- *Corresponding Author:
- S. Banerjee
Department of Pharmaceutical Sciences and Technology, Birla Institute of Technology, Mesra, Ranchi-835 215, India
E-mail: sbanerjee@bitmesra.ac.in
| Date of Submission | 21 September 2016 |
| Date of Revision | 18 February 2017 |
| Date of Acceptance | 28 July 2017 |
| Indian J Pharm Sci 2017;79(5):785-793 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Among all the secondary complications of type 2 diabetes, cognitive impairment associated with diabetes is less well studied and characterized. We made an attempt to investigate the molecular mechanisms involved in cognitive decline in high fat diet-streptozotocin model of type 2 diabetes. High-fat diet and a single low dose streptozotocin (25 mg/kg) were used to induce diabetes in Sprague-Dawley rats. Developments of cognitive deficit in these animals were measured using change in transfer latency in elevated plus maze test and step-down latency in passive avoidance test. This was followed by biochemical estimation of acetylcholinesterase and reduced glutathione levels followed by histological evaluation of cortical and hippocampal slices. The high fat diet-streptozotocin animals developed type 2 diabetes, characterized by an increase in fasting blood glucose levels >250 mg/kg, marked increase (P<0.001) in triglyceride (207±7.2) and total cholesterol level (125.3±2.7) with a decrease (P<0.001) in high-density lipoproteins (14.27±1.1) when compared to control animals. After 4 weeks of development of type 2 diabetes high fat diet-streptozotocin animals showed signs of cognitive deficit with a significant decline in step-down latency (P<0.001) and a significant increase in transfer latency (P<0.001) compared to controls. The high fat diet-streptozotocin animals also showed significant decrease (P<0.001) in cortico-hippocampal glutathione levels and increase in (P<0.001) acetylcholinesterase activity as compared to control animals suggesting type 2 diabetes-associated oxidative stress and decrease in hippocampal Ach activity. Immunohistochemical studies in these animals showed pronounced astrogliosis and microglial activation in the cortical and hippocampal slices suggesting neuroinflammatory changes. We also found significant loss (P<0.001) of hippocampal neurons at CA1 and CA3 region in the diabetic rats as compared with healthy controls. Antidiabetic compound glibenclamide (10 mg/kg for 3 weeks) partly reversed the above neurobehavioral, biochemical and histological changes in the diabetic rat brain. Hence we show that high fat diet-streptozotocin type 2 diabetic rats may develop signs of cognitive impairment. This cognitive decline was found to be associated with an increase in acetylcholinesterase activity and loss of hippocampal neurons. Oxidative stress and neuroinflammatory changes in the brain of these animals may be responsible for hippocampal neuronal loss, which could be partly reversed upon glibenclamide treatment.
Keywords
Brain, learning and memory, neuroinflammation, neuron, streptozotocin, type 2 diabetes
Diabetes mellitus is a progressive metabolic disorder. It may be due to defects in insulin secretion or insulin action resulting in altered glucose metabolism and hyperglycaemia. Diabetes mellitus may also affect lipid and protein metabolism and vice-versa. In both developed and developing nations diabetes remains a highly prevalent metabolic disorder. The number of type 2 diabetes (T2D) cases continue to rise, about 371 million people worldwide are suffering from diabetes, and this figure is steadily rising [1]. T2D associated secondary complications are also on the rise. It is associated with various secondary complications including: cardiomyopathy, retinopathy, neuropathy, nephropathy and cognitive impairment [2].
Diabetes-associated learning and memory deficit is a relatively less studied complication found in patients with prolonged diabetes [1]. Mechanisms associating T2D and cognitive impairment may be broadly classified into cerebrovascular and noncerebrovascular. Cerebrovascular abnormalities primarily include brain infarcts [3] and white matter hyperintensities (WHI) associated cerebral amyloid angiopathy [4-6]. Prolonged hyperglycaemia may also enhance advanced glycation end products (AGEs) and their receptor (RAGE) [7,8]. Enhanced AGE may lead to oxidative stress and neuroinflammatory response. During brain injury microglial cells may secrete neurotoxic proinflammatory cytokines. While activated astrocytes may highly express glial fibrillary acidic protein (GFAP), which may prevent axonal regeneration [9,10] and release chemokine’s like monocyte chemoattactant protein (MCP)-1, thus recruiting more microglia to the site of injury. This creates an inflammatory milieu detrimental to the hippocampal neurons. Current rodent models of T2D are mostly transgenic (db/db mouse, ob/ob mouse, Zucker fatty rat), which are not only expensive to maintain but also fail to mimic the human form of this multifactorial disease [11]. Thus understanding the pathogenesis of cognitive impairment associated with T2D in patients continues to be a challenge using transgenic animals. Reed et al. [12] first proposed a non-transgenic animal model for T2D, which was later standardized by Srinivasan et al. [13]. It involved a high-fat diet (HFD), to bring about insulin resistance and glucose intolerance. Followed by treatment with low dose streptozotocin (STZ), responsible for the moderate β-cell damage. This partial damage causes β-cells to secrete less insulin resulting in glucose tolerance resembling the human form of T2D. However, the dose of STZ is critical for development of type 1 or T2D in rodents. A study reported that even relatively low dose of STZ (>25 mg/kg) may induce type 1 diabetes like features including hypoinsulinemia, hyperkalaemia and weight loss due to β-cell damage in rats [14]. They suggested 25 mg/kg be a more appropriate dose for Sprague Dawley (SD) rats that mirrored the pathological and metabolic features of T2D patients. Thus animal models of diabetes using >25 mg/kg STZ seems to mimic more of the human form of type 1 diabetes. Datusalia and Sharma in 2014 [15] reported the development of cognitive impairment (15 w after induction of diabetes) in SD rats using HFD (2 w) and a relatively high dose of STZ (35 mg/kg), which may depict more of a type 1-diabetes associated cognitive impairment in human.
Here we successfully induced T2D in SD rats using HFD (4 w) followed by single low dose STZ (25 mg/kg). These animals showed signs of cognitive impairment 4 w after induction of diabetes (earlier than previously reported non-transgenic models), which was then characterized by various behavioural parameters. We further determined the mechanisms involved in the pathogenesis of diabetes-associated cognitive decline and studied the effect of hypoglycaemic agent, glibenclamide on diabetes associated cognitive changes in these animals.
Materials and Methods
SD male rats weighing 160-180 g were housed for acclimatization in the institute animal house maintained at controlled room temperature (22±2°) and humidity (55±5%) with 12:12 h light and dark cycle and provided with commercially available normal pellet diet (NPD, Amrut Diet, New Delhi, India) and water ad libitum before dietary manipulation. The guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Govt. of India were followed and prior permission was sought from the Institutional Animal Ethics Committee (IAEC) for conducting the study.
Animal model development
SD rats (n=10/group) were allocated into two different dietary regimens A) NPD and B) HFD;NPD+310 g/kg lard; Baroda Earth Private Limited, Vadodara, India. Animals were primarily divided into three different groups: control on NPD; diabetic group (HFD+25 mg/kg STZ; Sigma-Aldrich, St. Louis, MO, USA); diabetic group+glibenclamide (10 mg/kg for 3 w). For neurobehavioral studies scopolamine was used as amnestic agent while piracetam (Alkem Laboratories Limited, Pune, India) as a memory enhancer.
Every week after the initiation of diabetes neurobehavioral studies were performed to identify signs of cognitive impairment.
Induction of diabetes
One month of dietary manipulation (HFD) makes the animals insulin resistant and then a single dose of STZ (25 mg/kg) dissolved in citrate buffer (pH 4.4) was given i.p after 12 h of fasting. It was followed by administration of 5% glucose solution to prevent hypoglycaemic shock. One week after application of STZ blood glucose levels were measured. Animals with more than 250 mg/dl were considered to develop diabetes. Circulating glucose levels were measured by tail prick every week for 4 w using one touch glucose strips (OneTouch Ultra, Johnson & Johnson Private Ltd, India). After 4 w of development of diabetes the animals were subjected to neurobehavioral studies.
Animals were allowed to remain diabetic for 4 w. This was followed by treatment with oral hypoglycaemic agent, glibenclamide (10 mg/kg; gift sample from Wockhardt Ltd., India) for 3 w. After 3 w, of treatment animals were subjected to neurobehavioral studies.
Estimation of lipid profile
Since patients with T2D and animal models of T2D may show altered lipid profile [16]; total cholesterol, high-density lipoproteins (HDL), and triglyceride levels were measured in diabetic and control rats. The blood was collected by the retro-orbital method. The serum was separated from the plasma by centrifugation (Remi Industries Ltd, Mumbai, India) at 15 000 rpm for 10 min. The serum thus obtained was then used for further analysis. Triglyceride, total cholesterol, HDL, and low-density lipoproteins (LDL) cholesterol was measured using kit as per manufacturer's instructions (Span Diagnostics; Span Diagnostics Ltd., Surat, India).
Estimation of reduced glutathione (GSH) level
GSH levels were estimated as described by Moron et al. [17]. Brain tissue homogenates were prepared by adding brain tissue in the phosphate buffer (pH 8) with 1% Triton X in it followed by 1 ml of 10% trichloroacetic acid along with 1 ml distilled water and 1 ml tissue homogenate. The mixture was cooled for 10 min and centrifuged at 2000 rpm for 15 min at 4°. To the supernatant 4 ml of 5,5'-dithiobis- [2-nitrobenzoic acid] (DTNB) and 1.5 ml phosphate buffer was added and absorbance was measured at 412 nm. Different concentrations of standard GSH was prepared for the standard plot. GSH was measured as μg GSH/mg protein.
Estimation of acetylcholinesterase (AchE)
AchE was estimated by employing Ellman's method with slight modification [18]. Brain tissue homogenate (10% w/v diluted to 2% w/v) was centrifuged at 1 00 000×g at 4°. To 0.4 ml of supernatant aliquots 2.6 ml phosphate buffer (pH 8, 0.1 M) was added followed by100 μl DTNB. The absorbance of the above solution was measured at 412 nm. Acetylthiocholine iodide (0.075 M) 20 μl was then added to above solution and mixed thoroughly, again absorbance was observed at 412 nm for 10 min at 30 s interval. The change in absorbance per min was calculated. One unit of AchE activity was defined as the number of micromoles of Ach iodide hydrolysed/min/mg of protein. The specific activity of AchE was expressed in μ mol/min/mg protein. Calculation of enzyme activity was done using the following formula, R=δA×total reaction volume (3.12 ml) 13.33 cm/μ mol×1 cm×sample volume (0.4 ml)×mg of protein where, R=rate in micro moles of substrate hydrolysed/min/mg of protein, δA=change in absorbance/min. Absorption coefficient for the nitrobenzoic acid at 412 nm=13.33 cm/μ mol.
Passive avoidance test
Passive avoidance test was done using apparatus, which consisted of 2 chambers connected with a door. One compartment was well lit whereas the other one was made dark and was fitted with metal grid floor, supplied with electric current (Medicraft Electromedical Inc). Both mice and rats have a preference for dark compartment. All the animals of each group entered the dark chamber after placing them in the illuminated chamber. As soon as animals entered the dark chamber, the door was closed. Animals which took more than 60 s to enter the dark chamber were excluded from the study. On the second day animals were allowed to travel from illuminated to the dark chamber, but this time, metal grid floor was supplied with electric current of (1 mA for 5 s). The animals were checked for memory retention (1, 2 and 7 d after administration of shock) as measured by latencies to enter the dark compartment (step through latency). Time taken by the animals to travel from illuminated to dark chamber with all the four paws in the dark compartment, i.e., step-through latency was recorded. During the observation period no current was applied. The step through latency on day 7 is reported. A cut off time of the experiment was kept as 300 s.
Elevated plus maze
Elevated plus maze apparatus (Medicraft Electromedical Inc) was used for this study, consisted of 2 open arms and 2 closed arms with side walls. A pair of 2 arms was extended from a central platform and the maze was elevated to a height of 60 cm from the floor. Prior to training animals of each group were allowed to explore all the arms for 10 s and then animals were kept on the open arm gently and made to move from open arm to closer ones, which are referred to as transfer latency (TL). Animals which showed TL of more than 90 s were excluded from the study. One day after training, memory retention of the above task was checked by observing TL of the animals of each group at days 1, 2 and 7. The TL on day 7 has been reported.
Histopathology
Animals were sacrificed by decapitation and brain was isolated out. The brain was then sliced into two halves horizontally at the centre plane, so that region of hippocampus gets exposed. Then both halves were kept in buffered formalin (pH 7.6) for fixation. Then tissue was dehydrated employing increasing concentration of alcohol (70 to 100%). The tissue was then embedded in molten wax, keeping the temperature of the wax 60- 70°. After hardening of the wax, tissue was sectioned into 5 μm thin sections. Sections were fixed on the slide and deparaffinised using decreasing concentration of alcohol to water so that water-soluble dye can penetrate into the tissue sections. Prepared slides were then treated with haematoxylin and eosin. CA1, CA2 and CA3 neurons were observed under the light microscope for any structural alterations. The number of neurons in CA1, CA2, and CA3 regions were counted/mm2 using camera lucida under 40X magnification (Alcon Scientific Industries, Ambala, India).
Immunohistochemistry
After performing the procedure explained above, 5 μm thin sections were fixed on the slide and deparaffinised in xylene and rehydrated using decreasing concentration of alcohol and xylene (100 to 50%). Tissue was passed through different steps of antigen retrieval by putting them on pre-heated antigen retrieval buffer. Tissues were blocked and permeabilized by incubating in phosphate buffer saline with 0.25% Triton X-100. Incubated in presence of primary antibodies diluted in 1% bovine serum albumin (BSA) in phosphate buffer saline and 0.1% Tween 20 at 4° overnight. GFAP (1:150; Abcam) was used as primary antibody for astrocytes while cd11b (1:200; Abcam) was used as primary antibody for activated microglial cells. To visualize astrocytes tissues were incubated with secondary antibody conjugated with fluorochrome Alexa fluor 488 (excitation: 495 nm and emission: 519 nm; Abcam). For activated microglia cells secondary antibody conjugated with Alexa Fluor 647 (excitation: 652 nm, emission: 668 nm; Abcam) were used. Incubation time with secondary antibody was for 1 h at room temperature in dark followed by washing with phosphate buffer saline.
Statistical analysis
The data were evaluated by one way ANOVA followed by Tukey's multiple comparison tests. P<0.001 was considered to be statistically significant. All statistical analysis was carried out using GraphPad Prism 5.0 software (GraphPad Software Inc., USA). The data were expressed as mean±SEM.
Results and Discussion
Fasting blood glucose (FBG) levels were measured for animals in all groups (n=10/group). The control animals showed normal blood glucose levels throughout the experiment. However, the HFD-STZ group animals showed a marked increase in FBG as compared to healthy animals. The FBG levels were >250 mg/dl throughout for HFD-STZ group. However, treatment with glibenclamide for 3 w reduced FBG to <150 mg/dl. The lipid profile for all the animals was measured. The control groups showed normal triglyceride (84.4±1.7), total cholesterol (49±3.5) and HDL levels 45.13±1.8. However, there was marked increase in triglyceride (207±7.2) and total cholesterol level (125.3±2.7) in animals on HFD-STZ (P<0.001) whereas the HDL (14.27±1.1) level was found to decrease (P<0.001) in these animals when compared to controls. Glibenclamide treatment decreased triglyceride levels significantly (148.3±7.2; P<0.001) with little effect on total cholesterol and HDL levels in diabetic animals. These diabetic animals did not show any change in systolic or diastolic blood pressure when compared to healthy control.
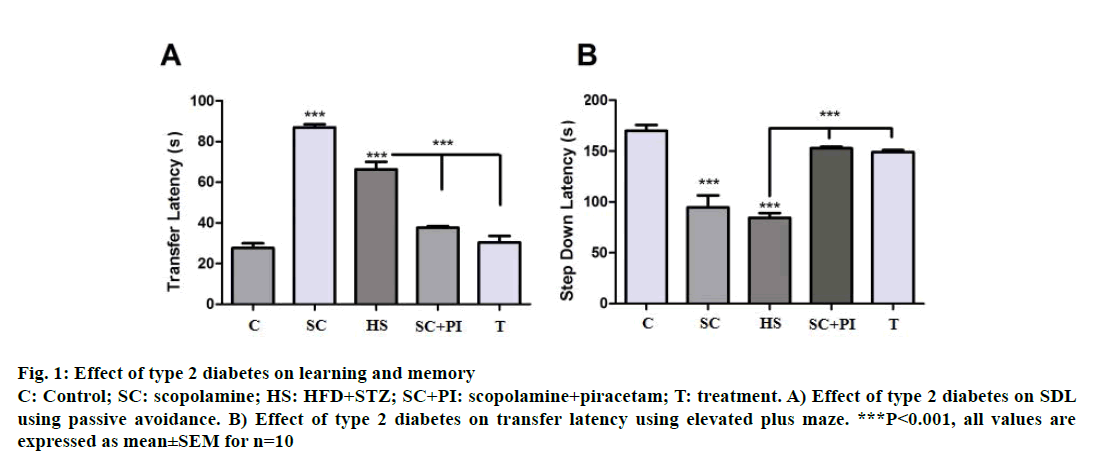
In passive avoidance increase in memory leads to increase in step-down latency (SDL), i.e., rats tend to stay on the shock free zone (SFZ) for a longer period. Hence scopolamine, an amnestic agent at a dose of 1 mg/kg was found to reduce SDL significantly (P<0.001) compared to control rats. Scopolamine along with piracetam significantly increased SDL when compared to scopolamine alone (P<0.001). HFD-STZinduced diabetic animals also showed a significant decline in SDL (P<0.001) when compared to control groups suggesting memory impairment. Diabetes associated memory impairment was significantly reversed after 3 w of glibenclamide treatment as shown by a significant increase in SDL (P<0.001) when compared to diabetic group (Figure 1A).
Figure 1: Effect of type 2 diabetes on learning and memory
C: Control; SC: scopolamine; HS: HFD+STZ; SC+PI: scopolamine+piracetam; T: treatment. A) Effect of type 2 diabetes on SDL
using passive avoidance. B) Effect of type 2 diabetes on transfer latency using elevated plus maze. ***P<0.001, all values are
expressed as mean±SEM for n=10
In elevated plus maze increase in memory reflects a decrease in TL, i.e., rats take less time to reach the closed arms when they are kept in the open space between the closed and open arms with their head facing the open arm. Here scopolamine at a dose of 1.0 mg/kg acting as an amnesic agent increased TL significantly i.e. rats did not remember the task hence took longer to travel from open arms to closed arms. Piracetam at a dose of 200 mg/kg acted as memoryenhancing drug, which reversed the scopolamineinduced amnesia hence reversed TL back to normal. HFD-STZ animals showed a significant increase in TL (P<0.001) compared to controls indicating memory impairment. Glibenclamide significantly reversed the memory impairment in the diabetic animals with the treated group showing a significant reduction in TL (P<0.001) as compared diabetic animals (Figure 1B).
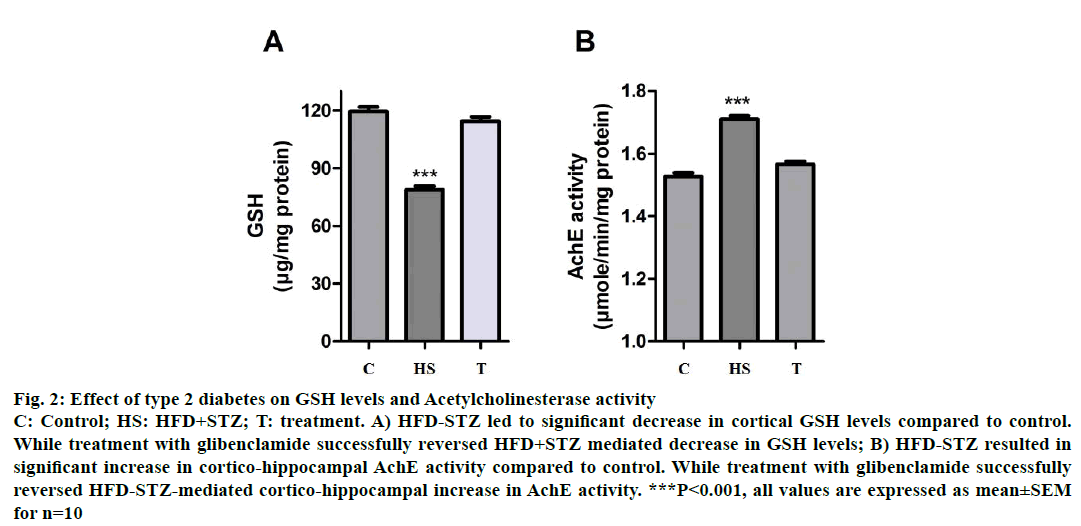
Decrease in cortical GSH levels have been positively correlated with increased oxidative stress of the CNS, which may result in neuronal damage leading to cognitive decline. HFD-STZ-induced diabetic animals showed a significant decrease (P<0.001) in cortical GSH levels as compared to control animals suggesting T2D-associated oxidative stress. While treatment with glibenclamide successfully reversed in the type HFD-STZ-induced animals, decrease in GSH levels (P<0.001, Figure 2A).
Figure 2: Effect of type 2 diabetes on GSH levels and Acetylcholinesterase activity
C: Control; HS: HFD+STZ; T: treatment. A) HFD-STZ led to significant decrease in cortical GSH levels compared to control.
While treatment with glibenclamide successfully reversed HFD+STZ mediated decrease in GSH levels; B) HFD-STZ resulted in
significant increase in cortico-hippocampal AchE activity compared to control. While treatment with glibenclamide successfully
reversed HFD-STZ-mediated cortico-hippocampal increase in AchE activity. ***P<0.001, all values are expressed as mean±SEM
for n=10
Increase in AchE activity has been correlated with decreased levels of Ach in the brain resulting in cognitive decline. HFD-STZ-induced diabetic animals showed a significant increase (P<0.001) in cortical AchE activity as compared to control animals suggesting T2D-associated cognitive decline. While treatment with glibenclamide (3 w) successfully reversed the T2D-associated increase in AchE activity (P<0.001, Figure 2B).
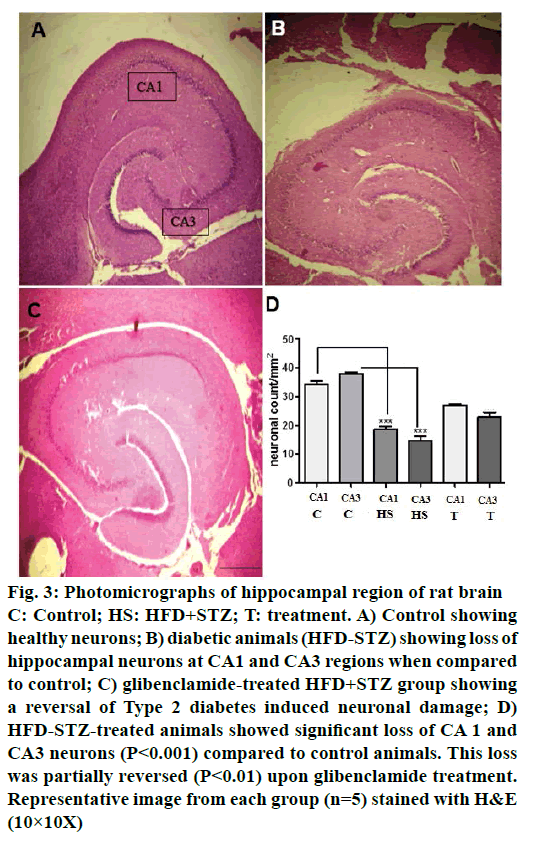
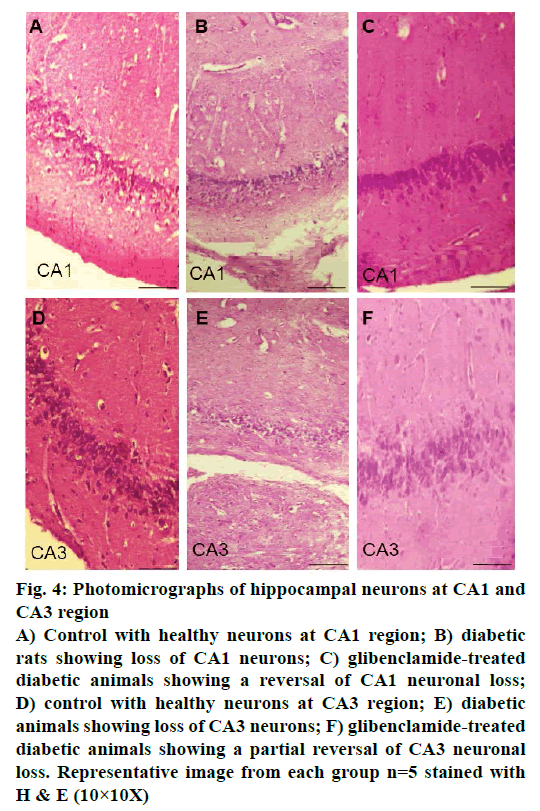
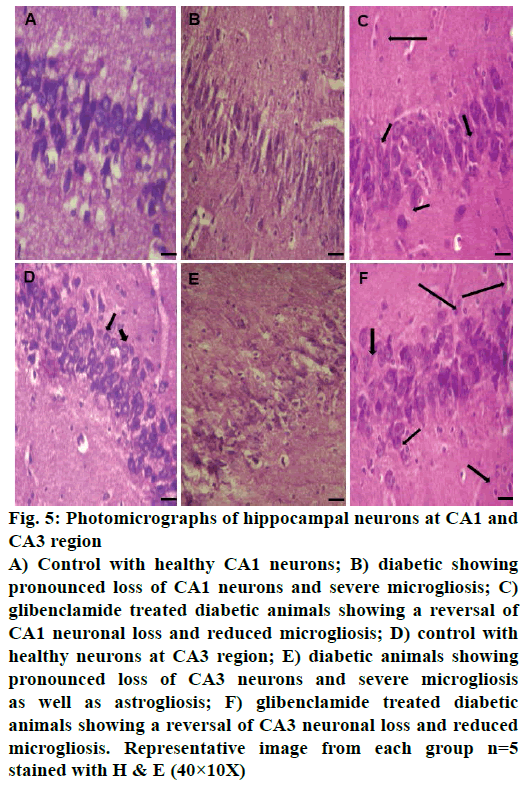
Control animals showed normal hippocampal neuronal density (Figures 3A and 4A) while HFD-STZ-induced T2D led to significant loss (P<0.001) of hippocampal neurons both at CA1 and CA3 region (Figures 3B and 4B). Treatment with glibenclamide for 3 w showed an ameliorating (P<0.01) effect on hippocampal neuronal damage in diabetic animals (Figures 3C and 4C). Under higher magnification (40X) while control animals showed normal CA1 and CA3 neuronal architecture (Figure 5A), pronounced neuronal loss, neuronal swelling as well as neuronophagia (engulfment of neurons by microglia) and severe microgliosis, was observed in CA1 and CA3 region of HFD-STZ-induced T2D diabetic rat hippocampus (Figure 5B). However, after glibenclamide treatment there was significant reduction in neuronal damage along with reduced microgliosis. However, chromatolysis and eccentric nuclei, which are characteristics of unhealthy neurons, were still present after glibenclamide treatment, which suggests partial reversal of the neuronal health (Figure 5C).
Figure 3: Photomicrographs of hippocampal region of rat brain
C: Control; HS: HFD+STZ; T: treatment. A) Control showing
healthy neurons; B) diabetic animals (HFD-STZ) showing loss of
hippocampal neurons at CA1 and CA3 regions when compared
to control; C) glibenclamide-treated HFD+STZ group showing
a reversal of Type 2 diabetes induced neuronal damage; D)
HFD-STZ-treated animals showed significant loss of CA 1 and
CA3 neurons (P<0.001) compared to control animals. This loss
was partially reversed (P<0.01) upon glibenclamide treatment.
Representative image from each group (n=5) stained with H&E
(10×10X)
Figure 4: Photomicrographs of hippocampal neurons at CA1 and
CA3 region
A) Control with healthy neurons at CA1 region; B) diabetic
rats showing loss of CA1 neurons; C) glibenclamide-treated
diabetic animals showing a reversal of CA1 neuronal loss;
D) control with healthy neurons at CA3 region; E) diabetic
animals showing loss of CA3 neurons; F) glibenclamide-treated
diabetic animals showing a partial reversal of CA3 neuronal
loss. Representative image from each group n=5 stained with
H & E (10×10X)
Figure 5: Photomicrographs of hippocampal neurons at CA1 and
CA3 region
A) Control with healthy CA1 neurons; B) diabetic showing
pronounced loss of CA1 neurons and severe microgliosis; C)
glibenclamide treated diabetic animals showing a reversal of
CA1 neuronal loss and reduced microgliosis; D) control with
healthy neurons at CA3 region; E) diabetic animals showing
pronounced loss of CA3 neurons and severe microgliosis
as well as astrogliosis; F) glibenclamide treated diabetic
animals showing a reversal of CA3 neuronal loss and reduced
microgliosis. Representative image from each group n=5
stained with H & E (40×10X)
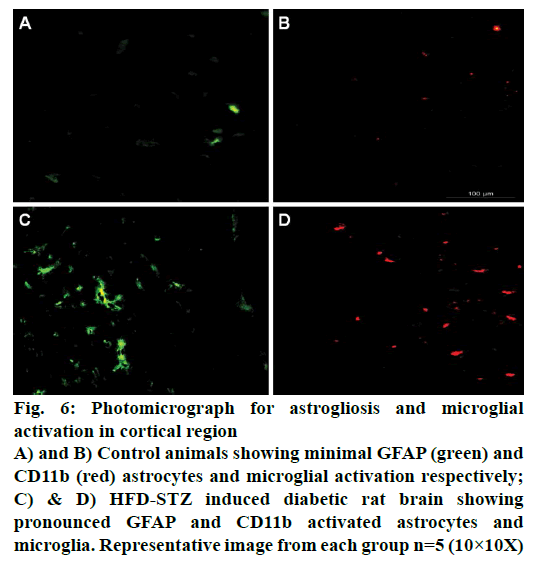
Immunohistochemical studies showed pronounced cortical astrogliosis as indicated by increased GFAP immunofluorescence (green; Figure 6) when compared to non-diabetic animal brain (Figure 6A). Significant microglial activation as indicated by increased cd11b immunofluorescence (red; Figure 6) was also observed in the cortical region of HFD-STZ-induced diabetic rats when compared to normal animals (Figure 6B).
Figure 6: Photomicrograph for astrogliosis and microglial
activation in cortical region
A) and B) Control animals showing minimal GFAP (green) and
CD11b (red) astrocytes and microglial activation respectively;
C) & D) HFD-STZ induced diabetic rat brain showing
pronounced GFAP and CD11b activated astrocytes and
microglia. Representative image from each group n=5 (10×10X)
T2D is highly prevalent among all age groups, but its prevalence is more among older (>65 y) individuals. Both type 1 and T2D have been shown to cause a decline in various domains of cognitive functions. The risk of cognitive decline is higher for people who are diabetic, prediabetic and suffering from metabolic syndrome [19]. Current pharmacological interventions may only partially improve the symptoms of cognitive decline associated with diabetes and other neurodegenerative disorders like AD. Various therapeutic approaches including pharmacological agents, implantation of stem cell, as well as compounds which may promote neuronal stem cell proliferation leading to regeneration of nerve fibres are being tried to reverse neurodegeneration [20]. However, a treatment which may ultimately reverse the neuronal damage and prevent the disease progression is still a distant reality. Hyperglycemia, neurovascular complication, AGE and altered insulin signalling all may have a role to play in the pathogenesis of cognitive impairment. However, the molecular mechanism may be highly complex and far from being clear. Lack of suitable animal models, which may mimic the human form of the disease is a major impediment towards understanding the pathogenesis of the disease.
This study was initiated with the objective to develop a non-transgenic murine model of T2D, which, upon prolonged hyperglycaemia may develop signs of cognitive decline. This model may be used to study the pathogenesis of diabetes associated cognitive impairment and utilized to screen novel pharmacological agents. Thus HFD (4 w) in combination with a low dose of STZ (25 mg/kg) was chosen for generating the rat model of T2D. As reported by other groups the animals showed insulin resistance, hyperglycaemia, and hypercholesteremia [13]. After 4 w of elevated blood glucose levels, the animals started showing signs of cognitive decline as measured by a decrease in SDL in passive avoidance test and increased TL in elevated plus maze.
The passive avoidance test used in this study is amygdala-dependent and evaluates emotional memory. It had been reported to be related to ‘long-term' or reference memory [21]. Increase in memory leads to increase in SDL, i.e., rat tends to stay on the SFZ for a longer period. The diabetic animals did not remember the task and thus stepped down more readily from SFZ to shock zone recording a significantly lower SDL time over normal rats. The elevated plus maze served as the exteroceptive behavioural model (wherein the stimulus existed outside the body) to evaluate learning and memory in rats. Increase in memory reflects a decrease in TL i.e., rats take less time to reach the closed arms of the plus maze. The STZ-HFD animals showed an increase in the TL compared to controls i.e. the diabetic rats did not remember the task and thus took more time to go from open arm to closed arm. However, three weeks of glibenclamide treatment significantly decreased SDL and decreased TL thus suggesting considerable improvement in cognition in these animals and signifying that the cognitive impairment was mediated by T2D. Previous human and animal studies have demonstrated that learning and memory can be modified by drugs affecting the central cholinergic system. Cholinergic transmission is terminated mainly by acetylcholine hydrolysis via the enzyme AchE. The action of the enzyme could influence the progression of Alzheimer’s disease. Here, we show a clear increase in AchE levels in HFD-STZ-induced diabetes. Various reports correlate CNS oxidative stress with the pathogenesis of Alzheimer’s disease. Thus, free radical scavengers and antioxidants have been shown to prevent neurodegeneration. Reduced GSH has been demonstrated to be an important marker for oxidative stress in the cortex and hippocampus [22,23]. Here, we show decreased GSH level in cortico-hippocampal lysates of HFD-STZ animals. Glibenclamide treated HFD-STZ animals showed a decrease in AchE and increase in GSH levels, which was comparable to control animals.
One of the standard features of neurodegenerative disorder like Alzheimer's disease is neuroinflammation, characterized by astrogliosis resulting in the release of chemokine’s followed by recruitment and activation of microglial cells at the injury site. Activated microglial cells release many pro-inflammatory cytokines, which may prove to be neurotoxic eventually leading to loss of hippocampal neurons [10]. Histopathological studies of HFD-STZ animals showed a marked loss of CA1 and CA3 hippocampal neurons, neuronal swelling, neuronophagia and severe microgliosis. We also report marked astrogliosis and microglial activation in HFD-STZ cortex as reported previously in Zucker diabetic fatty rats [24]. Previous high levels of TNF-α and IL-6 have been reported in HFD-STZ animals [15]. Sulphonylureas has been shown to modulate diabetes associated neurological complications both in human and animals. Glibenclamide has also been shown to improve diabetic encephalopathy [25]. While, glipizide may prevent kainic acid-induced hippocampal neuronal cell death [26], sulphonylurea therapy in neonatal diabetic individuals show improvements in neuropsychomotor impairment. Glyburide has been shown to improve high fat diet-induced anxiety and cognitive impairment [27] and reduce the expression of myosin-IIB in the frontal cortex and hippocampus of diabetic rats [28]. In our study glibenclamide treatment partially reversed cognitive impairment in diabetic rat thus confirming the cognitive impairment in these animals to be mediated by T2D [29].
In the present study, we show that HFD-STZ-induced T2D rats may develop cognitive impairment. This cognitive decline may be associated with increased AchE activity and loss of hippocampal neurons. Oxidative stress and neuroinflammatory changes in the brain may be responsible for neuronal damage leading to loss of hippocampal neurons. We also shown that glibenclamide treatment may partially reverse diabetes associated cognitive impairment in these animals. Future studies may determine specific signaling molecules which may be responsible for neurocognitive impairment in these animals.
Financial assistance
The study was funded by DST-SERB (project no SB/FT/LS-202/2012).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Alberti KG, Zimmet P. Epidemiology: global burden of disease--where does diabetes mellitus fit in? Nat Rev Endocrinol 2013;9:258-60.
- Kodl CT, Seaquist ER. Cognitive dysfunction and diabetes mellitus. Endocr Rev 2008;29:494-511.
- Honig LS, Tang MX, Albert S, Costa R, Luchsinger J, Manly J, et al. Stroke and the risk of Alzheimer disease. Arch Neurol 2003;60:1707-12.
- Nakata-Kudo Y, Mizuno T, Yamada K, Shiga K, Yoshikawa K, Mori S, et al. Microbleeds in Alzheimer disease are more related to cerebral amyloid angiopathy than cerebrovascular disease. Dement Geriatr Cogn Disord 2006;22:8-14.
- Haglund M, Englund E. Cerebral amyloid angiopathy, white matter lesions and Alzheimer encephalopathy - a histopathological assessment. Dement Geriatr Cogn Disord 2002;14:161-6.
- Carvalho C, Machado N, Mota PC, Correia SC, Cardoso S, Santos RX, et al. Type 2 diabetic and Alzheimer's disease mice present similar behavioural, cognitive, and vascular anomalies. J Alzheimers Dis 2013:35:623-35.
- Basta G, Schmidt AM, De Caterina R. Advanced glycation end products and vascular inflammation: implications for accelerated atherosclerosis in diabetes. Cardiovasc Res 2004;63:582-92.
- Negrean M, Stirban A, Stratmann B, Gawlowski T, Horstmann T, Gotting C, et al. Effects of low- and high-advanced glycation end product meals on macro- and microvascular endothelial function and oxidative stress in patients with type 2 diabetes mellitus. Am J Clin Nutr 2007;85:1236-43.
- Buffo A, Ronaldo C, Ceruti S. Astrocytes in the damaged brain: Molecular and cellular insights into their reactive response and healing potential. Biochem Pharmacol 2010;79:77-89.
- Mamik MK, Banerjee S, Walseth TF, Hirte R, Tang L, Borgmann K, et al. HIV-1 and IL-1β regulate astrocytic CD38 through mitogen-activated protein kinases and nuclear factor-κB signaling mechanisms. J Neuroinflammation 2011;8:145.
- Bugger H, Abel ED. Molecular mechanisms of diabetic cardiomyopathy. Diabetologia 2014;57:660-71.
- Reed MJ, Scribner KA. In vivo and in vitro models of type 2 diabetes in pharmaceutical drug discovery. Diabetes Obes Metab 1999;1:75-86.
- Srinivasan K, Viswanad B, Lydia A, Kaul CL, Ramarao P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: A model for type 2 diabetes and pharmacological screening. Pharmacol Res 2005;52:313-20.
- Mansor LS, Gonzalez ER, Cole MA, Tyler DJ, Beeson JH, Clarke K, et al. Cardiac metabolism in a new rat model of type 2 diabetes using high fat diet with low dose streptozotocin. Cardiovasc Diabetol 2013;12:136.
- Datusalia AK, Sharma SS. Amelioration of diabetes-induced cognitive deficits by GSK-3β inhibition is attributed to modulation of neurotransmitters and neuroinflammation. Mol Neurobiol 2014;50:390-405.
- Bardini G, Rotella CM, Giannini S. Dyslipidemia and diabetes: reciprocal impact of impaired lipid metabolism and beta-cell dysfunction on micro- and macrovascular complications. Rev Diabet Stud 2012;9:82-93.
- Moron MS, Depierre JW, Mannervile B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta 1979;582:67-78.
- Ellman GL, Courtney KD, Andres V Jr, Featherstone RM. A new and rapid colorimetric determination of acetycholineesterase activity. Biochem Pharmacol 1961:7:88-95.
- Feinkohl I, Price JF, Strachan MWJ, Frier BM. The impact of diabetes on cognitive decline: potential vascular, metabolic, and psychosocial risk factors. Alzheimers Res Ther 2015;7:46.
- Khalifa SA, de Medina P, Erlandsson A, El-Seedi HR, Silvente-Poirot S, Poirot M. The novel steroidal alkaloids dendrogenin A and B promote proliferation of adult neural stem cells. Biochem Biophys Res Commun 2014: 446:681-6.
- Nassiri-AM, Zamansoltani F, Javadi A, Ganjvar M. The effects of rutin on a passive avoidance test in rats. Prog Neuropsychopharmacol Biol Psychiatry 2010;34:204-7.
- Pocernich CB, Butterfield DA. Elevation of glutathione as a therapeutic strategy in Alzheimer disease. Biochi Biophys Acta 2012;1822:625-30.
- Salama SM, Gwaram NS, AlRashdi AS, Khalifa SAM, Abdulla MA, Ali HM, El-Seedi. A Zinc morpholine complex prevents HCl/ethanol-induced gastric ulcers in a rat model. Sci Rep 2016:6:29646-60.
- Hwang K, Choi JH, Nam SM, Park OK, Yoo DY, Kim W, et al. Activation of microglia & induction of pro-inflammatory cytokines in the hippocampus of types 2 diabetic rats. Neurol Res 2014;36:824-32.
- Motawi TK, Darwish HA, Hamed MA, El-Rigal NS, Naser AF A Therapeutic Insight of Niacin and Coenzyme Q10 against Diabetic Encephalopathy in Rats. Mol Neurobiol 2016:1-11.
- Kim CH, Park SH, Sim YB, Kim SS, Kim SJ, Lim SM, Jung JS, et al. Effect of tolbutamide, glyburide and glipizide administered supraspinally on CA3 hippocampal neuronal cell death and hyperglycemia induced by kainic acid in mice. Brain Res 2014:1564:33-40.
- Beltrand J, Elie C, Busiah K, Fournier E, Boddaert N, Bahi-Buisson N, et al. Sulfonylurea Therapy Benefits Neurological and Psychomotor Functions in Patients With Neonatal Diabetes Owing to Potassium Channel Mutations. Diabetes Care 2015;38:2033-41.
- Gainey SJ, Kwakwa KA, Bray JK, Pillote MM, Tir VL, Towers AE, et al. Short-Term High-Fat Diet (HFD) Induced Anxiety-Like Behaviors and Cognitive Impairment Are Improved with Treatment by Glyburide. Front Behav Neurosci 2016:11:156.
- da Costa AV, Calábria LK, de Souza Santos P, Goulart LR, Espindola FS. Glibenclamide treatment modulates the expression and localization of myosin-IIB in diabetic rat brain. J Neurol Sci 2014 15;340:159-64.