- *Corresponding Author:
- V. Rajesh
Department of Pharmacy Practice
Manipal College of Pharmaceutical Science, India
E-mail: rajesh.v@manipal.edu
| Date of Received | 18 May 2020 |
| Date of Revision | 12 August 2021 |
| Date of Acceptance | 03 March 2022 |
| Indian J Pharm Sci 2022;84(2):240-246 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Skin reactions pose a great challenge to the treating physician in the differential diagnosis. Cefuroxime with a good profile has the potential of being used as empirical therapy for a range of community acquired infections. The purpose of the review was to identify and assess various cases of drug eruptions caused by cefuroxime. The review was conducted in compliance with preferred reporting items for systematic reviews and meta-analyses guidelines. A total of nine case reports were included for the current systematic review in which the drug dose ranged from 500 mg/d to 1000 mg/d. The treatment duration varied from 1 d to 15 d across the studies. The indications for treatment were as per Food and Drug Administration guidelines. The presentation of the skin reactions was found to be symmetric in nature either in the form of erythematous rash alone or along with inflamed erosions and oozing. There was no fatal case reported. In most of the cases, the reaction subsided on the withdrawal of the intervening drug. The use of multiple doses of cefuroxime is prone to cause drug eruptions. Further research is necessary to understand the occurrence of reaction to prevent misdiagnosis of such reactions. Patients on antibiotics should be subjected to counseling on discharge regarding the occurrence of adverse drug reactions for better patient safety and qualitative care.
Keywords
Cefuroxime, immunology, pharmacokinetics, drug eruption
Cefuroxime is a broad-spectrum antibacterial agent, which is found to be effective in treating various infectious diseases[1]. Its pharmacokinetic profile provides a well-tolerated, twice-daily dosage regimen for providing better patient compliance[2]. Cefuroxime is a well-behaved and well-tolerated antibiotic with the potential of being used as an empirical therapy to treat a wide range of community-acquired infections, such as infections of the lower and upper respiratory tract, urinary tract, skin, and soft tissue[3]. In the year 1997, the drug was permitted by the Food and Drug Administration (FDA) in the treatment of respiratory tract infections due to its beneficial antibacterial activity. Being a second-generation cephalosporin, it covers an extensive range of both gram-negative and gram-positive bacteria, especially targeting organisms like gram-positive cocci and bacilli[4]. Effective treatment and ease of administration increased the use of cefuroxime regimen[5]. Based on the literature evidence, the incidence of Adverse Drug Reactions (ADR) occurring due to cefuroxime was noted to be 2.5 % of which the majority of the cases were found to be Gastrointestinal (GI) in nature followed by cutaneous reactions[6]. The nature of the cutaneous reaction is indistinguishable and poses a great challenge to the treating physician in the differential diagnosi[7]. The clinical presentation of drug eruptions can vary from mild maculopapular rashes to severe cutaneous ADRs, which may include drug-induced hypersensitivity reactions and also fatal reactions like Toxic Epidermal Necrolysis (TEN) and Stevens- Johnson Syndrome (SJS)[8]. Many drugs may induce SJS or TEN, some of which are infrequently prescribed and others that are widely used[9]. Cefuroxime is used commonly in a range of community-acquired infections and has been shown to develop cutaneous reactions in certain patients. Even though the reactions were not predictable, the withdrawal of drugs remains to be the superior choice for the treating physician[10]. There is no specific therapeutic option for the treatment of severe cutaneous reactions, whereas systemic corticosteroids serve as the gold standard of treatment. Corticosteroids act as the non-specific immunosuppressant to decrease the reaction progression in an individual[11]. The current review aimed to identify the various drug eruptions caused due to cefuroxime.
Materials and Methods
Sources of data and search:
A systematic review was carried out in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines[12]. Literature search was conducted from inception till May 2019 and was performed in PubMed, Ovid Medline and Scopus databases using the keywords “Cefuroxime” and “Drug eruption.” Use of Medical Subject Headings (MESH) along with relevant Boolean operators were utilized to build an appropriate search strategy. The literature search was conducted with restrictions on the English language. For additional records, the reference list of the included studies was screened manually as well.
Study selection:
The studies were screened based on the following criteria which were included in the review: Case report and case series; patients who developed drug eruptions; route of administration: Intravenous (IV) or oral cefuroxime. Based on the inclusion criteria, reviewers 1 and 2 had individually screened for potentially and relevant article titles along with its abstract. Full-text articles were also retrieved when needed. In all stages of the study selection, the authors were individually involved. Disagreements between the reviewers were resolved through discussion.
Data extraction and assessment of quality:
Two reviewers were self-reliantly involved in the process of data extraction. All the necessary information was tabulated in an excel sheet. The included studies were set identifiers based on the author and year of publication. Data regarding age, gender, the onset of action, route of administration and diagnosis were extracted accordingly. For this review, a previously applied tool, the Newcastle–Ottawa Scale (NOS), was used to evaluate the risk of bias (methodological quality) of case reports[13] and other relevant content[14-18]. The modified tool had eliminated items from the NOS that relayed to comparability, adjustment and retrieved items concerning selection, representativeness of cases based on individual outcomes and exposures. The tool comprised of 5 items, each needing a two-fold response to specify whether bias was expected and these items were practical to single-arm studies. We measured the quality of the study as ‘good’ when all five criteria were satisfied, ‘moderate’ when four were contented, and ‘poor’ when three or less were accomplished. The causality assessment of individual reports was carried out using Naranjo’s Sclae, World Health Organization (WHO) probability scale and the severity using Hartwig scale unless mentioned in the studies. Preventability for the included case reports was done using Schumock and Thornton scale[19-22]. The categorical data were represented descriptively and continuous data were represented as mean and standard deviation.
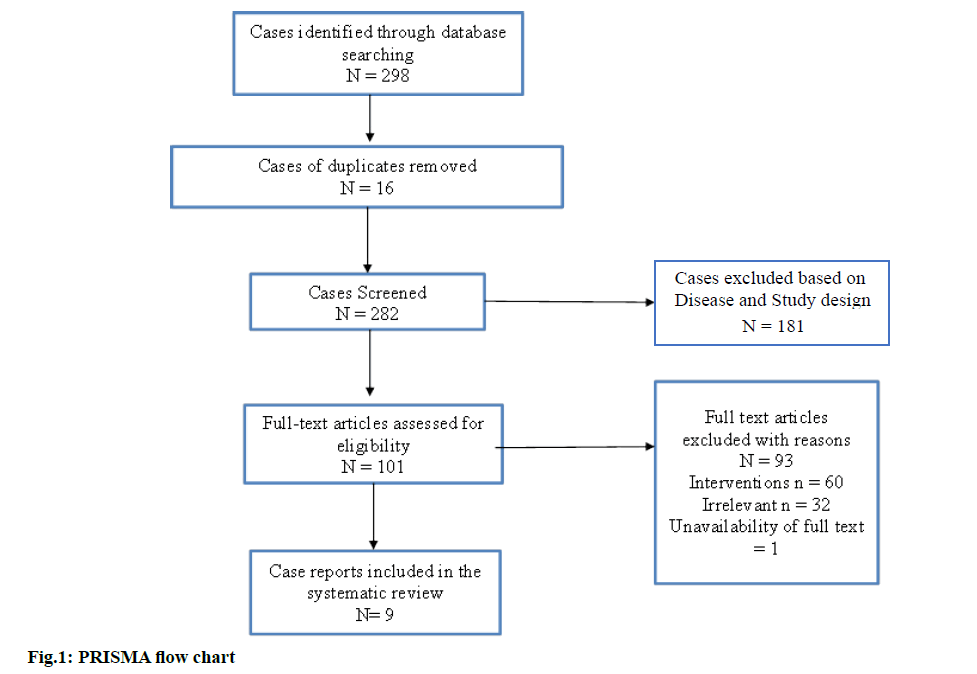
Through various electronic databases, a literature search was conducted, which resulted in a total number of 298 case reports, among which 16 case reports were excluded as duplicates. Upon screening further for titles and abstracts, 181 articles were excluded based on diagnosis and the design of the study. A sum of nine case reports was included for the current systematic review based on the inclusion criteria[23-31]. Out of the nine included case reports, one study was found to be a case series of various antibiotics causing Baboon syndrome. The screening process is concisely represented in fig. 1.
Characteristics of included studies:
The publication date of the included studies ranged from 2000 to 2015. The study characteristics are represented in Table 1. The quality of studies was rated to be moderate. There were no criteria to judge the selection bias, which was not available from the tool. Three of the studies failed to report vital information on differential diagnosis in the individual case reports. The questions utilized to measure the risk of bias and the risk of bias graph have been depicted in Table 2. The patients that were included in the study had a mean age of (54.62±18.15) y, which comprised of 6 females and two males. One of the studies reported an occurrence of reaction in a male newborn. In most of the studies, the favored route of administration was oral rather than IV. The onset of drug eruption was estimated to be ranging between 2-8 d from the day of administration.
| S. No. | Author | Country | Type of skin reaction | Drugs involved |
|---|---|---|---|---|
| 1 | Akman et al.[23] | Turkey | Systemic hypersensitivity reaction | Cefuroxime |
| 2 | Butler et al.[24] | California | Postcoital allergic reaction | Cefuroxime |
| 3 | Grgurevic et al.[25] | Croatia | Toxic epidermal necrolysis | Cefuroxime |
| 4 | Hossain et al.[26] | Bangladesh | Congenital fixed drug eruption | Cefuroxime |
| 5 | Montero et al.[27] | Spain | Cutaneous pustular leukocytoclastic vasculitis | Cefuroxime |
| 6 | Pastuszczak et al.[28] | Poland | IgA bullous dermatosis | Cefuroxime |
| 7 | Saeed et al.[29] | UK | Purpuric and erythematous macular rashes | Cefuroxime |
| 8 | Sahoo et al.[30] | India | Reddish-brown colored erythema | Cefuroxime |
| 9 | Wolf et al.[31] | Israel | Symmetric erythematous rash | Cefuroxime |
Table 1: Study Characteristics
| S. No. | Author | Question 1 | Question 2 | Question 3 | Question 4 | Question 5 | Risk of Bias | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Y | N | Y | N | Y | N | Y | N | Y | N | |||
| 1 | Akman et al.[23] | Low | Low | Low | High | Low | Moderate | |||||
| 2 | Butler et al.[24] | Low | Low | High | Low | Low | Moderate | |||||
| 3 | Grgurevic et al.[25] | Low | Low | Low | High | Low | Moderate | |||||
| 4 | Hossain et al.[26] | Low | Low | High | Low | Low | Moderate | |||||
| 5 | Montero et al.[27] | Low | Low | Low | High | Low | Moderate | |||||
| 6 | Pastuszczak et al.[28] | Low | Low | High | Low | Low | Moderate | |||||
| 7 | Saeed et al.[29] | Low | Low | Low | High | Low | Moderate | |||||
| 8 | Sahoo et al.[30] | Low | Low | Low | High | Low | Moderate | |||||
| 9 | Wolf et al.[31] | Low | Low | Low | High | Low | Moderate | |||||
Note: 1: Did the patient(s) represent the whole case(s) of the medical centre?; 2: Was the diagnosis correctly made?; 3: Where other important diagnoses excluded?; 4: Were all important data cited in the report? and 5: Was the outcome correctly ascertained?
Table 2: Tool for Risk of Bias Assessment of Included Studies
Indication for administration:
In the included studies, the dose ranged from 500 mg/d to 1000 mg/d. The treatment duration varied from 1-15 d. The indication for treatment was as per FDA guidelines. Among the included studies, IV administration was seen in only one study[29]. The prescribed dose was mostly indicated for urinary tract and upper respiratory tract infections. The occurrence of the reactions was independent of the treatment duration. In two of the studies, the drug transfer was observed to be through semen and placenta, respectively[24,26]. Even though sparse data existed regarding the transmission of the drug, the identified reports serve as an awareness to the prescribing physicians. The indications of treatment have been descriptively presented in Table 3.
| S. No. | Author | Age | Gender | Route of administration | Dose | Treatment duration | Diagnosis |
|---|---|---|---|---|---|---|---|
| 1 | Akman et al.[23] | 30 | Female | Oral | NR | NR | Acute tonsillitis |
| 2 | Butler et al.[24] | 38 | Female | Oral | 500 mg×2 od | 7 d | Acute bronchitis |
| 3 | Grgurevic et al.[25] | 73 | Female | Oral | 500 mg×2 od | 10 d | Urinary tract infection |
| 4 | Hossain et al.[26] | Newborn | Male | Oral | 750 tid | 3 d | Urinary tract infection |
| 5 | Montero et al.[27] | 66 | Female | Oral | 500 mg×2 od | 15 d | Urinary tract infection |
| 6 | Pastuszczak et al.[28] | 37 | Female | Oral | NR | NR | Rhinosinusitis |
| 7 | Saeed et al.[29] | 84 | Female | Intravenous | 500 mg×2 od | 1 d | Acute diverticulitis |
| 8 | Sahoo et al.[30] | 47 | Male | Oral | 500 mg×2 od | 10 d | Upper respiratory tract infection |
| 9 | Wolf et al.[31] | 62 | Male | Oral | 250 mg×2 od | 2 d | Upper respiratory tract infection |
Note: Od: Once daily; Tid: Thrice in a day and NR: Not Reported
Table 3: Treatment Indication
Clinical presentation and severity:
The clinical presentation of drug eruptions varied between the cases. One of the included studies presented with TEN with granulocytopenia identified to be severe and life-threatening when compared to other cases. The report also included a post-coital allergic reaction caused due to the transfer of drugs through body fluids during sexual intercourse. In another report, we found a reaction of congenital fixed drug eruption, which was precipitated through the placental transmission. The presentation of the skin reactions was found to be symmetric in appearance either in the form of erythematous rash alone or along with inflamed erosions and oozing[27,28,31]. Mild to moderate skin reactions in the form of purpuric rashes were also observed among the included patients. Individual causality assessments were undertaken for all the included studies using the Naranjo’s causality assessment scale which classified the drug reactions as probable for seven studies and possible for the other two studies[26,30]. The WHO probability scale assessment showed that eight studies were classified as probable ADRs whereas one ADR was found to be unclassifiable. The severity of the reactions were assessed using Modified Hartwig and Siegel scale, which categorized one ADR as severe[25] and the rest as moderate. Preventability for the included case reports was done using Schumock and Thornton scale, which categorized the ADRs as not preventable. The included studies classified the ADRs as not predictable.
Treatment pattern:
Based on the included studies, we observed that IV or intramuscular corticosteroids used in combination with topical corticosteroids were the preferred choices of treatment. Oral or IV antihistamines were also used as add-on therapy depending on the severity of the skin rashes and the presence of itching. There were no cases of mortality in the reported cases. In most of the cases, the reactions subsided on the withdrawal of the intervening drug.
The current systematic review identified nine case reports which focused on cefuroxime induced drug eruptions in various subsets of patients. In the included case reports, the drug was administered as per the labeled indications. In all the cases, the reaction subsided on the withdrawal of the drug. The reactions were managed with symptomatic treatments. The peak effect of cefuroxime was found to be within 2-3 h after the oral dose and 2-3 min after an intravenous dose. The average duration of action 4-8 h makes it an easier drug to administer[32]. In order to classify a type of drug allergy presented by the individual patients, the Gell and Coombs classification of human hypersensitivity can be applied. The classification comprises type I (Immunoglobulin E (IgE)-mediated), type II (cytotoxic), type III (immune complex) and type IV (cellular mediated). It is said that cellular immune mechanisms are responsible for mediating delayed hypersensitivity type IV reactions. Adverse reactions of cefuroxime are associated with the involvement of central nervous system, transient hematological changes, and renal toxicity[33]. Cutaneous reactions in the form of erythema multiforme, urticaria, angioedema, pustular eruptions, exanthema, pruritus, glossitis, fixed drug eruptions, oral ulceration and vaginitis are not detected much often[34]. The beta-lactam ring present in cephalosporins is known to cause a certain degree of instability in the cephalosporin molecule. This ring opens up to conjugate with proteins and later becomes a hapten and immunogenic, which further induces a delayed type of hypersensitivity[35]. Other observed features of delayedtype hypersensitive reactions include keratinocyte necrosis, papillary dermal edema, moderate spongiosis, focal and minimal exocytosis of cells overlying the inflamed dermal papillae in the epidermis[36]. As reported in our studies, the reactions were less severe except for a report where the patient had developed TEN[35]. A vigilant review should be thoroughly completed to determine if the patient has had any previous reactions of hypersensitivity to either cephalosporins and penicillins or any other antibiotics before instituting the therapy with cephalosporin antibiotics. If any antibiotic belonging to cephalosporins is to be given to a patient with penicillin-sensitivity, it must be implemented with caution because of the occurrence of cross-hypersensitivity among beta-lactam antibiotics. This type of reaction has been documented and perhaps might occur among 10 % of patients who have a history of allergy to penicillin. Based on the structure of the side chains, the cross-reactivity between a cephalosporin and other beta-lactam antibiotics can be partially explained. The test dosing for cephalosporins can be continued by administrating a minimal dose of the drug, which is expected to be less than the expected dose that would pose a severe reaction. Following this, the current strategy can be valuable in guiding therapeutic choices in the future. Among patients who have come across a previous allergic reaction to penicillin, skin testing is found to be helpful where an indication of cephalosporins is necessary. The patient may not be at an increased risk of reactions to cephalosporins if the skin test is found to be negative. Unlike the tests for penicillin-allergic skin reactions, the development of tests for skin allergy due to cephalosporins still remains obscure. Sparse predictive values were observed on skin reaction testing using the native drug alone[37]. In a few instances, standardization of skin testing for cephalosporin allergy has been failed as the diagnosis of severe allergic reaction were not identified well enough. Skin testing prior to administration of the medication and to understand the role of IgE antibody testing to cephalosporin or any other class of antibiotics remain uncertain[38]. The test dosing along with titrated doses could be measured as an alternative method to obtain the finest results. A detailed history through a review of medication records trailed by significant clinical examinations can help to guide therapeutic decisions for improving patient care[39]. The diagnostic approaches that are currently available such as IgE, skin prick test and skin patch test show a significant percentage of non-diagnostic results. The application technique of the skin patch test is simple and consists of applying a small amount of dilution of the test substance, which is usually referred to as the allergen reactogenicity onto the patient’s whole skin surface for a 48 h time period under bandage occlusion. In order to obtain an optimal bioavailability, the following factors that must be considered include the applied penetration ability of the molecule's strong allergen, its dose and concentration, the transport vehicle that was used, the type of occlusion patch and exposure. Based on the morphological criteria, the results of the patch test can be determined but the readings must be interpreted comprehensively. This also includes the patient's clinical observations and exposure assessment and the patient’s medical history[40]. Appropriate measures must be taken for the implementation of effective testing and accurate diagnosis of the ADR to prevent differential diagnosis. In certain cases, the reactions were presented after a prolonged duration, which made it difficult for the treating physician to identify the causative drug. Desensitization techniques to cephalosporins among patients who are at higher risk could be considered, but this procedure is yet to be standardized and the experience of implementation of this procedure is found to be limited. It can be considered for those patients who have experienced a life-threatening reaction to penicillin or other cephalosporins.
This study had its own limitations. The search was restricted to the English language, which might have excluded significant cases that might have added to the current review. Since the study planned to identify case reports, sparse information was available. Further inclusion of trials and observational studies should be considered to report the relative significance. Overall, the study collectively portrayed the drug reactions in various populations with different ethnicities, which could serve as a useful tool for the treating physicians before prescribing the drug.
Conclusion
This review highlights the pattern of drug eruptions caused by multiple doses of IV or oral cefuroxime in various populations. In most of the cases, the causality assessments of the reactions were categorized as probable. The study suggests that there is a need for antibiotic sensitivity testing before the administration of antibiotics that are known to cause severe reactions, which can be fatal if not identified. Further research is necessary to understand the occurrence of reaction to prevent misdiagnosis. Patients on antibiotics should be counseled upon discharge regarding the occurrence of adverse reactions for better patient safety and quality care.
Conflict of interests:
The authors declared no conflicts of interest.
References
- Badhwar VR, Ganapathy S, Prabhudesai PP, Tulara NK, Varaiya AY, Vyas D. A relook of cefuroxime in community infections: An option still beneficial. J Assoc Physicians India 2016;64(7):95-101.
- Scott LJ, Ormrod D, Goa KL. Cefuroxime axetil: An updated review of its use in the management of bacterial infections. Drugs 2001;61(10):1455-500.
[Crossref] [Google Scholar] [Pub Med]
- Perry CM, Brogden RN. Cefuroxime axetil. A review of its antibacterial activity, pharmacokinetic properties and therapeutic efficacy. Drugs 1996;52(1):125-58.
[Crossref] [Google Scholar] [Pub Med]
- Richards DM, Heel RC, Brogden RN, Speight TM, Avery GS. Ceftriaxone. A review of its antibacterial activity, pharmacological properties and therapeutic use. Drugs 1984;27(6):469-527.
[Crossref] [Google Scholar] [Pub Med]
- Adam D, Scholz H, Helmerking M. Comparison of short-course (5 day) cefuroxime axetil with a standard 10 day oral penicillin V regimen in the treatment of tonsillopharyngitis. J Antimicrob Chemother 2000;45(1):23-30.
[Crossref] [Google Scholar] [Pub Med]
- del Villar-Guerra P, Moreno Vicente-Arche B, Castrillo Bustamante S, Santana Rodriguez C. Anaphylactic reaction due to cefuroxime axetil: A rare cause of anaphylaxis. Int J Immunopathol Pharmacol 2016;29(4):731-3.
[Crossref] [Google Scholar] [Pub Med]
- Brockow K, Pfutzner W. Cutaneous drug hypersensitivity: Developments and controversies. Curr Opin Allergy Clin Immunol 2019;19(4):308-18.
[Crossref] [Google Scholar] [Pub Med]
- Roujeau JC, Stern RS. Severe adverse cutaneous reactions to drugs. N Engl J Med 1994;331(19):1272-85.
[Crossref] [Google Scholar] [Pub Med]
- Howard RL, Avery AJ, Slavenburg S, Royal S, Pipe G, Lucassen P, et al. Which drugs cause preventable admissions to hospital? A systematic review. Br J Clin Pharmacol 2007;63(2):136-47.
[Crossref] [Google Scholar] [Pub Med]
- Wilke RA, Lin DW, Roden DM, Watkins PB, Flockhart D, Zineh I, et al. Identifying genetic risk factors for serious adverse drug reactions: Current progress and challenges. Nat Rev Drug Discov 2007;6(11):904-16.
[Crossref] [Google Scholar] [Pub Med]
- Law EH, Leung M. Corticosteroids in Stevens-Johnson Syndrome/toxic epidermal necrolysis: Current evidence and implications for future research. Ann Pharmacother 2015;49(3):335-42.
[Crossref] [Google Scholar] [Pub Med]
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009;339:b2700.
- Murad MH, Sultan S, Haffar S, Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med 2018;23(2):60-3.
[Crossref] [Google Scholar] [Pub Med]
- Haffar S, Bazerbachi F, Prokop L, Watt KD, Murad MH, Chari ST. Frequency and prognosis of acute pancreatitis associated with fulminant or non-fulminant acute hepatitis A: A systematic review. Pancreatology 2017;17(2):166-75.
[Crossref] [Google Scholar] [Pub Med]
- Bazerbachi F, Sawas T, Vargas EJ, Prokop LJ, Chari ST, Gleeson FC, et al. Metal stents vs. plastic stents for the management of pancreatic walled-off necrosis: A systematic review and meta-analysis. GastrointestEndosc 2018;87(1):30-42.
[Crossref] [Google Scholar] [Pub Med]
- Bazerbachi F, Haffar S, Szarka LA, Wang Z, Prokop LJ, Murad MH, et al. Secretory diarrhea and hypokalemia associated with colonic pseudo-obstruction: A case study and systematic analysis of the literature. NeurogastroenterolMotil 2017;29(11):e13120.
[Crossref] [Google Scholar] [Pub Med]
- Bazerbachi F, Haffar S, Hussain MT, Vargas EJ, Watt KD, Murad MH, et al. Systematic review of acute pancreatitis associated with interferon-a or pegylated interferon-a: Possible or definitive causation? Pancreatology 2018;18(7):691-9.
[Crossref] [Google Scholar] [Pub Med]
- Bazerbachi F, Leise MD, Watt KD, Murad MH, Prokop LJ, Haffar S. Systematic review of mixed cryoglobulinemia associated with hepatitis E virus infection: Association or causation? GastroenterolRep2017;5(3):178-84.
[Crossref] [Google Scholar] [Pub Med]
- Schumock GT, Thornton JP, Witte KW. Comparison of pharmacy-based concurrent surveillance and medical record retrospective reporting of adverse drug reactions. Am J HospPharm1991;48(9):1974-6.
[Crossref] [Google Scholar] [Pub Med]
- Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981;30(2):239-45.
[Crossref] [Google Scholar] [Pub Med]
- Hartwig SC, Siegel J, Schneider PJ. Preventability and severity assessment in reporting adverse drug reactions. Am J Hosp Pharm 1992;49(9):2229-32.
[Crossref] [Google Scholar] [Pub Med]
- The use of the WHO-UMC system for standardized case causality assessment. World Health Organization; 2013.
- Akman C, Duran A, Kalafat UM, Ocak T. Uveitis attack and drug reaction due to cefuroxime axetil. Cutan Ocul Toxicol 2016;35(3):254-6.
[Crossref] [Google Scholar] [Pub Med]
- Butler RH, Gupta R. Postcoital allergic reaction in a woman. J Emerg Med 2005;28(2):153-4.
[Crossref] [Google Scholar] [Pub Med]
- Grgurevic I, Pejsa V, Morovic-Vergles J, Dobric I, Gasparovic V, Tudoric N. Fatal toxic epidermal necrolysis and severe granulocytopenia following therapy with cefuroxime. Acta Dermatovenerol Croat 2008;16(3):133-7.
- Hossain D. Cefuroxime-induced congenital fixed drug eruption: A case report. J Pak Assoc Dermatol 2012;22(4):379-83.
- Montero I, Gutierrez-Gonzalez E, Alvarez-Perez A, Sanchez-Aguilar D, Ginarte M, Toribio J. Cefuroxime-induced cutaneous pustular leukocytoclastic vasculitis with Koebner phenomenon on the donor area of a skin graft. Int J Dermatol 2015;54(11):1338-9.
[Crossref] [Google Scholar] [Pub Med]
- Pastuszczak M, Lipko-Godlewska S, Jaworek AK, Wojas-Pelc A. Drug-induced linear IgA bullous dermatosis after discontinuation of cefuroxime axetil treatment. J Dermatol Case Rep 2012;6(4):117-9.
[Crossref] [Google Scholar] [Pub Med]
- Saeed SA, Bazza M, Zaman M, Ryatt KS. Cefuroxime induced lymphomatoid hypersensitivity reaction. Postgrad Med J 2000;76(899):577-9.
[Crossref] [Google Scholar] [Pub Med]
- Sahoo HB, Rath B, Behera JP, Moharana CS. Cefuroxime axetil associated oral eruption: A case report. Apollo Med 2016;13(2):133-4.
- Wolf R, Orion E, Matz H. The baboon syndrome or intertriginous drug eruption: A report of eleven cases and a second look at its pathomechanism. Dermatol Online J 2003;9(3):2.
[Crossref] [Google Scholar] [Pub Med]
- Vogel F, Droszcz W, Vondra V, Reisenberg K, Marr C, Staley H. Sequential therapy with cefuroxime followed by cefuroxime axetil in acute exacerbations of chronic bronchitis. J Antimicrob Chemother 1997;40(6):863-71.
[Crossref] [Google Scholar] [Pub Med]
- Herishanu YO, Zlotnik M, Mostoslavsky M, Podgaietski M, Frisher S, Wirguin I. Cefuroxime-induced encephalopathy. Neurology 1998;50(6):1873-5.
[Crossref] [Google Scholar] [Pub Med]
- Manley HJ, Bailie GR, Eisele G. Bilateral renal cortical necrosis associated with cefuroxime axetil. Clin Nephrol 1998;49(4):268-70.
- Oakley AM, Krishnamurthy K. Stevens Johnson syndrome. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2020.
- Deshpande AD, Baheti KG, Chatterjee NR. Degradation of ß-lactam antibiotics. CurrSci 2004;87(12):1684-95.
- Magro CM, Crowson AN. Drug-induced immune dysregulation as a cause of atypical cutaneous lymphoid infiltrates: A hypothesis. HumPathol 1996;27(2):125-32.
[Crossref] [Google Scholar] [Pub Med]
- Joint Task Force on Practice Parameters; American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology; Joint Council of Allergy, Asthma and Immunology. Drug allergy: An updated practice parameter. Ann Allergy Asthma Immunol 2010;105(4):259-73.
[Crossref] [Google Scholar] [Pub Med]
- Kelkar PS, Li JT. Cephalosporin Allergy. N Engl J Med2001;345(11):804-9.
[Crossref] [Google Scholar] [Pub Med]
- Constantin MM. Value and impact of patch testing in patients with allergic contact dermatitis. Revista Romana de Medicina de Laborator 2012;20(3/4):287-92.