- *Corresponding Author:
- Swathi Nageswara
Pharmaceutical Biotechnology Division, Andhra University College of Pharmaceutical sciences, Andhra University, Visakhapatnam-530003, Andhra Pradesh, India
E-mail: swathinageswara@gmail.com
| Date of Received | 07 September 2021 |
| Date of Revision | 08 October 2022 |
| Date of Acceptance | 16 November 2022 |
| Indian J Pharm Sci 2022;84(6):1463-1475 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Serralysin is well known to exhibit anti-inflammatory and fibrinolytic properties. The current research designed a cost-effective serralysin production medium from Streptomyces hydrogenans var. MGS13 with the aid of solid state fermentation. Four pre-screened factors, namely horse gram flour concentration, inoculum size, initial moisture content and soya bean meal were modeled by central composite design for optimizing in order to predict their influence on serralysin production. Analysis of variance results showed a high coefficient of determination (R2) value of 0.9611, ensuring a satisfactory adjustment of the quadratic model with the experimental data and F value 26.45 (p value of ?0.0001) indicated that the model was significant. The design of experiment assisted production process enhanced 1.3 fold productivity at the best possible conditions consisting 5.0 g of horse gram flour, 1.2 ml of inoculum (1×106 CFU/ml), 44 % of initial moisture content and soya bean meal 1.0 % w/w. Besides this study, the in vitro fibrinolytic and antiinflammatory activities were carried with purified serralysin of Streptomyces hydrogenans var. MGS13. The results revealed that the purified enzyme exhibited fibrinolytic and anti-inflammatory activity in a dose dependent manner. Further one-way analysis of variance and Dunnett’s multiple comparisons statistically justify the data p<0.05 in both activities.
Keywords
Horse gram flour, Streptomyces hydrogenans var. MGS13, central composite design, solid state fermentation, fibrinolytic activity, anti-inflammatory activity
“Healing power of nature has been well-known since ages, as the source of all medicine has been lying within Mother Nature”. Particularly, enzymes occur universally in wide variety of sources like microbes, plants and animals. Microbes are appeared as promising sources for production of peptidases over plants and animals. Peptidases such as trypsin, chymotrypsin and serralysin (also called as serratiopeptidase and serrapeptase) belong to the class of hydrolases and used clinically as anti-inflammatory agents[1,2]. Among these peptidases, serralysin has been effectively employed in disorders such as inflammation, pain and atherosclerosis[3,4]. Anti- inflammatory mechanism of serralysin involves the hydrolytic cleavage of inflammatory mediators such as bradykinin, serotonin and histamine[5]. Fibrinolytic mechanism of serralysin is due to enhancing plasmin activity by antagonizing the plasmin inactivators[6]. Serratiopeptidase (EC 3.4.24.40) comes under metzincin class of proteases as per the MEROPS database[7] which was initially produced from Serratia marcescens furthermore, it was reported from other bacterial strains such as Pseudomonas aeruginosa, Proteus mirabilis, Escherichia freundii[8], Xenorabdus[9,10], Deinococcus radiodurans[11] and Bacillus subtilis[12].
Till date serralysin produced from an opportunistic pathogen is available in market which is being associated with unwanted effects such as lung and corneal damage[13] therefore, it arises an increased need of novel microbial sources for bio-better serralysin production. Even though surplus numbers of reports are accessible for serralysin production from bacterial strains there is meager reports available pertaining serralysin production from Strepomyces sp. In this regard Strepomyces. sp gained much attention due to ability for the bioactive metabolites (proteases, cellulose, keratinase, and fibrinolytic enzymes) production[14,15]. Previously, serralysin producing isolate was screened and identified as Streptomyces hydrogenans (S. hydrogenans) var. MGS13 in Pharmaceutical Biotechnology laboratory[16], further produced the same enzyme by Sub-Merged Fermentation (SMF)[17]. This investigation chooses, Solid State Fermentation (SSF) technique for serralysin production from the same isolate because, the preferred technique not only resembles as a natural habitat for growth of organism but also reduces the production cost of metabolite. In this regard an increasing emphasis is being laid on the microbial proteases production by SSF where cheap agro residues were employed to produce worthy bioactive metabolites[18,19].
In the previous study, authors have reported serralysin production using this novel isolate (S. hydrogenans), chromatographically purified, characterized and structure of the enzyme was also predicted further confirmed that the produced enzyme is a serralysin like alkaline metalloprotease[20]. The optimization of growth medium components exerts a huge impact on cell growth and yield of bioactive metabolites. The One Factor At a Time (OFAT) approach is frequently used classical approach for designing the initial medium composition, but this strategy does not identify the interaction effects among the variables. To overcome this drawback, statistical method (Response Surface Method (RSM)) has been efficiently employed for achieving maximum productivity[21]. Hence in the present investigation, Central Composite Design (CCD) based RSM model was opted to enhance the serralysin yield by optimizing the cultivation medium of S. hydrogenans var. MGS13. Further, the biological activities of purified serralysin of S. hydrogenans var. MGS13 such as in vitro fibrinolytic and in vitro anti-inflammatory activity were also investigated in detail.
Materials and Methods
Source of the microorganism:
The novel strain was screened and isolated from Koringa mangrove soil which was used in previous study for the production of serralysin by SMF in Pharmaceutical Biotechnology division, Andhra University. For identification the strain was sent to Institute of Microbial Technology, Chandigarh showed 99.91 % identity to homologous fragments of S. hydrogenans hence, it was identified as S. hydrogenans var. MGS13[16]. The same strain was selected in the present study and deposited in National Collection of Industrial Microorganisms, Pune India with deposit number 5745.
Preparation of seed culture:
The seed culture for fermentation was prepared by scrapping the spore surface of 7 d old well sporulated cultures with 10 ml sterile distilled water, then the cell suspension was diluted and made up to 50 ml which was used as inoculum for execution of 30 treatment combinations. The cell density of cell suspension was determined in terms of CFU/ml by using colony counter which was found to be 1×106 CFU/ml and the same was maintained for all experiments based on the earlier findings of several other researchers[22].
Production of serralysin by SSF:
Horse gram flour (5g each) of average particle size 425 microns were placed in 250 ml Erlenmeyer flasks (B.S.S 25 American Standard Test Sieve Series (ASTM) sieve passed and B.S.S 60 ASTM sieve retained particles) hydrated to initial moisture content 40 % v/w and 1 % w/w of soya bean meal was added. The initial medium pH 6.5-7.0 was noticed and all the contents were vigorously mixed and autoclaved at 121° for 20 min, cooled and inoculated with 1 ml of cell suspension of S. hydrogenans var. MGS13 carrying 1×106 CFU/ ml and incubated at 28° for 96 h. All these fermentation parameters were selected based on the results obtained in the classical method (OFAT)[23] and the trails were done in triplicates. After incubation, the culture contents were extracted with sodium borate buffer (pH 9.0) (50 ml/ flask) by squeezing through a wet sterile cloth and centrifuged at 8000 ×g at 4° for 20 min. The cell free supernatant was collected and used for the determination of serralysin assay.
Quantitative assessment of serralysin assay:
The quantitative assay for the evaluation of serralysin activity was done as per Indian Pharmacopoeia 2010[24]. One serralysin unit is defined as the amount of enzyme required to liberate 1 µmol of free tyrosine per ml per min under the specified experimental conditions.
Optimization process of serralysin with the aid of CCD based RSM design:
In order to achieve sample yield of serralysin, individual as well as interaction effects[25] of variables were determined with the aid of central composite design. In the proposed experimental model horse gram flour (substrate) concentration, inoculum size, initial moisture content and soya bean meal were selected from the OFAT study[23] and these selected variables are considered for further optimization by implementing RSM based CCD in order to ascertain the individual and interaction effects on serralysin production. The code for each independent variable and their ranges in the design are given in Table 1. The CCD design studying 4 significant variables was generated using design Expert 12.0 software (Stat- Ease Inc. Minneapolis, USA) for execution of 30 experiments.
| Variables | Range of levels | ||||
|---|---|---|---|---|---|
| -2 | -1 | 0 | 1 | 2 | |
| Horse gram flour concentration (g) | 3 | 4 | 5 | 6 | 7 |
| Inoculum size (ml) | 0.5 | 0.75 | 1 | 1.25 | 1.5 |
| Initial moisture content (% v/w) | 30 | 35 | 40 | 45 | 50 |
| Soya bean meal (% w/w) | 0.5 | 0.75 | 1 | 1.25 | 1.5 |
Table1: Experimental Variables and Their Levels used in CCD
For four-factor system, the regression model equation (Eq. (1)) is derived as below.
Y=β0+β1A+β2B+β3C+β4D+β11A2+β22B2+β33C2+β44D2+β12AB+β13AC+β14AD +β23BC+β24BD+β34CD (1)
Where Y: Predicted response; β0: Intercept, A: Horse gram flour concentration, B: Inoculum level, C: Initial moisture content, D: Soya bean meal, β1, β2, β3, β4: linear coefficients; β11, β22, β33, β44 are the squared coefficients; β12, β13, β14, β23, β24, β34: interaction coefficients.
Each independent variable was separated into three levels, namely positive level, middle level or zero level and negative level. Every level was included in the run matrix for the study on effect of various independent variables on serralysin production by S. hydrogenans var. MGS13. Here, each experiment was done in three sets.
Statistical analysis and validation of experimental model:
Analysis of Variance (ANOVA) was employed for analyzing the data to identify the most effective independent variables and its interaction effects for maximum serralysin production. The experimental model was validated with respect to all variables within the design space. The optimized combinations were experimented each in triplicate and the observed mean response was compared with the predicted value.
Purification and characterization of serralysin:
Extracellualr enzyme of S. hydrogenans var. MGS13 was extracted from fermented bran, which was further precipitated and purified by gel filteration method using Sephadex G-100[20].
Native Polyacrylamide Gel Electrophoresis (PAGE) (non-denaturing gel electrophoresis):
As a trail native PAGE was done according to the method of Laemmli et al.[26] with various percentages of polyacrylamide resolving gel i.e., 10, 12 and 15 % to know the exact sieving gel percentage of the crude enzyme under non-denaturing conditions. The sample was prepared in Sodium Dodecyl Sulfate (SDS) free buffer, under non-reducing (without 2-βmercaptoethanol) and non-denaturing conditions (without heating) and the stacking and separating gel was also prepared without using SDS.
SDS-PAGE:
Evaluation of biological activities of serralysin:
The purified serralysin has been examined for its biological properties by performing in vitro fibrinolytic and anti-inflammatory activities.
In vitro fibrinolytic activity of purified serralysin:
The fibrinolytic activity of the purified serralysin was assessed by in vitro ‘fibrin clot lytic method’ according to Rajput et al.[27] with slight modifications. The preparation of fibrin clot was done as per Sigma- Aldrich procedure by cross-linking of fibrinogen with thrombin. Both fibrinogen and thrombin solutions were prepared in 0.9 % NaCl. About 5 ml aliquot of fibrinogen solution (10 mg/ ml in saline) was added to 0.5 ml of 50 U/ml solution of thrombin in saline. Then the solution was mixed thoroughly and permitted to stand for 1 h incubation at 37° to ensure the polymerization of fibrin clot. Fibrinolytic activity was performed with different concentrations of purified serralysin (250, 500, 750, 1000 U/500 µl) and commercial serratiopeptidase (100, 200, 300, 400 and 500 U/500 µl). All test tubes containing fibrin clot were labeled properly and thereafter, 500 µl of each sample was added separately into each tube and allowed to stand 8 h incubation at 37°. As a control, borate buffer pH 9.0 (500 µl) was added to fibrin clot and incubated under same experimental conditions. After incubation, volume of fluid obtained was measured to perceive the change in volume after of lysis fibrin clot. The percentage fibrinolysis was calculated by measuring the difference obtained in volume of fluid taken before and after fibrin clot lysis. All trials were repeated in triplicate. The software Graph Pad Prism version 5.0 has been used to analyze the obtained data. All values are expressed as mean±standard error of mean. Data were analyzed by one-way ANOVA and difference between means was assessed by Dunnett’s multiple comparison. p<0.05 was considered statistically significant.
In vitro anti-inflammatory activity of purified serralysin:
The anti-inflammatory activity of purified serralysin enzyme was assessed by in vitro Human Red Blood Cell (HRBC) membrane stabilization method according to Shinde et al.[28] with minor modifications. Sample solutions of purified serralysin enzyme (100, 200, 300, 400 and 500 IU/ml), commercial serratiopeptidase (100, 200 IU/ml) and diclofenac (200 µg/ml) were prepared by dissolving the corresponding stock solutions made up of hypotonic and isotonic buffers.
The erythrocyte suspension was prepared by drawing fresh blood sample (3 ml) from healthy volunteer who is not any drug therapy for the past 2 w and transferred into the heparinized tubes to prevent clotting and centrifuged at 3000 rpm for 10 min at 4°. After centrifugation, supernatant was measured, discarded and an equivalent volume of normal saline solution was used to dissolve red blood pellet. The acquired quantity of red blood cell pellet was measured and re-constituted as a 40 % v/v cell suspension with isotonic buffer (10 mM sodium phosphate buffer, pH 7.4). Haemolysis was induced by dissolving the samples under test in hypotonic solution (distilled water) and isotonic solution respectively. About 5 ml of hypotonic solution containing graded concentration of enzyme solutions (100, 200, 300, 400 and 500 U/ml) were set into 2 pairs (per concentration) of small test tubes. About 5 ml of isotonic solution also containing graded concentration of enzyme solutions (100, 200, 300, 400 and 500 IU/ml) were also set into 2 pairs (per concentration) of the small test tubes.
The control tubes were containing 5 ml of hypotonic solution and 5 ml of graded dose (100 and 200 IU/ ml) of commercial serratiopeptidase and diclofenac (200 µg/ml) were prepared with hypo and isotonic solutions respectively. About 0.1 ml of erythrocytic suspension was incorporated to the mixture of each of test tube and mixed gently. Further the sample mixtures were incubated for 1 h at 37° and centrifuged for 10 min at 3000 rpm at 4°. The supernatant was collected further absorbance (Optical Density (OD)) of the hemoglobin content of supernatant was valued at 540 nm using visible spectrophotometer. The haemolysis produced in the presence of hypotonic solution was considered as 100 %. The inhibition percentage of haemolysis was estimated by using the given formula.
Percentage of inhibition of haemolysis=1-(OD2-OD1/OD3-OD1)×100
Where OD1=Absorbance of the test sample in isotonic solution; OD2=Absorbance of the test sample in hypotonic solution; OD3=Absorbance of the control sample in hypotonic solution
The software Graph Pad Prism version 5.0 has been used to analyze the obtained data. All values are expressed as mean±standard deviation. Data were analyzed by using one-way ANOVA and difference between means was assessed by Dunnett’s multiple comparison. p<0.05 was considered statistically significant.
Results and Discussion
Serralysin has been extensively employed in the treatment of inflammation and atherosclerotic disease (by dissolving fibrin clot)[2]. RSM based CCD design matrix was applied to identify the best possible level of individual parameters and their combined effects on serralysin production from S. hydrogenans var. MGS13 with four process variables namely horse gram flour concentration, initial moisture content, inoculum level and soya bean meal by employing SSF. Based on the results of traditional OVAT method[23], the process variables were selected and considered as center point in CCD design. Identification of appropriate solid substrate is a vital step in SSF process. So the preliminary screening was conducted by using various particle sizes (ranging from 850-250) of various solid substrates (Rice bran, wheat bran, green gram husk, black gram husk, rice flour and horse gram flour) were used. Among them 425 microns of horse gram flour was found to be suitable for serralysin production from S. hydrogenans var. MGS13. There were no reports available on production of serralysin using horse gram flour as a solid substrate in the SSF. Although horse gram is an excellent source of proteins but its consumption is very less due to unacceptable taste and flavor of cooked products, so it remained as an underutilized crop. Thus utilizing this crop residue as a nutrient source will not only support the growth of organism but also reduces the overall production cost. The CCD based RSM model was designed with the result of 30 treatments (runs) then actual and predicted response of serralysin activity (U/gds) are presented in Table 2. The significance of this model was checked with ANOVA using F test and multiple regression analysis was applied to obtain a second order polynomial equation (1). Depending on the selected variable combination, serralysin activity was varied over a range (from 7.24 to 93.78). After regression analysis, the regression equation for serralysin production in terms of coded variable and actual variables was obtained as presented below in equation (2).
| Std | Runs | A: Horse gram flour concentration (g) | B: Inoculum size (ml) | C: Initial moisture content (%v/w) | D: Soya bean meal (% w/w) | Serratiopeptidaseactivity (U/gds) | |
|---|---|---|---|---|---|---|---|
| Experimental | Predicted | ||||||
| 12 | 1 | 6 | 1.25 | 35 | 1.25 | 59.21 ±0.02 | 59.47 |
| 15 | 2 | 4 | 1.25 | 45 | 1.25 | 68.98±0.02 | 73.4 |
| 1 | 3 | 4 | 0.75 | 35 | 0.75 | 21.31±0.03 | 23.26 |
| 5 | 4 | 4 | 0.75 | 45 | 0.75 | 65.90±0.05 | 62.9 |
| 24 | 5 | 5 | 1 | 40 | 1.5 | 55.29±0.01 | 59.2 |
| 3 | 6 | 4 | 1.25 | 35 | 0.75 | 30.33±0.01 | 31.35 |
| 25 | 7 | 5 | 1 | 40 | 1 | 78.85±0.00 | 78.85 |
| 23 | 8 | 5 | 1 | 40 | 0.5 | 60.24±0.03 | 58.88 |
| 2 | 9 | 6 | 0.75 | 35 | 0.75 | 13.46±0.03 | 6.3 |
| 7 | 10 | 4 | 1.25 | 45 | 0.75 | 78.01±0.08 | 79.6 |
| 27 | 11 | 5 | 1 | 40 | 1 | 78.85±0.00 | 78.85 |
| 26 | 12 | 5 | 1 | 40 | 1 | 78.85±0.00 | 78.85 |
| 29 | 13 | 5 | 1 | 40 | 1 | 78.85±0.00 | 78.85 |
| 8 | 14 | 6 | 1.25 | 45 | 0.75 | 89.93±0.09 | 78.12 |
| 17 | 15 | 3 | 1 | 40 | 1 | 30.33±0.02 | 30.1 |
| 19 | 16 | 5 | 0.5 | 40 | 1 | 25.22±0.03 | 29.1 |
| 20 | 17 | 5 | 1.5 | 40 | 1 | 93.78±0.05 | 92.45 |
| 18 | 18 | 7 | 1 | 40 | 1 | 7.24±0.01 | 10.03 |
| 9 | 19 | 4 | 0.75 | 35 | 1.25 | 22.34±0.11 | 31.41 |
| 4 | 20 | 6 | 1.25 | 35 | 0.75 | 18.24±0.51 | 30.09 |
| 11 | 21 | 4 | 1.25 | 35 | 1.25 | 67.88±0.02 | 62.38 |
| 22 | 22 | 5 | 1 | 50 | 1 | 55.45±0.02 | 64.34 |
| 10 | 23 | 6 | 0.75 | 35 | 1.25 | 14.26±0.02 | 12.82 |
| 21 | 24 | 5 | 1 | 30 | 1 | 20.22±0.05 | 13.88 |
| 30 | 25 | 5 | 1 | 40 | 1 | 78.85±0.00 | 78.85 |
| 16 | 26 | 6 | 1.25 | 45 | 1.25 | 72.09±0.01 | 70.29 |
| 28 | 27 | 5 | 1 | 40 | 1 | 78.85±0.00 | 78.85 |
| 13 | 28 | 4 | 0.75 | 45 | 1.25 | 45.54±0.00 | 33.84 |
| 14 | 29 | 6 | 0.75 | 45 | 1.25 | 18.78±0.09 | 15.03 |
| 6 | 30 | 6 | 0.75 | 45 | 0.75 | 40.09±0.09 | 45.74 |
Table 2: Experimental and Predicted Values of Serralysin Yield Recorded in the Experimental Setup of RSM
Serralysin activity= +78.85×A-5.02×A+15.84×B+12.61×C+0.0796×D+3.92×AB-0.0531×AC- 0.4106×AD+2.15×BC+5.72×BD-9.31×CD-14.70×A2-4.52×B2-9.93×C2-4.9×D2 (2)
Where Y: Response (Serralysin activity in (U/gds), A: Horse gram flour concentration (g); B: Inoculum size (ml); C: Initial moisture content (% v/w); D: Soya bean meal (% w/w) respectively.
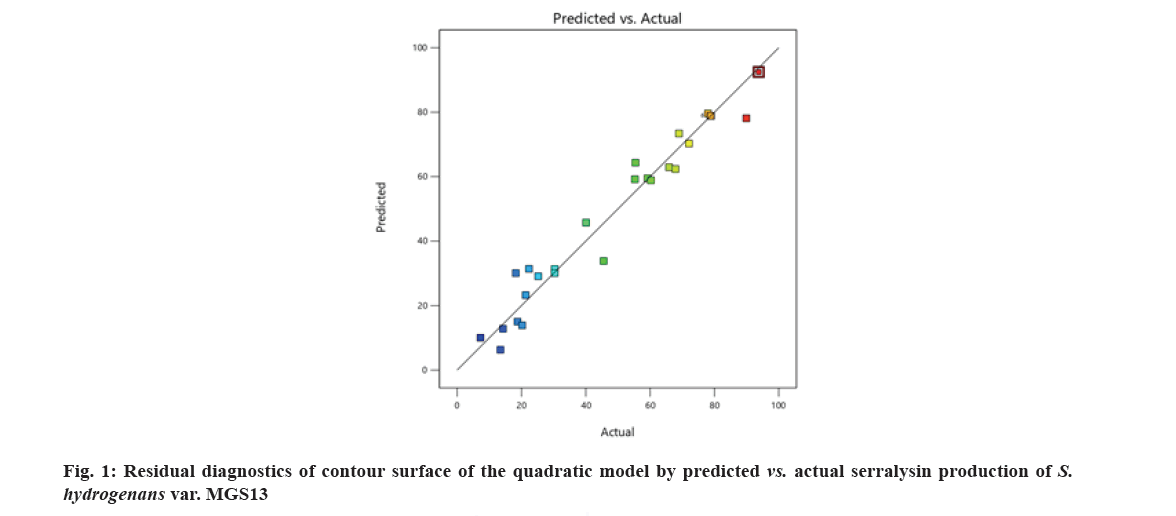
The ANOVA of the regression model expressed the significance of the parameters with effect of serralysin production as evident from the high F value 26.45 and low p value of ?0.0001 (Table 3). There is only 0.01 % probability of the model F-value could happen due to noise. The values of ?Prob>F’ less than 0.0500 implies that the model terms such as linear terms horse gram flour concentration (A); inoculum size (B); initial moisture content (C); interactive terms BD (inoculum size and soya bean meal); CD (initial moisture content and soya bean meal) and the quadratic terms horse gram flour concentration2 ((A2); inoculum size2 (B2); initial moisture content2 (C2) and soya bean meal2 (D2) were found to be significant for serralysin production. The predicted and experimental values plot illustrated that actual values were nearer to the straight line (fig. 1) indicating that experimental serralysin activity (93.78) under optimized condition was in strong agreement with predicted serralysin activity (92.45).
| Source of variance | Sum of Squares | df | Mean Square | F-value | p-value | |
|---|---|---|---|---|---|---|
| Model | 20459.81 | 14 | 1461.42 | 26.45 | <0.0001 significant | |
| Linear effects | A-Horse gram flour concentration | 604.11 | 1 | 604.11 | 10.93 | 0.0048 |
| B-Inoculum size | 6020.15 | 1 | 6020.15 | 108.95 | <0.0001 | |
| C-Initial moisture content | 3819.07 | 1 | 3819.07 | 69.11 | <0.0001 | |
| D-Soya bean meal | 0.152 | 1 | 0.152 | 0.0028 | 0.9589 | |
| Interaction effects | AB | 246.25 | 1 | 246.25 | 4.46 | 0.052 |
| AC | 0.0452 | 1 | 0.0452 | 0.0008 | 0.9776 | |
| AD | 2.7 | 1 | 2.7 | 0.0488 | 0.8281 | |
| BC | 74 | 1 | 74 | 1.34 | 0.2653 | |
| BD | 523.15 | 1 | 523.15 | 9.47 | 0.0077 | |
| CD | 1385.51 | 1 | 1385.51 | 25.07 | 0.0002 | |
| Quadratic effects | A2 | 5923.26 | 1 | 5923.26 | 107.19 | <0.0001 |
| B2 | 559.52 | 1 | 559.52 | 10.13 | 0.0062 | |
| C2 | 2706.12 | 1 | 2706.12 | 48.97 | <0.0001 | |
| D2 | 672.15 | 1 | 672.15 | 12.16 | 0.0033 | |
| Residual | 828.86 | 15 | 55.26 | |||
| Lack of Fit | 828.86 | 10 | 82.89 | |||
| Pure Error | 0 | 5 | 0 | |||
| Corrected Total | 21288.67 | 29 | ||||
Note: *The model F-value 26.45 implies the model is significant; A: Horse gram flour concentration B: Inoculum size; C: Initial moisture content and D: Soya bean meal
Table 3: Analysis of Variance for CCD Results of Serralysin Production
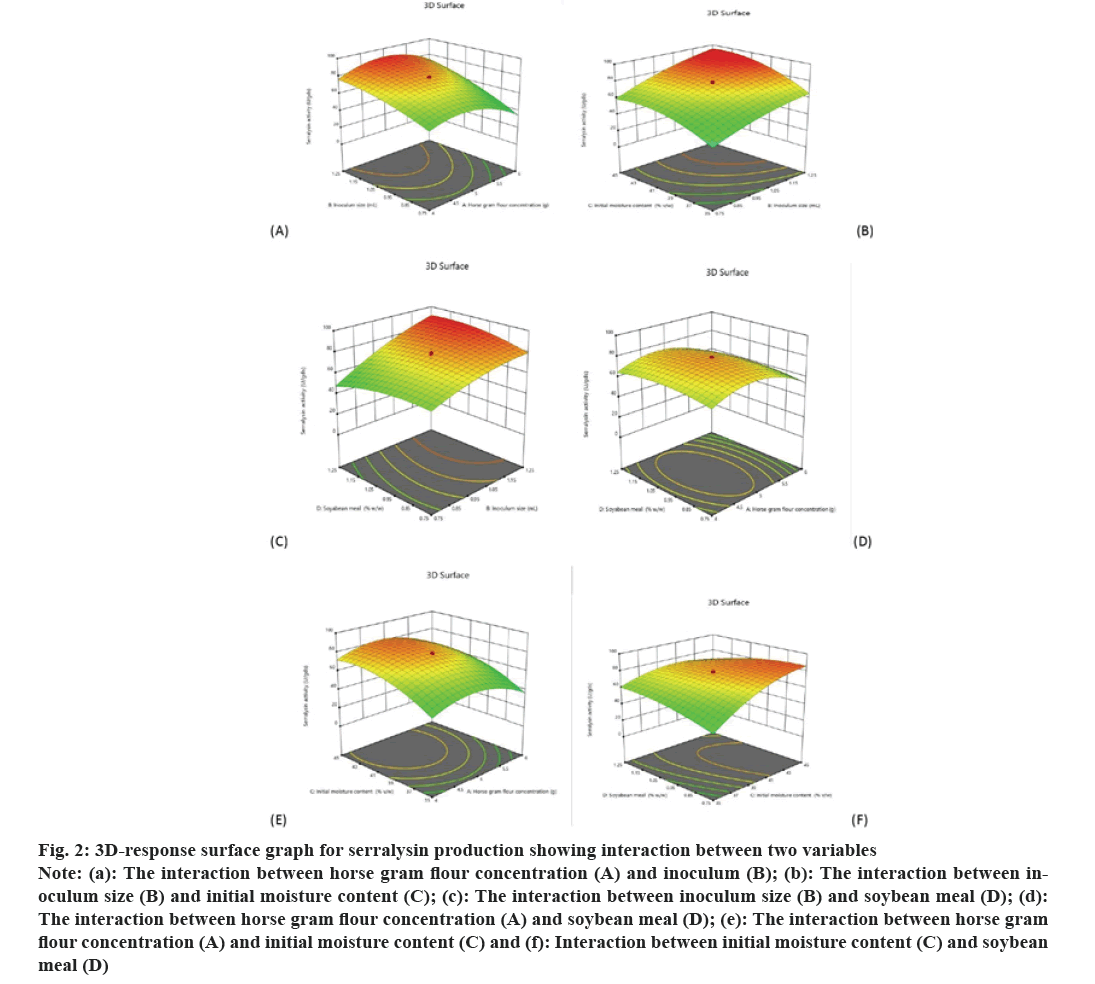
The coefficient of determination (R2) was 0.9611 indicated that 96.11 % variability in the response data could be elucidated by this model. The R2 value 0.9611 which is closer to 1 depicted that the model is statistically good. The predicted R2 of 0.7757 is in reasonable agreement with the adjusted R2 of 0.9247 because the difference is less than 0.2. An “adequate precision” measures the signal to noise ratio and the desirable value should be more than 4. A ratio16.390 indicates that the proposed model is significant. The ANOVA results depicted that the horse gram flour concentration had a significant influence on serralysin production. An optimum yield of serralysin was obtained with 5.0 g of horse gram flour. The concentration of solid substrate plays an essential role in enzyme production because it provides essential nutrients, growth factors to microbes and also minimizes the manufacturing cost[19,29,30]. Like other solid substrates horse gram flour also supports same substrate concentration for SSF. The ANOVA results depicted that inoculum size had a remarkable influence on serralysin production and maximum yield of serralysin was achieved with 1.2 ml of inoculums S. hydrogenans var. MGS13. Beyond 1.2 ml of inoculum size a decrease of enzyme yield was observed which could be due to exhaustion of nutrients in the fermented medium whereas lower inoculum level of S. hydrogenans var. MGS13 required a more time for its growth. Numerous studies have detailed that optimum size of inoculum is crucial for achieving maximum biomass and yield of metabolite[19,31]. Depending up on the type of microorganism, and concentration of substrate appropriate size of inoculums was varied Bacillus sp. showed maximum protease production with 20 % v/ w inoculum[32-34] whereas maximum yield of rhamnolipid was achieved with 2.5 ml inoculum of Serratia rhubidaea[29]. The ANOVA results revealed initial moisture content had a significant influence on serralysin production and optimum serralysin yield was achieved at 44 % of initial moisture content whereas at lower and higher moisture contents resulted in reduction of serralysin production. This may be because of less solubility of nutrients in solid substrate medium at low moisture level; while at higher moisture levels low protease yield was noticed because of stickiness and decreased porosity of solid substrate[19,34,35]. The 3D-response surface plot (fig. 2a) obtained as a function of horse gram flour concentration versus inoculum size, where maximum serralysin yield (90 U/gds) was noticed with 5.1 g horse gram flour concentration and 1.2 ml inoculum.
The 3D-response surface plot (fig. 2b) obtained as a function of inoculum size versus initial moisture content, where maximum serralysin yield (95 U/gds) was observed with 1.2 ml inoculum size and 44 % v/ w initial moisture content. The 3D response plot (fig. 2c) obtained as a function of inoculum size vs. soya bean meal where maximum serralysin yield by 91 U/ gds was noticed with inoculum size 1.2 ml and soya bean meals 1.2 % w/w further increasing or decreasing their levels resulted in decrease in serralysin yield. The 3D-response surface plot (fig. 2d) depicted the interaction between horse gram flour concentration and soya bean meal where no improvement of serralysin yield was noticed with this interaction. The 3D response plot (fig. 2e) acquired as a function of horse gram flour concentration vs. initial moisture content and (fig. 2f) acquired as a function of initial moisture content versus soya bean meal where serralysin yield was improved with increasing initial moisture content up to 44 %, further increasing its level resulted in gradual decrease in serralysin yield.
Fig 2: 3D-response surface graph for serralysin production showing interaction between two variables
Note: (a): The interaction between horse gram flour concentration (A) and inoculum (B); (b): The interaction between inoculum size (B) and initial moisture content (C); (c): The interaction between inoculum size (B) and soybean meal (D); (d): The interaction between horse gram flour concentration (A) and soybean meal (D); (e): The interaction between horse gram flour concentration (A) and initial moisture content (C) and (f): Interaction between initial moisture content (C) and soybean meal (D)
The developed design was validated by performing the experiment with predicted optimized parameters such as horse gram flour (5.0 g), inoculum (1.2 ml), initial moisture content (44 % v/ w), soya bean meal (1.0 % w/w). Under these optimized conditions the experimental serralysin activity (94 U/gds) was determined to be in quite close the predicted serralysin activity (93 U/gds) which confirmed that the developed model was reliable. This study resulted in 1.3 fold increase on serralysin production (94 U/ gds) than initial level (78 U/gds).
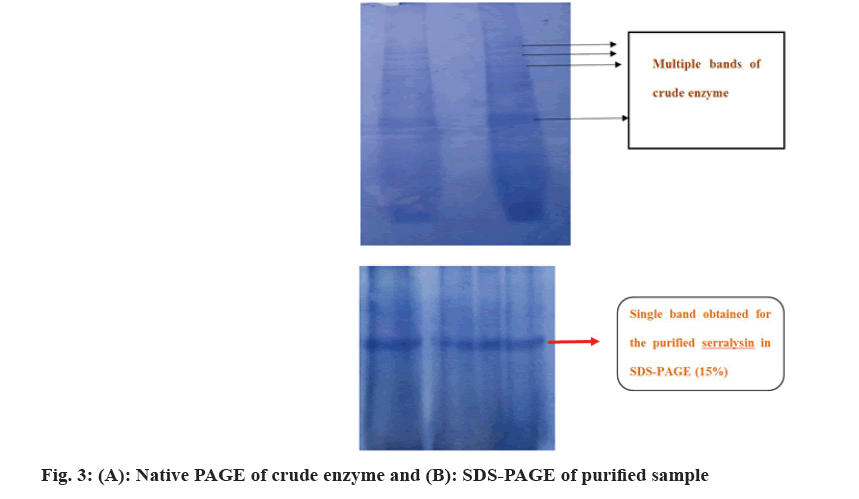
The extracellular supernatant of enzyme was extracted from fermented bran, precipitated, further purified by gel filtration using Sephadex G100 and the process was clearly reported by authors in the previous reports[20]. An appropriate resolving gel percentage is required for good mobility and separation of sample. So, as a trail in the present study Native PAGE of crude enzyme was carried with various percentage of resolving gel (10, 12 and 15 %). The observed multiple bands in native PAGE represents that 15 % resolving gel was found to be appropriate sieving gel percentage for the crude enzyme (fig. 3a). Further the purity of the enzyme was verified by performing a SDS PAGE with 15 % resolving gel where a single band was observed (fig. 3b) which indicates that the enzyme is homogenous in nature. Previously we have reported the characterization studies, peptide mapping analysis and bioinformatics studies based on that we confirmed that this purified enzyme is an alkaline metalloprotease[20].
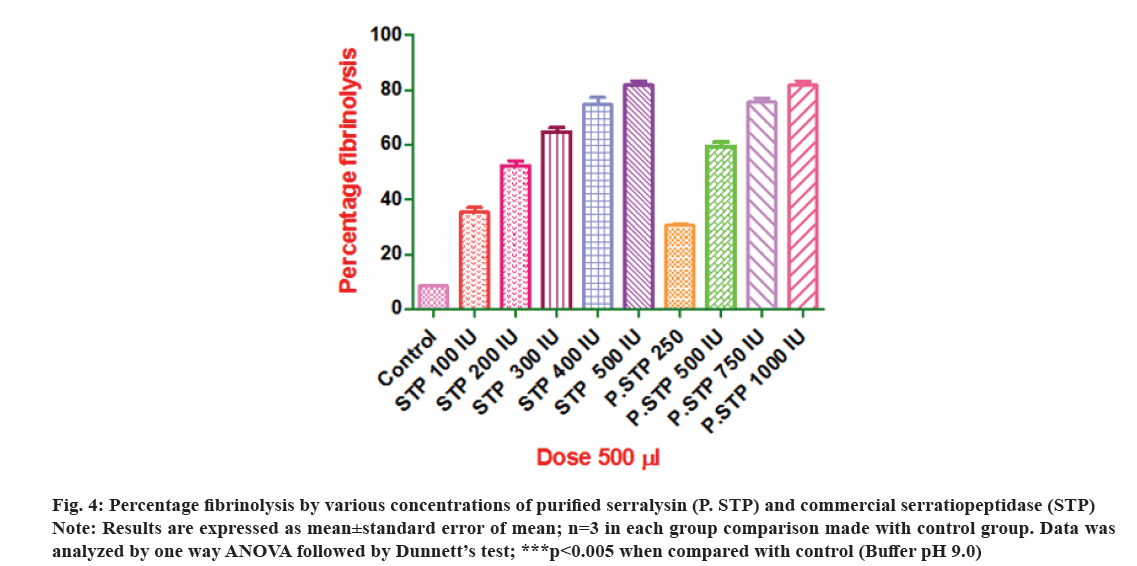
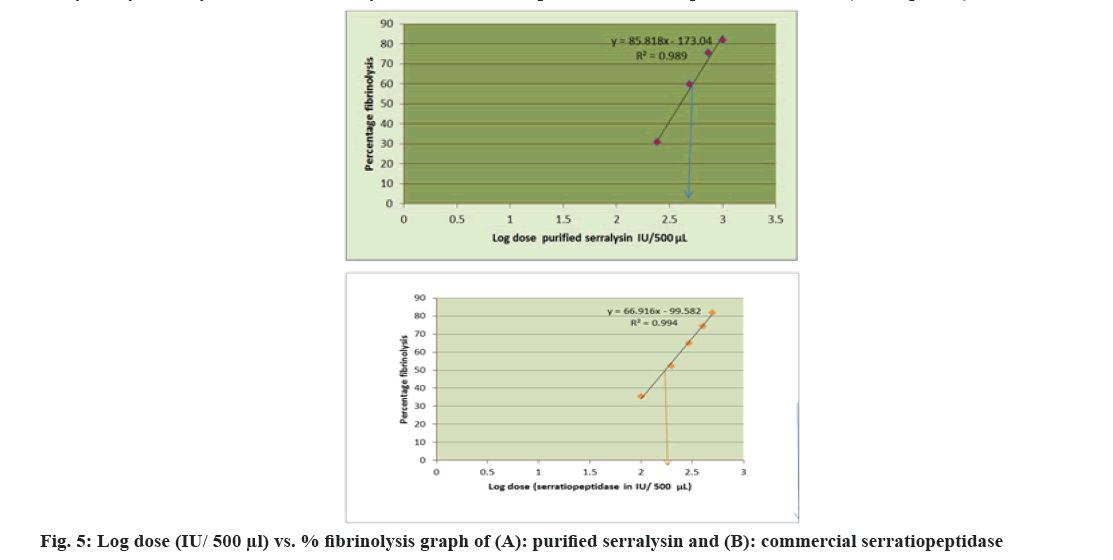
In vitro fibrinolytic and anti-inflammatory activities of purified serralysin were further performed in order to verify the biological properties of serratiopeptidase. Fibrin clot lytic method was employed for assessment of fibrinolytic activity using various concentrations of standard (commercial serratiopeptidase) as positive control and purified serralysin of S. hydrogenans var. MGS13 as test and buffer as negative control. The results (Table 4) were validated by demonstrating a dose dependent graph (fig. 4) using Prism-software (Version 5.0). The investigation suggested that both purified serralysin (250-1000 IU/500 µl) and commercial serratiopeptidase (100-500 IU/500 µl) showed dose dependent fibrinolysis because the activity was increased with the increasing concentrations. Clots were treated with borate buffer pH 9.0 showed negligible clot lysis. The mean difference in percentage clot lysis between each dose of purified serralysin enzyme, commercial serratiopeptidase enzyme and buffer (control) was significant (p<0.05). Further half maximal inhibitory concentration (IC50) values of purified serralysin (fig. 5a) and commercial serratiopeptidase (fig. 5b) for fibrinolysis were calculated by plotting log dose vs. fibrinolysis respectively and found to be 403.1 and 172.2 IU respectively. After incubation of 8 h, the purified serralysin showed 81 % fibrinolysis at a concentration of 1000 IU/500 µl when compared to commercial serratiopeptidase same activity was exhibited at concentration of 500 IU/500 µl, further the lysis of clot was visually observed during incubation. Serrapeptase is a plasmin like protease which could directly dissolves fibrin clot[36], hence it is the more advantageous over plasminogen activators such as urokinase, streptokinase and tissue plasminogen activators (t-PA) where the enzyme converts the blood plasminogen to clot dissolving plasmin. From the present research, it is denoted, that the purified serralysin has potent fibrinolytic activity like commercial serratiopeptidase, which conforms that purified serralysin of S. hydrogenans var. MGS13 shares serralysin kind of nature. As mentioned above some reports on serralysin like alkaline metalloprotease from various sources such as Serratia sp. and Xenorhabdus also exhibited strong fibrinolytic property[36,10].
| Dose (IU/500 µl) | Percentage fibrinolysis purified serralysin | Commercial serratiopeptidase |
|---|---|---|
| 100 | - | 35.55±0.89*** |
| 200 | - | 52.47±0.93*** |
| 250 | 30.50±0.80*** | - |
| 300 | - | 64.87±0.86*** |
| 400 | - | 74.65±1.50*** |
| 500 | 59.55±0.89*** | 81.77±0.86*** |
| 750 | 75.54±0.88*** | - |
| 1000 | 81.89±0.86*** | - |
| Control | 8.7±0.03*** | - |
Note: Results are expressed as mean±standard error of mean; n=3 in each group comparison made with control group. Data was analysed by one way ANOVA followed by Dunnett’s test; ***p<0.005 when compared with control (Buffer pH 9.0)
Table 4: Percentage Of Fibrinolysis Exhibited by Various Concentrations of Purified Serralysin and Commercial Serratiopeptidase
Fig 4: Percentage fibrinolysis by various concentrations of purified serralysin (P. STP) and commercial serratiopeptidase (STP)
Note: Results are expressed as mean±standard error of mean; n=3 in each group comparison made with control group. Data was analyzed by one way ANOVA followed by Dunnett’s test; ***p<0.005 when compared with control (Buffer pH 9.0)
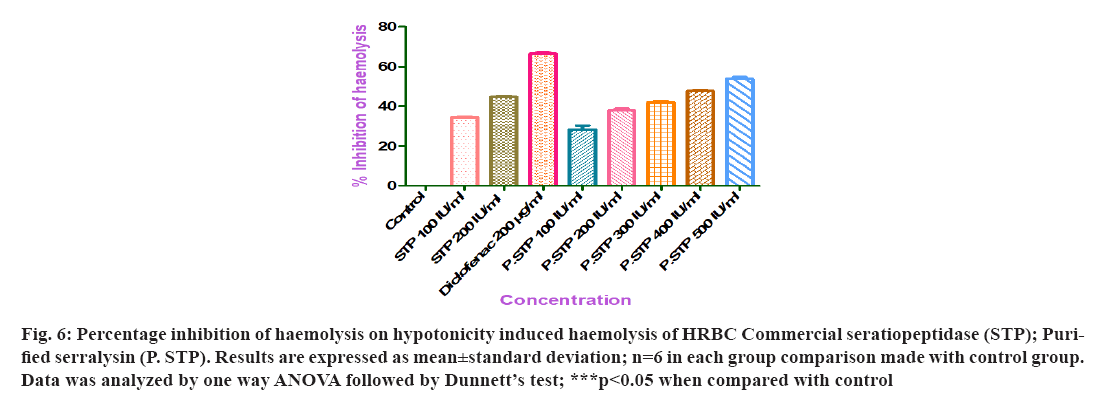
In vitro anti-inflammatory activity was studied by measuring the percentage inhibition of hemolysis for all concentrations of purified serralysin enzyme, commercial serratiopeptidase and diclofenac (Table 5). Various concentrations (100-500 IU/ml) of purified serralysin of S. hydrogenans var. MGS13 exhibited varying percentage inhibition of haemolysis were 28, 38, 42, 48 and 54 (fig. 6). Control groups treated with diclofenac (200 µg/ml) and commercial serratiopeptidase (100, 200 IU/ml) showed 67, 34 and 44 percentage inhibition of haemolysis respectively. A concentration dependent anti-inflammatory activity was noticed with both purified enzyme and commercial serratiopeptidase. The data revealed that purified serralysin significantly (p value <0.05) inhibited hemolysis induced by hypotonic solution (water). The percentage inhibition of haemolysis by purified serralysin enzyme was 0.85 times when compared to the commercial serratiopeptidase.
| Concentration (IU/ml) | Purified Serralysin solution | Commercial Serratiopeptidase solution | Diclofenac Solution |
|---|---|---|---|
| 100 | 28.3±4.3 | 34.4±0.62 | - |
| 200 | 38.0±2.1 | 44.4±1.1 | - |
| 300 | 42.0±1.3 | - | - |
| 400 | 47.0±1.3 | - | - |
| 500 | 54.3±1.7 | - | - |
| 200 | - | - | 66.6±1.0 |
Note: Level of significance***p<0.05. Percent inhibition of haemolysis was calculated relative to control. Results are expressed as mean±standard deviation; n=6 in each group comparison made with control group. Data was analysed by one way ANOVA followed by Dunnett’s test; ***p<0.05 when compared with control
Table 5: Percentage Inhibition of Haemolysis on Hypotonicity Induced Haemolysis of HRBC
Fig 6: Percentage inhibition of haemolysis on hypotonicity induced haemolysis of HRBC Commercial seratiopeptidase (STP); Purified serralysin (P. STP). Results are expressed as mean±standard deviation; n=6 in each group comparison made with control group. Data was analyzed by one way ANOVA followed by Dunnett’s test; ***p<0.05 when compared with control
The results depicted that the purified serralysin exhibited membrane stabilization effect by protecting the human erythrocyte membrane against hemolysis induced by hypotonic solution. As HRBC membrane is comparable with the lysosomal membrane[37], the percentage inhibition of hemolysis was taken for the assessment of anti-inflammatory activity. Hypotonic solution induces hemolysis by accumulating excess fluid within the cells, this result in rupturing of cell membrane. When there is any damage to RBC membrane, it will make the cell more vulnerable to secondary damage and this damage is occurred due to free radical induced lipid peroxidation. During inflammatory conditions, the vascular permeability of membrane was increased by inflammatory mediators like serotonin, bradykinin and histamine so more fluid is accumulated in tissues. The study revealed that purified serralysin perhaps stabilized the HRBC membrane by hydrolyzing serotonin, bradykinin and histamine. The results are in accordance with other reports where serratiopeptidase obtained from S. marcescens protease and L-asparaginase[38-40] obtained from Aspergillus sp. also showed excellent membrane stabilizing property.
A cost effective medium was designed for production of serralysin and evaluated for its anti-inflammatory (in vitro) and fibrinolytic activity (in vitro). The CCD based RSM model was proved to be effective in enhancing serralysin yield by S. hydrogenans var. MGS13. The DOE approach assisted in 1.3 fold increases in serralysin yield compared with initial level. The purified enzyme of S. hydrogenans var. MGS13 exhibited significant anti-inflammatory and fibrinolytic activity like commercial serratiopeptidase. The biological activities (anti- inflammatory and fibrinolytic) and structural features were similar to serratiopeptidase suggesting it could serves as potential bio-similar for therapeutic and industrial relevance. Further this enzyme formulation may also find many pharmaceutical applications in topical formulation to reduce the inflammation on skin.
Acknowledgements:
The authors want to acknowledge A.U. College of Pharmaceutical Sciences, Andhra University for providing laboratory facilities to carry out the entire research work. The authors are extremely grateful to T. Prabhakar, Retd. Professor, A.U. College of Pharmaceutical Sciences, for giving his valuable suggestions in preparing this manuscript.
Conflict of interests:
The authors declared no conflict of interests.
References
- Viswanathswamy AH, Patill PA. Effect of some clinically used proteolytic enzymes on inflammation in rats. Indian J Pharm Sci 2008; 70(1):114-117.
[Crossref] [Google Scholar] [PubMed]
- Swathi BJ, Neha S, Ankit Rvic R, Abhijit R. Serratiopeptidase: Insights into the therapeutic applications. Biotechnol Rep 2020 Dec; 28 e00544.
- Mazzone A, Catalani M, Drusian A, Mandoli A, Russo S. Evaluation of serralysin in acute or chronic inflammation of otorhinolaryngology pathology: A multicentre, double-blind, randomized trail versus placebo. J Int Med Res 1990; 18(5):379-388.
[Crossref] [Google Scholar] [PubMed]
- Kakinuma A, Moriya N, Kawahara K, Sugino H. Repression of fibrinolysis in scalded rats by administration of Serratia protease. Biochem Pharmacol 1982;31(18):2861-6.
[Crossref] [Google Scholar] [PubMed]
- Wols RL, Bond JS. Phe5 (4-nitro)-bradykinin: A chromogenic substrate for assay and kinetics of the metalloendopeptidase meprin. Anal Biochem 1990;191(2):314-20.
[Crossref] [Google Scholar] [PubMed]
- Jadav SP, Patel NH, Shah TG, Gajera MV, Trivedi HR, Shah BK. Comparison of anti-inflammatory activity of serratiopeptidase and diclofenac in albino rats. J Pharmacol Pharmacother 2010;1(2):116-7.
[Crossref] [Google Scholar] [PubMed]
- Mansfeld J. Metalloproteases. In: Polania J, MacCabe AP (ed). Industrial enzymes: Structure, function and applications. The Netherlands: Springer; 2007.
- Maeda H. Role of microbial proteases in pathogenesis. Micrbiol Immunol 1996; 40(10):685-699.
[Crossref] [Google Scholar] [PubMed]
- Massaoud MK, Marokházi J, Venekei I. Enzymatic characterization of a serralysin-like metalloprotease from the entomopathogen bacterium, Xenorhabdus. Biochim Biophys Acta 2011;1814(10):1333-9.
[Crossref] [Google Scholar] [PubMed]
- Pranaw K, Singh S, Dutta D, Chaudhuri S, Ganguly S, Nain L. Statistical optimization of media components for production of fibrinolytic alkaline metalloproteases from Xenorhabdus indica KB-3. Biotechnol Res Int 2014;2014:293434.
[Crossref] [Google Scholar] [PubMed]
- Basu B, Apte SK. A novel serralysin metalloprotease from Deinococcus radiodurans. Biochim Biophys Acta. 2008;1784(9):1256-64.
[Crossref] [Google Scholar] [PubMed]
- Kyöstiö SR, Cramer CL, Lacy GH. Erwinia carotovora subsp. carotovora extracellular protease: characterization and nucleotide sequence of the gene. J Bacteriol 1991;173(20):6537-46.
[Crossref] [Google Scholar] [PubMed]
- Kreger AS, Griffin OK. Cornea-damaging proteases of Serratia marcescens. Invest Ophthalmol Vis Sci 1975;14(3):190-8.
[Google Scholar] [PubMed]
- Hatanaka T, Usuki H, Arima J, Uesugi Y, Yamamoto Y, Kumagai Y, et al. Extracellular production and characterization of two Streptomyces L-asparaginases. Appl Biochem Biotechnol 2011;163(7):836-44.
[Crossref] [Google Scholar] [PubMed]
- Chitte RR, Deshmukh SV, Kanekar PP. Production, purification, and biochemical characterization of a fibrinolytic enzyme from thermophilic Streptomyces sp. MCMB-379. Appl Biochem Biotechnol 2011;165(5):1406-13.
[Crossref] [Google Scholar] [PubMed]
- Vanama J, GirijaSankar G, Prabhakar T, Ameena B. Isolation of novel mutant strain for enhanced production of extracellular serratiopeptidase from mangrove soil. Int J Pharm Sci Rev Res 2014;24(2):302-5.
- Vanama J, Chintada H, Guntuku G, Tadimalla P. Application of response surface methodology in medium components optimization to enhance serratiopeptidase production by Streptomyces hydrogenans MGS13. Eur Sci J 2014;10(12):196-209.
[Google Scholar] [PubMed]
- Rani SR, Kumar PA, Soccol Carlos R, Ashok P. Review: Recent advances in solid-state fermentation. Biochem Eng J 2009;44:13-8.
- Kumar V, Ahluwalia V, Saran S, Kumar J, Patel AK, Singhania RR. Recent developments on solid-state fermentation for production of microbial secondary metabolites: Challenges and solutions. Bioresour Technol 2021;323:124566.
[Crossref] [Google Scholar] [PubMed]
- Nageswara S, Guntuku G, Yakkali BL. Purification, characterization, and structural elucidation of serralysin-like alkaline metalloprotease from a novel source. J Genet Eng Biotechnol 2019;17(1):1-5.
[Crossref] [Google Scholar] [PubMed]
- Singh S, Bajaj BK. Medium optimization for enhanced production of protease with industrially desirable attributes from Bacillus subtilis K-1. Chem Eng Commun 2015;202(8):1051-60.
- MM Silva G, Bezerra RP, Teixeira JA, Porto TS, Lima-Filho JL, Porto AL. Fibrinolytic protease production by new Streptomyces sp: DPUA 1576 from Amazon lichens. Electron J Biotechnol 2015;18(1):16-9.
- Swathi N, Girijasankar G, Ramya S. Design of low cost fermentation medium for the production of serratiopeptidase enzyme using a novel Streptomyces sp. WJPPS 2016;5(10):829-42.
- Indian Pharmacopoeial Commission: Indian Pharmacopoeia, 6th edn. Govt. of India, Ministry of health and family welfare, Ghaziabad, Delhi: India. 2010.
- Dutta JR, Dutta PK, Banerjee R. Optimization of culture parameters for extracellular protease production from a newly isolated Pseudomonas sp. using response surface and artificial neural network models. Proc Biochem 2004;39(12):2193-8.
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970;227(5259):680-5.
[Crossref] [Google Scholar] [PubMed]
- Sarkar PD, Rajput MS, Nirmal NP. Tailored methods for preclinical assessment of fibrinolytic agents in vitro. J Chem Pharm Res. 2015;7(5):1130-5.
- Shinde UA, Phadke AS, Nair AM, Mungantiwar AA, Dikshit, Sarsf MN. Membrane stabilization activity: A possible mechanism of action for the anti- inflammatory activity of Cedrus deodara wood oil. Fitoterapia1999;70:251-7.
- Nalini S, Parthasarathi R. Production and characterization of rhamnolipids produced by Serratia rubidaea SNAU02 under solid-state fermentation and its application as biocontrol agent. Bioresour Technol 2014;173:231-8.
[Crossref] [Google Scholar] [PubMed]
- Cerda A, Artola A, Barrena R, Font X, Gea T, Sánchez A. Innovative production of bioproducts from organic waste through solid-state fermentation. Front Sustainable Food Systems. 2019 Aug 9;3:63.
[Google Scholar] [PubMed]
- Palla MS, Guntuku GS, Sahu PK, Kota P, Panda J. Statistical optimization of anticandida metabolite production process using Streptomyces hydrogenans strain from mangrove soils. SN Applied Sciences. 2020 Nov;2(11):1-2.
- Kashyap P, Sabu A, Pandey A, Szakacs G, Soccol CR. Extra-cellular L-glutaminase production by Zygosaccharomyces rouxii under solid-state fermentation. Proc Biochem. 2002;38(3):307-12.
- Uyar F, Baysal Z. Production and optimization of process parameters for alkaline protease production by a newly isolated Bacillus sp. under solid state fermentation. Proc Biochem 2004;39(12):1893-8.
- Lonsane BK, Ghildyal NP, Budiatman S, Ramakrishna SV. Engineering aspects of solid state fermentation. Enzy Microbiol Technol 1985;7(6):258-65.
- Zadra?il F, Brunnert H. Investigation of physical parameters important for the solid state fermentation of straw by white rot fungi. Eur J Appl Microbiol Biotechnol 1981;11(3):183-8.
- Bhargavi PL, Prakasham RS. A fibrinolytic, alkaline and thermostable metalloprotease from the newly isolated Serratia sp RSPB11. Int J Biol Macromol 2013;61:479-86.
[Crossref] [Google Scholar] [PubMed]
- Shenoy S, Shwetha K, Prabhu K, Maradi R, Bairy KL, Shanbhag T. Evaluation of anti-inflammatory activity of Tephrosia purpurea in rats. Asian Pacific J Tropical Med 2010;3(3):193-5.
- El-abd MA, Ibrahim EA. Production and one step- purification of serratiopeptidase enzyme from Serratia marcescens with potent anti-inflammatory and anti-oxidant power. Egypt Pharmaceut J 2020; 19(3): 238-243.
- Sundar SK, Sudarkodi C. In vitro anti-inflammatory activity of protease from Aspergillus sp. Int J Curr Adv Res 2018; 7:11134-7.
- Mahabal NS, Kaliwal BB. In vitro Anti-inflammatory Activity of Extracellular L-asparaginase from Soil Rhizosphere Fungus Aspergillus tamarii. Int J Drug Develop Res 2017;9(1): 35-38.