- *Corresponding Author:
- Lingarkar Silpavathi
School of Pharmaceutical Sciences, Siksha “O” Anusandhan Deemed to be University, Bhubaneshwar-751003, India
E-mail: shilpa_pharma@rediffmail.com
| Date of Submission | 18 January 2019 |
| Date of Revision | 22 April 2019 |
| Date of Acceptance | 02 September 2019 |
| Indian J Pharm Sci 2019;81(6):981-987 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Non-classical herbal formulations have gained momentum in the recent past. These formulations, however, have not been well integrated with the modern system of medicine in most of the countries in the world. This is due to the lack of enough scientific evidence pertaining to its long-term safety and efficacy. Besides, the paucity of authentic monographs on the impurity profiling, standardization protocols, lack of guideline on the fixed-dose combinations and absence of programmed pharmacovigilance plan for herbal drugs are among the major caveats. In recent years, few issues pertaining to the classical herbal formulations are being addressed to some extent. However, the issues on non-classical modern formulations remain the same. Hence, the objective of this mini-review is to shed light on the major flaws and challenges of these formulations and provide some expert opinion to counter these issues.
Keywords
Non-classical herbal formulations, challenges, standardization
Drugs from natural sources have been used by most of the world population since time immemorial for the prophylaxis and treatment of various dreaded ailments. Modern medicine despite substantial development has failed to cater to the needs of people from diverse socio-economical class[1]. Hence, the majority of the population from the weaker section of the society, residing in a rural area largely depends on herbal medicine. Moreover, these traditional medicines have also become popular among a larger world population due to its lesser side effects and cost. The herbal medicine market is expected to grow at a compound annual growth rate of ~7.2 % during 2017-2023 and is predicted to reach $ 111 billion by the end of 2023[2]. Despite escalating growth, these medications are not the primary choice of treatment to date. However, these are used as adjunctive therapy along with primary treatment in many developed countries. This is due to the lack of integration of traditional medicine with the modern treatment, lack of explicit regulatory framework[3] and quality standards. In addition to this, the most profound caveats of traditional medicine are lack of established official standardization techniques and evidence-based safety and efficacy data[4]. Even in the new WHO traditional medicine strategy 2014-2023, the paucity of research data is one among the topmost challenges faced by the majority of member states[5]. Hence, the objective of this review is to shed light on the persistent challenges encountered in standardizing these herbal preparations, emphasizing more on nonclassical proprietary herbal formulations.
Challenges in the standardization of non-classical proprietary herbal formulations:
Classical herbal formulations are those which are prepared according to the formula given in the traditional books of alternative systems of medicines like Charaka Samhita, Sushruta Samhita, Bhaishajya ratnavali and Shanghan Lun (classical Chinese medical treatise). The manufacturers of the classical herbal formulations follow the same formula to prepare the formulations and standardize as per the guideline of official monographs. On the contrary, non-classical or proprietary formulations are prepared as per manufacturers own formula and many a time the ingredients and additives are not found in the traditional literature. Most of the proprietary herbal preparations consist of a complex heterogeneous mixture. Though some of the materials are included in different official monographs, their chemical markers and chromatographic specifications are not well documented[6]. The analytical limits for the active constituents present in the modern herbal formulation cannot be so precise like pure chemicals. This is due to the inherent inconsistency of the active constituents present in the raw materials due to variation in the age and origin of the medicinal plant, method of cultivation and processing[7]. Hence, standardization of these products should include monitoring of the raw material right from its origin until its clinical application. Besides the challenges in herbal drug standardization has been discussed in detail by Chawla et al.[4], hence, this review emphasizes more on the standardization of non-classical proprietary herbal formulations. Majority of these formulations composed of a varied number of components. Table 1 shows the composition of 4 established brands of herbal cough formulations available in the Indian market. Few ingredients in all the 4 formulations are common, but their strengths vary. There is no specific guideline on the strength of the components to be used in these formulations. Though sporadically the safety and efficacy of these formulations are studied[8,9], the patient population and study design is not enough to establish the long-term safety and efficacy of these preparations. Standardization of these formulations requires a state-of-the-art research facility with high end modern analytical instruments. For this activity, either the manufactures should have their own in-house center or can depend on the government approved testing laboratories. Majority of the small and medium scale manufacturers of herbal drugs have financial constraints to have their own in-house testing facility. The limited number of governments approved testing laboratories for herbal drugs is a major hurdle for chemical characterization and biological evaluation of the proprietary formulations.
| Formulation1 constituents | Each 5 ml contains | Formulation2 constituents | Each 5 ml contains | Formulation3 constituents | Each 5 ml contains | Formulation 4 constituents | Each 5 ml contains |
|---|---|---|---|---|---|---|---|
| Ocimum tenuiflorum | 50 mg | Mel despumatum | 1.25 g | Adhatoda vasica | 200 mg | Anacyclus pyrethrum Rt., | 10 mg |
| Glycyrrhiza glabra | 50 mg | Balsamodendron mukul | 35 mg | Solanum xanthocarpum | 200 mg | Cubea officicinalis Fr., | 10 mg |
| Viola odorata | 50 mg | Vitis vinifera | 35 mg | Piper chaba | 10 mg | Piper nigrum Fr. | 10 mg |
| Solanum virginianum | 50mg | Ocimum sanctum | 25 mg | Pistacia integerrima | 10 mg | Zingiber officinale Rz. | 10 mg |
| Abies webbiana | 50 mg | Hyssopus officinalis | 25 mg | Fagonia arabica | 10 mg | Curcuma longa | 20 mg |
| Zingiber officinale | 25 mg | Tinospora cordifolia | 20 mg | Clerodendron serratum | 10 mg | Piper longum | 30 mg |
| Curcuma longa | 25 mg | Adhatoda vasica | 15 mg | Pluchea lanceolata | 10 mg | Clerodendron serratum | 40 mg |
| Justicia adhatoda | 25 mg | Myristica fragrans | 15 mg | Curcuma zedoaria | 10 mg | Viola odorata | 50 mg |
| Curcuma zedoaria | 25 mg | Glycyrrhiza glabra | 15 mg | Plumbago zeylanica | 10 mg | Juniperus communis | 50 mg |
| Mentha piperita | 3 mg | Onosma bracteatum | 10 mg | Cyperus rotundus | 10 mg | Ocimum sanctum | 60 mg |
| Shudha Madhu | 1.75 g | Viola odorata | 10 mg | Zingiber officinale | 10 mg | Terminalia bellirica | 100 mg |
| Flavoured syrup base | q.s. | Triphala | 9 mg | Piper nigrum | 10 mg | Solanum xanthocarpum | 120 mg |
| - | - | Trikatu | 9 mg | Piper longum | 10 mg | Glycyrrhiza glabra | 150 mg |
| - | - | Embelia Ribes | 6 mg | Glycyrrhiza glabra | 10 mg | Adatoda vasica | 200 mg |
| - | - | Solanum xanthocarpum | 8 mg | - | - | Navsar | 15 mg |
| - | - | Cinnamomum cassia | 3 mg | - | - | Sodium benzoate | 15 mg |
| - | - | Nausadar | 3 mg | - | - | Sodium methyl paraben | 8 mg |
| - | - | - | - | - | - | Sodium propyl paraben | 1 mg |
| - | - | - | - | - | - | Sugar | 2550 mg |
| - | - | - | - | - | - | Liquid glucose | 1800 mg |
| - | - | - | - | - | - | Citric acid monohydrate | q.s |
| - | - | - | - | - | - | flavour grape 1400 | 17.5 mg |
| - | - | - | - | - | - | Purified water | q.s |
Table 1: Composition of Some Proprietary Herbal Cough Remedies
In India, along with the indigenous Ayurvedic system of medicine other alternative systems of medicine like Siddha and Unani are in practice since a prehistoric period. Although there is a similarity between the basic principle of treatment in these three systems of medicine, there are differences in formulations and method of standardization. The modern Ayurvedic formulations include tablets, capsules, syrups, solutions. Similarly, most of the Siddha formulations utilize mineral and metal in the formulations. These formulations are categorized into Uppu, Pashanam, Uparasam, Ratnas and Uparatnas, Loham, Gandhakam. Siddha system of medicine also includes drugs of animal and plant origin having a similar profile as that of Ayurvedic drugs. However, standards pertaining to the limit of impurities, heavy metals, and toxins in modern Siddha formulations are not defined in the Siddha Pharmacopeia of India. Hence, the standardization of Siddha formulations becomes a challenge. Due to the proximity of a few Siddha drugs with Ayurvedic preparations, the earlier can be standardized in a similar fashion as that of the later preparations. Unani system of medicine got introduced into India during 1350 AD. Since then this system of medicine has undergone many folds of development and modernization. The Government of India, Ministry of AYUSH has developed the Unani Pharmacopeia of India. This official monograph constitutes 50 classical Unani formulations and their standardization techniques, and limits of heavy metal content are also mentioned. However, the modern aspects of good manufacturing practice for herbal drugs were not addressed sufficiently in the published monographs.
Beside active ingredients, several excipients are used in modern herbal formulations to enhance the palatability, bio-absorption, and shelf-life of the formulations. These excipients vary based on the type of formulations. Solid dosage forms require diluents, binder or adhesives, lubricants, glidants, disintegrants, superdisintegrants, coloring agents, sweeteners, coating material, plasticizers. Similarly, liquid and semiliquid preparations require solvents, co-solvents, buffers, antimicrobial preservatives, thickening agents, wetting agents, humectants, emulsifying agents, sweetening agents, emollients, flavors. These excipients are basically of synthetic and natural origin. The nonclassical modern herbal formulations contain both types of excipients. In many cases, lack of compatibility studies pertaining to additives leads to instability of the formulations. Hence, compatibility study of the additives in the formulation needs to be performed to ensure product quality.
Non-classical herbal formulations (modified herbal preparations) represent the modification of the indigenous classical herbal preparations either by changing dosage form or route of administration or method of preparation or using for different indications. Hence, these modified preparations most likely get impaired due to incompatibility, instability and impurity leading to serious adverse events due to toxicity. More than 16 000 suspected case report of adverse effects of herbal medicinal preparations have been reported in the WHO database till date. The most frequently reported adverse effects are face edema, hepatitis, hypertension, angioedema, convulsions, dermatitis, and death. The committee for proprietary medicinal products prepared a list of 33 drugs having serious risk factors and it was published by the European Commission in the year 1992. The WHO has come forward with a set of quality control standards for the modern herbal preparations. The safety and efficacy of herbal drugs are dependent on the extent of quality control. Hence the quality of the herbal drugs must be ensured right from the cultivation till preparation of the finished product. The WHO has published quality control methods for medicinal plant materials as a guide for quality control of botanicals. With reference to contaminants and residues WHO has developed a new guideline for assessing the quality of herbal medicines[10]. In Japan, where herbal drugs called Kampo medicine undergo stringent regulatory framework as that of the allopathic system of medicine and are well integrated. The manufacturer of a new herbal product needs to submit details of heavy metal content, aflatoxin details, mycotoxin, and pesticide details to the regulatory authority. Along with these documents the manufacturer needs to submit chemistry, manufacture and control (CMC) documents prior to market authorization. However, in the US submission of CMC document is not mandatory for herbal drugs manufacturers at investigational new drug stage. It becomes a herculean task to standardize the herbal formulation due to the presence of multiple components in a modern formulation. In Germany, phytoequivalence, a newer concept was developed to ensure the consistency of the herbal formulations[11].
Modern approaches in the Standardization of proprietary Herbal Formulations
Chemical methods of characterization are more acceptable for standardization of herbal formulations in the modern era. Some of the modern approaches and associated challenges are discussed below.
Chromatographic fingerprinting technique:
Since a long time, chromatographic fingerprinting techniques were used for identification of the single chemical entity. However, in recent years, these techniques have shown a new avenue in the phytopharmaceutical research. The techniques like thin-layer chromatography, high performance liquid chromatography, high-performance thin-layer chromatography, gas chromatography and liquid chromatography-mass spectrometry are broadly used for the identification of genuine crude drugs; active chemical components and their stability in proprietary herbal formulations. In case of the presence of a known constituent, it should be quantitated. On the contrary, a marker compound is used to standardize the drug when the active phytoconstituents are not known. To ensure the content uniformity of phytoconstituents across batches in a polyherbal formulation, single or multiple marker compounds are used. Numerous studies reported the use of chromatographic fingerprinting as a potential tool for standardizing complex polyherbal formulations[12-15]. The chromatographic fingerprinting for standardization of herbal formulations is an approved modern analytical technique by the USFDA and the WHO[16,17]. However, these techniques have some caveats, for example, nearly similar fingerprints do not always represent the same chemical constituent in the samples. This is because the chemical constituents in the preparation might have altered due to instability and diverse sources of crude drugs[18]. Hence, the simultaneous estimation of multiple components should be performed to estimate the chemical pattern of the components present in the formulation.
Impurities:
Impurities get into the herbal formulations at various stages of preparation[19] including deliberate adulteration of the herbal formulations with synthetic drugs[20,21]. The other sources of impurities include heavy metals, aflatoxins, pesticides and solvent residues in which the plant material is being extracted or fractionated. Limits of these impurities are prescribed in different official monographs presented in Tables 2 and 3[22-31]. However, complete information pertaining to the limit of residual solvents in individual plant extracts are lacking in most of the official monographs. Impurities produced in the herbal formulation due to degradation should be closely monitored. Few attempts were made in the recent past to perform the stability studies of the herbal formulations[32,33]. However, comprehensive guideline needs to be formulated exclusively for stability study of the herbal preparations.
| HM/Afla | IP | JP | EP | USP | AP | UP |
|---|---|---|---|---|---|---|
| Lead | NMT 20 ppm | NMT 20 ppm | 5 ppm | NMT 5 ppm | NMT 10 ppm | 10 ppm |
| Mercury | - | - | 0.1 ppm | NMT 20 ppm | NMT 1 ppm | 1 ppm |
| Bismuth | - | - | - | NMT 20 ppm | - | |
| Arsenic | NMT 10 ppm | - | - | NMT 3 ppm | NMT 3 ppm | 3 ppm |
| Antimony | - | - | - | NMT 20 ppm | - | |
| Tin | - | - | - | NMT 20 ppm | - | |
| Cadmium | - | - | 0.5 ppm | NMT 20 ppm | NMT 0.3 ppm | 0.3 ppm |
| Silver | - | - | - | NMT 20 ppm | - | - |
| Copper | - | - | - | NMT 20 ppm | - | |
| Molybdenum | - | - | - | NMT 20 ppm | - | |
| Vanadium | - | - | - | - | - | |
| Palladium | - | - | - | - | - | |
| Platinum | - | - | - | - | - | |
| Gold | - | - | - | - | - | |
| Ruthenium | - | - | - | - | - | |
| Afla B1 | - | 10 μg/kg | NMT 5 ppb | 0.5 ppm | ||
| Afla G1 | - | 10 μg/kg | NMT 20 ppb | 0.5 ppm | ||
| Afla B2 | - | 10 μg/kg | NMT 20 ppb | 0.1 ppm | ||
| Afla G2 | - | 10 μg/kg | NMT 20 ppb | 0.1 ppm |
HM- Heavy metal, Afla- aflatoxin, IP- Indian Pharmacopoeia, JP- Japanese Pharmacopoeia, EP- European Pharmacopoeia, USP-United states Pharmacopoeia, AP- the Ayurvedic Pharmacopoeia of India, UP- the Unani Pharmacopeia of India, NMT- not more than, Ppm-parts per million and ppb- parts per billion
Table 2: Limits of Heavy Metals and Aflatoxins [22-31]
| Class-I toxic and carcinogenic | Class-II less toxicity | Class-III low risk to human health | |||
|---|---|---|---|---|---|
| Solvent | limit (ppm) | Solvent | limit (ppm) | Solvent | limit (ppm) |
| Benzene | 2 | Acetonitrile | 410 | Acetic acid | 5000 ppm |
| Carbon tetra chloride | 4 | Chloroform | 60 | Acetone | 5000 ppm |
| 1,2-dichloroethane | 5 | Cyclohexane | 3880 | 1-Butanol | 5000 ppm |
| 1,1-dichloroethane | 8 | Hexane | 290 | Anisole | 5000 ppm |
| 1,1,1-trichloroethane | 1500 | Methanol | 3000 | 2-Butanol | 5000 ppm |
| - | - | Nitromethane | 50 | Dimethyl sulfoxide | 5000 ppm |
| - | - | Pyridine | 200 | Ethanol | 5000 ppm |
| - | - | Tetra hydro furan | 720 | Ethyl acetate | 5000 ppm |
| - | - | Toluene | 890 | Ethyl ether | 5000 ppm |
| - | - | Xylene | 2170 | Ethyl formate | 5000 ppm |
| - | - | - | - | Formic acid | 5000 ppm |
| - | - | - | - | Heptane | 5000 ppm |
| - | - | - | - | Isobutyl acetate | 5000 ppm |
| - | - | - | - | Isopropyl acetate | 5000 ppm |
| - | - | - | - | Methyl acetate | 5000 ppm |
| - | - | - | - | Pentane | 5000 ppm |
| - | - | - | - | 1-Pentanol | 5000 ppm |
| - | - | - | - | 1-Propanol | 5000 ppm |
Ppm- parts per million
Table 3: Limits of Residual Solvents
Limits for microbial count:
Presence of microorganisms in natural products is an inherent phenomenon. Hence, there should be guideline prescribing the limit of aerobic microorganisms in the herbal products. Till date, there is no substantial monograph, which defines the percent and extent of acceptable total yeast count, molds and other harmful bacteria in an individual plant or animal extract.
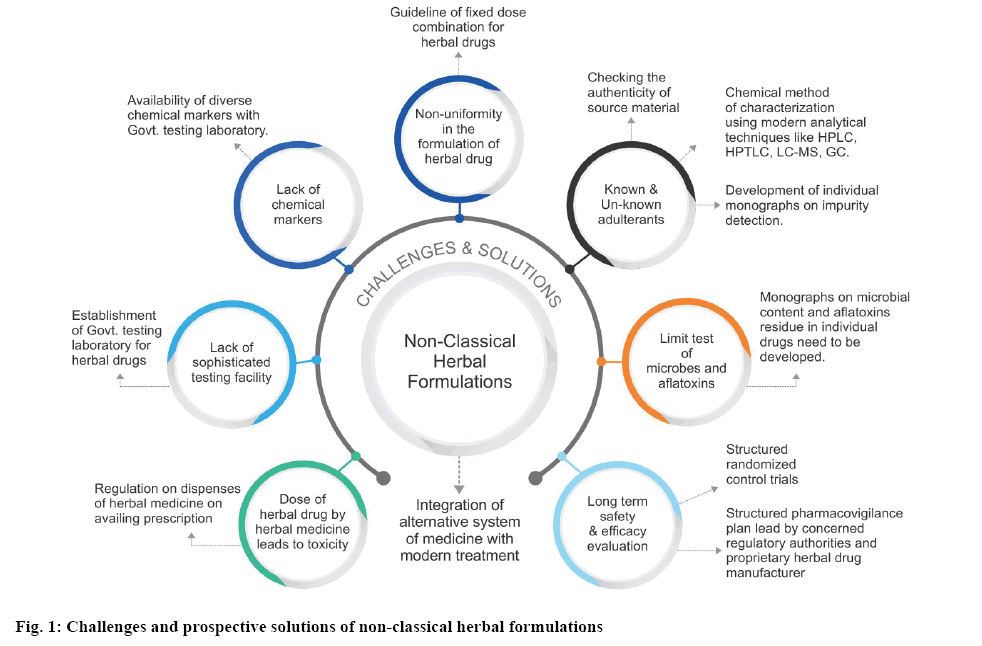
As per the discussion, most of the modern nonclassical herbal formulations are prepared according to the manufacturer’s own formula. Many a time these formulations contain impurities due to known or unknown adulterants. There is a lack of stringent guideline and monographs for the control of these adulterants in these formulations. Safety and longterm efficacy studies are among the pivotal concerns in evaluating the potency of these formulations. Checking the authenticity of the source material from which the drug is being extracted, purity of the extract and chemical assay of the finished formulation can control the quality of the preparations to a major extent. Lack of sophisticated testing facility of herbal drugs is also a major concern which needs to be addressed by the government agencies in underdeveloped and developing countries. Lack of chemical marker for individual components present in the formulation is a critical challenge encountered by a manufacturer. Hence, a few governments led policies for the easy availability of diverse marker compounds seems to open a wider avenue for standardization of these modern herbal preparations. There is a need to establish a fixed dose combination for non-classical herbal preparations to control the quality and uniformity of the formulations. More exhaustive monographs pertaining to the content of microbial and aflatoxin limits in individual herbal components need to be developed for reference. Figure 1 explains the challenges associated with non-classical herbal formulations and their prospective solutions. In most of the countries, herbal drugs are regarded as an alternative system of medicine and are not well integrated with the modern system of medicine. Hence, the regulations pertaining to the quality of these formulations are not stringent. Many a case, these preparations are used among the populations by word of mouth and dispensed without a prescription from a qualified physician. Hence the use of these formulations leads to toxicity many a time[34,35]. Lack of a well-structured pharmacovigilance plan for herbal drugs is also a major flaw in establishing the long-term safety and efficacy for these formulations. Hence effort from the concerned regulatory authority and proprietary herbal drug manufacturer are required in addressing these challenges and integrating this system of medicine with the modern treatment.
Conflicts of interest:
There are no conflicts of interest.t.
References
- Prakash J, Srivastava S, Ray RS, Singh N, Rajpali R, Singh GN. Current Status of Herbal Drug Standards in the Indian Pharmacopoeia. Phytother Res 2017;31;1817-23.
- Global Herbal Medicine Market Research Report - Forecast to 2023. Available from: https://www.marketresearchfuture.com/reports/herbal-medicine-market-3250.
- Zhang Q. Global situation and WHO strategy on traditional medicine. Tradit Med Modern Med 2018;1:11-3.
- Chawla R, Thakur P, Chowdhry A, Jaiswal S, Sharma A, Goel R. Evidence based herbal drug standardization approach in coping with challenges of holistic management of diabetes: a dreadful lifestyle disorder of 21st century. J Diabetes Metab Disord 2013;12:1-16.
- WHO traditional medicine strategy: 2014-2023 [cited 2018 Dec 30]. Available from: http://www.searo.who.int/entity/health_situation_trends/who_trm_strategy_2014-2023.pdf?ua=1.
- Yadav NP, Dixit VK. Recent Approaches in Herbal Drug Standardization. Int J Integr Biol 2008;2:195-203.
- Waldesch FG, Konigswinter BS, Remagen HB. Herbal Medicinal Products-Scientific and Regulatory Basis for Development Quality Assurance and Marketing Authorisation. Washington DC: Medpharm Stuttagart and CRC Press; 2003. p. 37-52.
- Gupta A, Gaikwad V, Kumar S, Srivastava R, Sastry J. Clinical validation of efficacy and safety of herbal cough formulation "Honitus syrup" for symptomatic relief of acute non-productive cough and throat irritation. Ayu 2016;37:206-14.
- Viswanatha GL, Rafiq M, Thippeswamy A, Yuvaraj HC, Kavya KJ, Baig. Ameliorative effect of Koflet formulations against pyridine-induced pharyngitis in rats. Toxicol Rep 2014;1:293-9.
- World Health Organization. Guidelines on good agricultural and collection practices (GACP) for medicinal plants; 2003. Available from: http://apps.who.int/medicinedocs/pdf/s4928e/s4928e.pdf.
- Liang Y, Xie P, Chan K. Quality control of herbal medicines. J Chromatogr B Analyt Technol Biomed Life Sci 2004;812(1-2):53-70.
- Pushpendra, Sunil Kumar KN, Priyadarshini, Holla BS, Ravishankar B, Yashovarma B. Quality standards for Hutabhugādi cūrṇa (Ayurvedic Formulary of India). J Tradit Complement Altern Med 2015;6:78-88.
- Pandit S, Kanjilal S, Awasthi A, Chaudhary A, Banerjee D, Bhatt BN, et al. Evaluation of herb-drug interaction of a polyherbal Ayurvedic formulation through high throughput cytochrome P450 enzyme inhibition assay. J Ethnopharmacol 2017;197:165-72.
- Biswas R, Mukherjee PK, Kar A, Bahadur S, Harwansh RK, Biswas S, et al. Evaluation of Ubtan – A traditional Indian skin care formulation. J Ethnopharmacol 2016;192:283-91.
- Muguli G, Gowda VD, Dutta V, Jadhav AN, Mendhe BB, Paramesh R, et al. A contemporary approach on design, development, and evaluation of Ayurvedic formulation- Triphala Guggulu. Ayu 2015;36:318-22.
- FDA Guidance for Industry—Botanical Drug Products (Draft Guidance) (2000), Rockville, US Food and Drug Administration [cited 2018 Dec 27]. Available from: https://www.fda.gov/downloads/Drugs/Guidances/UCM458484.pdf.
- World Health Organization, (1991) Guidelines for the Assessment of Herbal Medicines, Munich, 1991; WHO, Geneva [cited 2018 Dec 26]. Available from: http://apps.who.int/iris/handle/10665/58865.
- Wang P, Li L, Yang H, Cheng S, Zeng Y, Nie L, et al. Chromatographic fingerprinting and quantitative analysis for the quality evaluation of Xinkeshu tablet. J Pharm Anal 2012;2:422-30.
- Zhanga J, Wider B, Shang H, Li X, Ernst E. Quality of herbal medicines: Challenges and solutions. Complement Ther Med 2012;20:100-6.
- Wilson P, Masse C. Detection of Synthetic Drugs as Adulterants in Natural and Herbal Slimming Products by LC-ESI-MS/MS with Polarity Switching. J AOAC Int 2016;99:929-40.
- Calahan J, Howard D, Almalki AJ, Gupta MP, Calderón AI. Chemical Adulterants in Herbal Medicinal Products: A Review. Planta Med 2016;82:505-15.
- Anonymous. Indian Pharmacopeia. Vol. 1. Ghaziabad, India: Indian Pharmacopoeia Commission (IPC); 2018. p. 287-8.
- Quality control methods for medicinal plant materials-WHO guidelines [cited 2018 Dec 26]. Available from: http://apps.who.int/iris/bitstream/handle/10665/41986/9241545100.pdf;jsessionid=1BF52032CE79A1345C4232EA33E6FF18?sequence=1.
- Anonymous. The Ayurvedic Pharmacopoeia of India (API). Part I, volume VII. Ghaziabad, India: Pharmacopoeia Commission for Indian Medicine & Homoeopathy; 2016. p. 252-8.
- Elemental impurities updates. Available from: https://www.usp.org/chemical-medicines/elemental-impurities-updates.
- General Chapter Articles of Botanical Origin [cited 2018 Dec 28]. Available from: https://hmc.usp.org/sites/default/files/documents/HMC/GCs-Pdfs/c561.pdf.
- Monograph USP-Pharmacopeia Forum 2009. Available from: https://www.uspnf.com/pharmacopeial-forum.
- Japanese pharmacopoeia 16- Heavy Metals Limit Test-1.07:29. [cited 2018 Dec 28]. Available from: https://wenku.baidu.com/view/66b899e181c758f5f61f677c.html.
- Aflatoxins [cited 2018 Dec 28]. Available from http://www.famic.go.jp/ffis/oie/obj/hc_aflatoxin.pdf.
- Herbal drugs, monograph 1433 Pharmeuropa (2008). Available from: https://www.edqm.eu/medias/fichiers/Technical_Guide_for_the_Elaboration_of_Monographs_on_herbal_drugs_and_herbal_drug_preparations_2007.pdf.
- Residual Solvents. [cited 2019 Jan 1]. Available from https://hmc.usp.org/sites/default/files/documents/HMC/GCs-Pdfs/c467.pdf.
- Poetsch FA, Steinhoff B, Verlag C. Stability testing of herbal medicinal products: A report on problematic cases from practice with discussion of possible resolution approach. Pharm Ind 2006;68:1166-74.
- Heigl D, Franz G. Stability testing on typical flavonoid containing herbal drugs. Pharmazie 2003;58:881-5.
- Fathima N, Nayeem N. Toxic Effects as a Result of Herbal Medicine Intake. In: Larramendy M, editor. Toxicology - New Aspects to This Scientific Conundrum. London, UK: InTech Open; 2016. p. 193-207.
- Woo CSJ, Lau JSH, Nezami HE. Herbal Medicine: Toxicity and Recent Trends in Assessing Their Potential Toxic Effects. In: Shyur LF, Allan SY Lau, editors, Advances in Botanical Research. London, UK: Academic Press; Vol. 62. 2012. p. 365-84.