -OAc), 2.06 (3H, s, -OAc), 2.04 (3H, s, -OAc), 2.00 (3H, s, -OAc), 1.98 (3H, s, -OAc); GC-MS (m/z) 464 (M+), 279, 207, 170, 128, 97.
Kaemferol-3-O-α-D-glucoside and quercitin-3-O-α-D- glucoside have already been reported from this plant, while dodecanoic anhydride and methyl pentacosanoate are being reported for the first time from this plant. 3-O-methyl quercitin and quercitin are also being reported from the leaves of this plant [13].
Acknowledgements
Authors are thankful to Landscape Officer, CCS H.A.U., Hisar, for the supply of plant material.
References
- Bhattacharjee, S.K., In; Handbook of Medicinal Plants. Pointer Publishers, Jaipur, 1998, 228.
- Kirtikar, K.R. and Basu, B.D., In; Indian Medicinal Plants, Vol II, Bishan Singh Mahendra Paul Singh, Dehradun, 1984, 830.
- Agarwal, V.S., Drug Plants of India, Vol. I. Kalyani Publishers, New Delhi, 1997, 299.
- Drury, C.H., In; The Useful Plants of India, International Book Distributors, Dehradun, 1978, 353.
- Singh, R.G. and UshaKapoor, S., J. Res. Edu. Indian Med., 1991, 10, 35.
- Anand, R., Patnaik, G.K., Kulshershta, D.K. and Dhawan, B.N, Proceeding 24 th Indian Pharmacol. Soc. Conference, Ahmedabad, Gujarat, India, 1994, A10.
- Baskar, R., Malini, M.M., Varalakshmi, P., Bal Krishna, K., Rao, R.B., Meenalakshmi- Malini, M. and BhimaRao, R, Fitoterapia, 1996, 67, 121.
- Singh, S., Bani, S., Singh, G.B., Gupta, B.D., Banerjee, S.K. and Singh, B., Fitoterapia, 1997, 68, 9.
- Heilbron, I., Cook, A.H., Bunbury, H.M., Hey, D.H., Eds., In; Dictionary of Organic Compounds, Eyre and Spottiswoode, London, 1965, 1568.
- Sasaki, S., In; Handbook of proton-NMR Spectra and Data, Academic Press, Tokyo, 1986, 2618.
- Buckingham, J., Eds., In; Dictionary of Natural Products, Chapman and Hall, London, 1994, 2570.
- Buckingham, J., Eds., In; Dictionary of Natural Products, Chapman and Hall, London, 1994, 2831.
- Arya, Chitra, Daniel, M. and Arya, C., Natl. Acad. Sci. Lett. 1997, 20, 38.