- *Corresponding Author:
- Yang Xu
National Clinical Research Center for Chinese Medicine Acupuncture and Moxibustion, Xiqing, Tianjin 300387, People's Republic of China
E-mail: : tjzyxuyang@126.com
| This article was originally published in a special issue, “Recent Progression in Pharmacological and Health Sciences” |
| Indian J Pharm Sci 2024:86(2) Spl Issue “134-141” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Previous observational studies regarding atrial fibrillation and venous thromboembolism have yielded inconsistent conclusions, and the causal relationship between the two remains unclear. This study aimed to assess the causal effects of atrial fibrillation with venous thromboembolism (consisting of pulmonary embolism and deep vein thrombosis), by the bidirectional two-sample Mendelian randomization analysis. The bidirectional two-sample Mendelian randomization analysis was performed based on publicly available summary-level data obtained in large-scale genome-wide association studies conducted among the European populations. The primary method for estimating causal relationships was inverse variance weighting, supplemented by weighted median, weighted mode, and Mendelian randomization-Egger regression for assessing the result robustness. To guarantee the robustness of our findings, results obtained through the weighted median method were adopted, which indicated the absence of potential causal impact of atrial fibrillation on venous thromboembolism or pulmonary embolism. Besides, atrial fibrillation showed no significant causality with deep vein thrombosis. Similarly, venous thromboembolism did not exhibit any significant causality with atrial fibrillation, pulmonary embolism and atrial fibrillation, or deep vein thrombosis and atrial fibrillation. The present Mendelian randomization analysis suggests that atrial fibrillation and venous thromboembolism have no significant causal association in both directions.
Keywords
Venous thromboembolism, deep vein thrombosis, pulmonary embolism, atrial fibrillation, Mendelian randomization
Venous Thromboembolism (VTE), including Pulmonary Embolism (PE) and Deep Vein Thrombosis (DVT), ranks the 3rd place among factors leading to vascular diseases following stroke and acute myocardial infarction. According to existing data, VTE shows an incidence of up to 8 % in the United States (US) and European populations, significantly higher than that in other regions[1]. Besides, VTE has posed considerable clinical and economic burdens. The annual medical costs of this condition in the US are estimated to be as high as $10 billion[2].
Recent research demonstrates that numerous risk factors are linked to VTE, among which, Atrial Fibrillation (AF) is often undervalued. As shown by recent observational studies, AF is probably related to VTE. For example, according to Hornestam et al.[3], the VTE (comprising PE and DVT) risk may elevate in patients with new-onset AF. Additionally, a study carried out by Enga et al.[4] came to the same conclusion.
Although the above studies have suggested an association between AF and VTE, it is difficult to establish causality between the two due to the limitations of reverse causality and small sample size. Furthermore, multiple confounding factors have significant influence on causal inference. Mendelian Randomization (MR) analysis is the analytical method that uses genetic variation of identified phenotypes or functions for correlating causality between them and the disease outcome. Because individual alleles show random assignment and fixation at conception, MR approach allows to partially avoid reverse causality and environmental confounders compared with traditional epidemiology[5].
Large-scale Genome-Wide Association Studies (GWAS) on circulatory diseases have made it possible to investigate the relationships between related diseases through MR analysis. As discovered from a study by Lv et al.[6], there was a causality between Ulcerative Colitis (UC) and VTE. Furthermore, Hu et al.[7] and colleagues revealed no substantial causal relationship between type 1/type 2 diabetes and VTE risk, even after adjusting for possible confounding factors. However, to date, MR studies examining the role of AF in VTE risk are lacking. Therefore, the present work conducted a two-sample MR analysis based on recent GWAS data regarding AF and VTE (comprising DVT and PE), so as to investigate the relation between AF and VTE.
Materials and Methods
Study design:
The present work involved the bidirectional twosample MR analysis, wherein Single-Nucleotide Polymorphisms (SNPs) were used to be Instrumental Variables (IVs) for exploring bidirectional causal connections between AF and VTEs (including PE and DVT).
First, causal effects of AF-related SNPs on VTEs were assessed. Then, VTE, PE, and DVT-associated SNPs were utilized to be IVs separately during MR analysis for investigating the reverse causality between them.
Data sources:
Our summary statistics of AF were obtained based on a GWAS that involved 60 620 cases and 970 216 controls using Instrument Electronics Unit (IEU) open GWAS project (https://gwas.mrcieu.ac.uk/)[8].
While summary statistical of VTE (19 372 cases and 357 905 controls), DVT (9109 cases and 324 121 controls), and PE (9243 cases and 367 108 controls) were acquired based on R9 release of FinnGen GWAS results (https://r9.finngen.fi/) released in 2023[9]. The cases were diagnosed based on International Classification of Diseases (ICD) codes.
All data used in this study were previously collected and published. Therefore, no additional ethical approval was required. The details of the data source and definition are listed in Table 1.
| Phenotypes | Data source | Phenotypic code | Cases/controls | Population |
|---|---|---|---|---|
| Exposures | ||||
| AF | HUNT | ebi-a-GCST006414 | 6493/63 142 | European |
| deCODE | 13 471/358 161 | European | ||
| MGI | 1226/11 049 | European | ||
| DiscoverEHR | 6679/41 803 | European | ||
| UKB | 14 820/380 919 | European | ||
| AFGen consortium | 17 931/115 142 | European | ||
| Outcomes | ||||
| VTE | FinnGen | I9_VTE | 19 372/357 905 | European |
| PE | FinnGen | I9_PHLETHROMBDVTLOW | 9109/324 121 | European |
| DVT | FinnGen | I9_PULMEMB | 9243/367 108 | European |
Table 1: Data Sources
Instrumental variable selection:
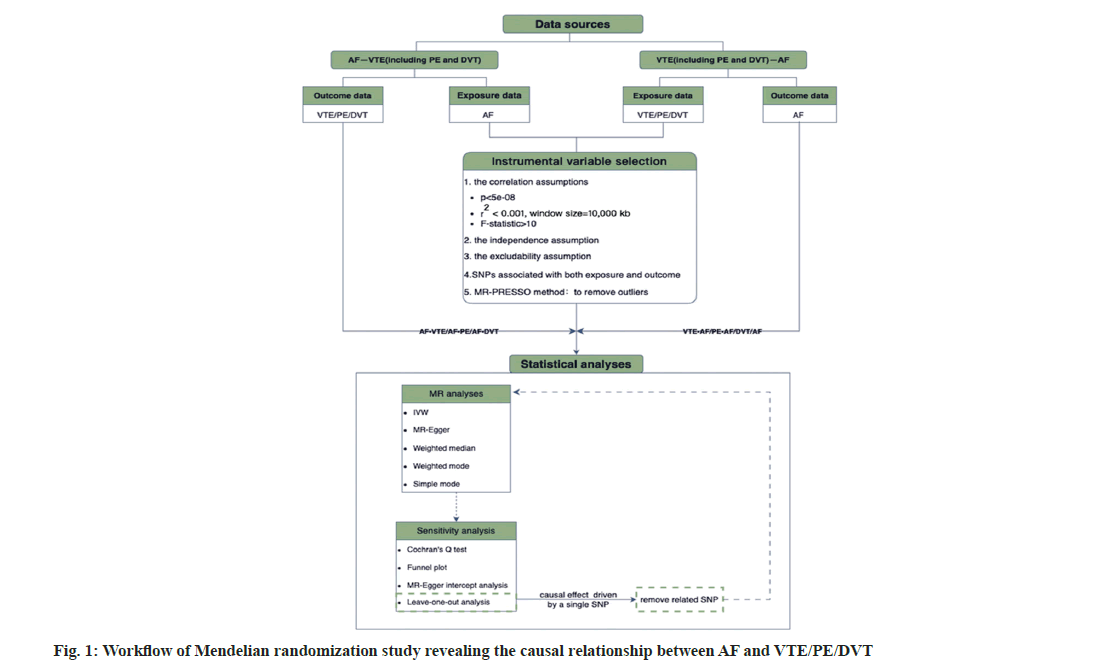
The fundamental objective of MR is to eliminate relevant confounding by using IVs (usually SNPs) to conclude the causality. Therefore, the selection of IVs is of paramount importance. In the classical MR stochastic analysis, IVS ought to satisfy three foundational assumptions (fig. 1); the assumption of association, IVs must be strongly related to exposure; the assumption of independence, IVs are unrelated to other confounders and the assumption of exclusivity, IVs just have an effect on outcome via exposure[10].
Based on correlation assumptions, exposure-related SNPs were chosen to be candidate IVs at a genomewide significance level (p<5×10-8).
For attaining independence between each IV utilized, SNPs with Linkage Disequilibrium (LD) were excluded. This was done by establishing the corresponding thresholds (r2<0.001, clumping window size=10 000 kb) according to the European 1000 genomes panel[11]. Meanwhile, F-statistic of each SNP was calculated separately to further validate the strength of its correlation with the exposure factors by the following equation[12,13]. IVs whose F-statistic was <10 were deemed to be weak IVs. Consequently, such IVs were excluded to avoid weak instrument bias. To satisfy the independence assumption, related phenotypes of each genetic variant were searched at PhenoScanner website (http://www.phenoscanner. medschl.cam.ac.uk/)[14,15]. SNPs with phenotypes linked to confounding factors were eliminated in accordance with relevant guidelines and clinical studies[1,16]. Moreover, exposure-related SNPs were extracted, while SNPs strongly related to outcome were excluded to satisfy the excludability assumption.
Additionally, for guaranteeing that effect alleles for both exposure and outcome were identical, the alleles for both exposure and outcome were coordinated to exclude palindrome and incompatible SNPs[14].
Finally, we employed MR Pleiotropy Residual Sum and Outlier (MR-PRESSO) approach for identifying and removing possible outliers, thereby reducing their potential impact on the causal estimates[17].
Statistical analysis: For evaluating causality of AF with three VTE phenotypes, different MR approaches were employed, such as inverse variance weighting, MR-Egger regression, weighted median and weighted mode.
Each of the above methods relies on distinct assumptions regarding IVs validity. To be specific, Inverse Variance Weighted (IVW) method can be primarily used for fundamental causal inference as it essentially meta-analyzes the Wald ratios of multiple SNPs[18]. It provides the most accurate estimates when all SNPs are valid instruments[19,20]. In addition, MR-Egger regression can provide correct estimates even when all IVs are invalid, provided that the assumptions of Instrument Strength Independent of the Direct Effect (In SIDE) are met. However, it tends to show lower precision in such cases[21,22]. When over 50 % of weight is derived from valid IVs, there is no need to satisfy the In SIDE assumption, and weighted median method offers the same estimates. Moreover, compared with MR-Egger regression, it exhibits a lower type I error rate and a higher causal estimation capability[19,23]. The weighted mode method can group SNP subsets with similar causal effects and estimate the causal effect of the subset with highest number of SNPs.
Hence, in this study, the IVW method was primarily used for estimating the causal relationship, while the other methods were used as supplementary tools or offered additional insights.
Sensitivity analysis:
The IVW method and MR-Egger regression were both employed to assess the potential heterogeneity, which was quantified using Cochran’s Q statistic (with p<0.05 being suggestive of heterogeneity). Besides, horizontal pleiotropy was detected using MR-Egger regression, and the intercept from this model was a common indicator for evaluation (with p<0.05 being indicative of directional pleiotropy). Furthermore, the symmetry of funnel plots can be used as a visual indicator for assessing horizontal pleiotropy. Lastly, a leave-one-out analysis was carried out for assessing if results were affected by single SNPs, and a forest plot was created to visually present the study findings.
Multiple comparisons were corrected with Bonferroni method. p<0.05/3 (adjusted for 1 exposure variable ×3 outcomes)=0.0167 indicated statistically significant differences, which provided strong evidence of a causal relationship; meanwhile, results of p values ranging from 0.0167 to 0.05 suggested the presence of correlation. We utilized the R software (version 4.3.1) “Two Sample MR” packages for MR analysis and sensitivity tests[24].
Results and Discussion
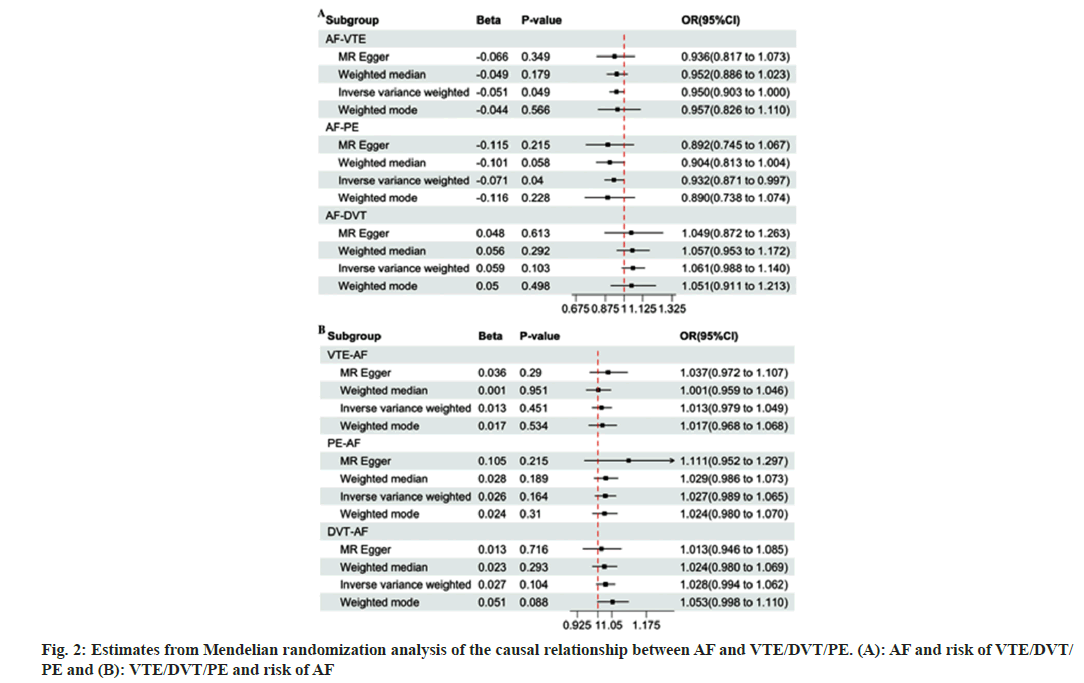
We conducted a bidirectional MR analysis for investigating relationship of AF with VTEs. Table 2 displays numbers of qualified SNPs related to the exposures (AF or VTEs). Following the method described above and satisfying the three main assumptions while harmonizing the direction of SNPs, we enrolled altogether 63 SNPs. As for AFVTE, through MR-PRESSO analysis, one outlier (rs6771054) was identified and removed, resulting in 62 SNPs for MR analysis. With regard to AFPE and AP-DVT, no outliers were found, so all the 63 SNPs were included for MR analysis. Besides, there were 3 causality pairs tested, including two showing statistical significance. According to fig. 2, IVW model indicated that genetic predisposition to AF was linked to an increased risk of VTE (Odds Ratio (OR)=0.950, 95 % Confidence Interval (CI): 0.902-1.000, p=0.049) and PE (OR=0.932, 95 % CI: 0.871-0.997, p=0.040). However, other statistical models, like MR-Egger regression, weighted mode, and weighted median, indicated that there was no causal effect of AF on VTE or PE. The IVW model is based on the assumption that all the SNPs are effective, which can hardly be achieved even with strict selection. Therefore, the study relied on the weighted median results (VTE, OR=0.952, 95 % CI: 0.886-1.023, p>0.05; PE, OR=0.904, 95 % CI: 0.813-1.004, p>0.05), which suggested that genetic predisposition to AF was not associated with the risk of VTE or PE.
| MR analysis | nIVs | Heterogeneity test | Pleiotropy test | |||||
|---|---|---|---|---|---|---|---|---|
| IVW | MR Egger | Egger_intercept | SE | P | ||||
| Q-pval | Q | Q-pval | Q | |||||
| AF-VTE | 62 | 0.113 | 74.611 | 0.098 | 74.548 | 0.001 | 0.005 | 0.823 |
| AF-PE | 63 | 0.343 | 65.918 | 0.32 | 65.625 | 0.003 | 0.006 | 0.604 |
| AF-DVT | 56 | 0.856 | 44.011 | 0.833 | 43.994 | 0.001 | 0.006 | 0.898 |
| VTE-AF | 16 | 0.314 | 17.082 | 0.296 | 16.284 | -0.004 | 0.005 | 0.422 |
| PE-AF | 11 | 0.178 | 13.895 | 0.19 | 12.426 | -0.019 | 0.019 | 0.329 |
| DVT-AF | 11 | 0.633 | 7.954 | 0.56 | 7.742 | 0.003 | 0.006 | 0.656 |
Table 2: Heterogeneity and pleiotropy tests for the associations between AF With VTE/DVT/PE
Upon Cochran Q test, the p-values for Q statistics during AF-VTE, AF-PE and AF-DVT analyses were higher than 0.05, indicating that there was no heterogeneity among the IVs. Additionally, MR egger regression-based pleiotropy test revealed no pleiotropy for causality of AF with VTEs (p>0.05). After leave-one-out sensitivity analysis, the causality of AF with DVT was mainly driven by a single SNP (from a total of 7 SNPs). After removing this SNP (leaving a total of 56 SNPs), MR analysis was conducted again.
The IVW analysis results indicated that AF did not significantly affect DVT (OR=1.061, 95 % CI: 0.988-1.140, p>0.05), besides, the results from other analysis methods were consistent with such finding (fig. 2).
Moreover, in the leave-one-out analyses for AF-PF and AF-VTE, no outlier SNP that drove the causal effect was identified.
To understand the impact of VTE on AF, the causality of AF with VTE was further evaluated. During IV selection, VTE-AF was identified and one outlier (rs72708961) was removed during MRPRESSO analysis, resulting in altogether 16 SNPs for MR analysis. Meanwhile, both PE-AF and DVTAF, without outliers, included 11 SNPs in the MR analysis. As shown in fig. 2, the genetic predisposition to VTEs did not significantly increase the risk of AF (VTE-AF, OR=1.013, 95 % CI: 0.979-1.049, p>0.05; PE-AF, OR=1.027, 95 % CI: 0.989-1.065, p>0.05; DVT-AF, OR=1.028, 95 % CI: 0.994-1.062, p>0.05). In the heterogeneity tests, there was no obvious evidence revealing heterogeneity. Moreover, MR Egger regression-based pleiotropy test did not reveal any obvious pleiotropy in VTEs with AF (intercept p>0.05) (Table 2). Furthermore, leave-one-out analysis verified that no specific IV drove the causal associations.
In recent years, the possible association between AF and VTE has received increasing attention, but there is still no definitive conclusion for the time being. Consequently, the bidirectional two-sample MR analysis was conducted in this work for assessing causality of AF with VTE. No clear evidence supporting the causality of genetic predisposition to AF with VTE was found.
Numerous previous cohort studies have investigated the link between AF and VTE, and many of these studies have reported a connection between the two. For instance, in a study led by Enga et al.[4] that included 29 975 participants, the results revealed an increased VTE risk associated with AF. Notably, this association was more prominent within 6 mo of AF diagnosis, and the risk significantly increased for PE compared with DVT. However, after 6 mo of AF diagnosis, the correlation with DVT diminished, though a certain level of association with PE persisted. In another case-control study that compared 463 244 AF patients with 887 336 individuals from the general population in Sweden, it was found that AF patients had an evidently increased VTE (including PE and DVT) risk relative to the general population, especially within the first 3 mo after diagnosis. Such result suggested the potential of AF, in particular the recent-onset AF, as the risk factor of VTE[3]. When it comes to the impact of VTE on AF, it is discovered that VTE patients are associated with an increased AF risk, especially within the first 6 mo following the thromboembolic event. Specifically, PE patients may show an evidently increased AF risk compared with DVT patients[25]. Furthermore, as indicated by a longitudinal study involving over 15 000 elderly individuals, there was a bidirectional association between AF and VTE[26]. However, some studies have offered a different perspective after considering confounding factors like fractures, surgeries, and tumors. It is proposed that the incidence of DVT and PE in AF is low, and there is no clear relation of AF with VTE[27]. However, the relationship between the two remains unclear since high-quality randomized controlled trial evidence that establishes a causal relationship between AF and VTE is lacking, and the aforementioned observational studies are poorly consistent.
In this study, while we did not directly observe a causality of AF with VTE, it was possible that they might exert an indirect influence on the disease processes of each other, which was not elucidated within the scope of this research. AF is associated with underlying pathophysiological changes, including blood stasis in the left atrium, gradual enlargement of the left atrium, and abnormal alterations in the vascular wall, such as endothelial shedding. Besides, there are abnormal changes in blood components, including platelet activation, inflammation, and the presence of growth factors. These changes essentially fulfill the Virchow’s triad for thrombus formation, and may indirectly increase the risk of VTE[28]. Furthermore, AF can promote venous stasis, and slow venous blood flow may activate coagulation factors and platelets, thereby increasing the risk of VTE[29]. VTE can induce an increase in pulmonary artery pressure, subsequently elevating stress on the right ventricular wall and pressure in the right atrium[25,30]. Right ventricular pressure overload can lead to sustained right ventricular dysfunction and tension, ultimately resulting in atrial stretching and subsequent AF[25]. Although we believe that there is no direct causal relationship between AF and VTE at the genetic level, we cannot rule out the possibility of their roles in indirectly increasing the risk of each other.
Our study has several strengths. Firstly, different from traditional observational studies, the IVs used in the MR design were SNPs related to random allocation at conception, which allowed for simulating the randomized controlled trial within the observational setting and thereby mitigating the impact of reverse causality and confounders to some extent. Secondly, all participants in GWAS data for both exposure and outcome were of European ancestry, which helped mitigate the bias arising from population stratification. Moreover, AF and VTE show relatively high incidence rates among the general population, therefore, uncovering the casualty of AF with VTE may contribute to establishing a foundation for the prevention and intervention of both conditions. Our findings suggest that it may be futile to intensify VTE screening in the genetically predisposed AF patients, and that using oral anticoagulants for VTE prevention may be more likely to induce negative effects.
Nevertheless, there were also several limitations in the study. All the GWAS data were derived from the European population, which might limit our result applicability to additional ethnicities. Additionally, as for summarized data we utilized, stratification by disease severity and specific demographic details, such as age, was lacking, further constraining our ability to conduct more detailed subgroup analyses.
This study is the first to assess the causal relationship between AF and VTE (including PE and DVT) using two-sample bidirectional MR analyses, which provides new genetic evidence for a relationship between the two. Our two-sample two-way MR analysis showed no significant evidence for a significant causal relationship between AF and VTE (including PE and VTE), i.e., our results do not support the hypothesis of a causal relationship between genetic susceptibility to AF and VTE (including PE and VTE). Although the above results suggest that there is no direct causal relationship between AF and VTE at the genetic level, many clinical cohort studies have reported the possibility of a correlation between the two, and therefore we cannot exclude the possibility that they indirectly increase each other’s risk. Future longitudinal clinical studies and experimental analyses are needed to confirm our findings.
Acknowledgments:
We appreciate the FinnGen and UK Biobank study participants and investigators, as well as the IEU Open GWAS project for sharing the summary data publicly.
Funding:
The study was supported by grants from National Natural Science Foundation of China (81973854), National Administration of Traditional Chinese Medicine (979022), Tianjin Municipal Science and Technology Bureau, Natural Science Foundation of Tianjin Municipality (18JCYBJC93600) and First Teaching Hospital of Tianjin University of Traditional Chinese Medicine (ZD202106).
Author’s contributions:
Shiyu Zhang and Yueqi Zhang have contributed equally to this work.
Conflict of interests:
The authors declared no conflict of interests.
References
- Lutsey PL, Zakai NA. Epidemiology and prevention of venous thromboembolism. Nat Rev Cardiol 2023;20(4):248-62.
- Secemsky EA, Rosenfield K, Kennedy KF, Jaff M, Yeh RW. High burden of 30-day readmissions after acute venous thromboembolism in the United States. J Am Heart Assoc 2018;7(13):e009047.
[Crossref] [Google Scholar] [PubMed]
- Hornestam B, Adiels M, Wai GK, Hansson PO, Bjorck L, Rosengren A. Atrial fibrillation and risk of venous thromboembolism: A Swedish nationwide registry study. Europace 2021;23(12):1913-21.
[Crossref] [Google Scholar] [PubMed]
- Enga KF, Rye-Holmboe I, Hald EM, Lochen ML, Mathiesen EB, Njolstad I, et al. Atrial fibrillation and future risk of venous thromboembolism: The Tromso study. J Thromb Haemost 2015;13(1):10-6.
[Crossref] [Google Scholar] [PubMed]
- Larsson SC, Bäck M, Rees JM, Mason AM, Burgess S. Body mass index and body composition in relation to 14 cardiovascular conditions in UK Biobank: A Mendelian randomization study. Eur Heart J 2020;41(2):221-6.
[Crossref] [Google Scholar] [PubMed]
- Lv X, Gao X, Liu J, Deng Y, Nie Q, Fan X, et al. Immune-mediated inflammatory diseases and risk of venous thromboembolism: A Mendelian randomization study. Front Immunol 2022;13:1042751.
[Crossref] [Google Scholar] [PubMed]
- Hu S, Tan JS, Hu MJ, Guo TT, Chen L, Hua L, et al. The causality between diabetes and venous thromboembolism: A bidirectional two-sample Mendelian randomization study. Thromb Haemost 2023;123(9):913-9.
[Crossref] [Google Scholar] [PubMed]
- Nielsen JB, Thorolfsdottir RB, Fritsche LG, Zhou W, Skov MW, Graham SE, et al. Biobank-driven genomic discovery yields new insight into atrial fibrillation biology. Nat Genet 2018;50(9):1234-9.
[Crossref] [Google Scholar] [PubMed]
- Kurki MI, Karjalainen J, Palta P, Sipila TP, Kristiansson K, Donner KM, et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature 2023;613(7944):508-18.
[Crossref] [Google Scholar] [PubMed]
- Davies NM, Holmes MV, Smith GD. Reading Mendelian randomization studies: A guide, glossary, and checklist for clinicians. BMJ 2018;362:k601.
- 1000 Genomes Project Consortium. A map of human genome variation from population scale sequencing. Nature 2010;467(7319):1061.
[Crossref] [Google Scholar] [PubMed]
- Li B, Martin EB. An approximation to the F distribution using the Chi-square distribution. Comput Stat Data Anal 2002;40(1):21-6.
- Bottigliengo D, Foco L, Seibler P, Klein C, Konig IR, Del Greco MF. A Mendelian randomization study investigating the causal role of inflammation on Parkinson’s disease. Brain 2022;145(10):3444-53.
[Crossref] [Google Scholar] [PubMed]
- Yin KJ, Huang JX, Wang P, Yang XK, Tao SS, Li HM, et al. No genetic causal association between periodontitis and arthritis: A bidirectional two-sample Mendelian randomization analysis. Front Immunol 2022;13:808832.
[Crossref] [Google Scholar] [PubMed]
- Kamat MA, Blackshaw JA, Young R, Surendran P, Burgess S, Danesh J, et al. PhenoScanner V2: An expanded tool for searching human genotype–phenotype associations. Bioinformatics 2019;35(22):4851-3.
[Crossref] [Google Scholar] [PubMed]
- Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist C, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2021;42(5):373-498.
[Crossref] [Google Scholar] [PubMed]
- Wu F, Huang Y, Hu J, Shao Z. Mendelian randomization study of inflammatory bowel disease and bone mineral density. BMC Med 2020;18(1):312.
[Crossref] [Google Scholar] [PubMed]
- Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol 2013;37(7):658-65.
[Crossref] [Google Scholar] [PubMed]
- Yuan S, Chen J, Ruan X, Sun Y, Zhang K, Wang X, et al. Smoking, alcohol consumption, and 24 gastrointestinal diseases: Mendelian randomization analysis. Elife 2023;12:e84051.
[Crossref] [Google Scholar] [PubMed]
- Zagkos L, Dib MJ, Pinto R, Gill D, Koskeridis F, Drenos F, et al. Associations of genetically predicted fatty acid levels across the phenome: A Mendelian randomisation study. PLoS Med 2022;19(12):e1004141.
[Crossref] [Google Scholar] [PubMed]
- Huang Y, Wang J, Yang H, Lin Z, Xu L. Causal associations between polyunsaturated fatty acids and kidney function: A bidirectional Mendelian randomization study. Am J Clin Nutr 2023;117(1):199-206.
[Crossref] [Google Scholar] [PubMed]
- Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int J Epidemiol 2015;44(2):512-25.
[Crossref] [Google Scholar] [PubMed]
- Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol 2016;40(4):304-14.
[Crossref] [Google Scholar] [PubMed]
- Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife 2018;7:e34408.
[Crossref] [Google Scholar] [PubMed]
- Hald EM, Enga KF, Lochen ML, Mathiesen EB, Njolstad I, Wilsgaard T, et al. Venous thromboembolism increases the risk of atrial fibrillation: The Tromso study. J Am Heart Assoc 2014;3(1):e000483.
[Crossref] [Google Scholar] [PubMed]
- Lutsey PL, Norby FL, Alonso A, Cushman M, Chen LY, Michos ED, et al. Atrial fibrillation and venous thromboembolism: Evidence of bidirectionality in the atherosclerosis risk in communities study. J Thromb Haemost 2018;16(4):670-9.
[Crossref] [Google Scholar] [PubMed]
- Basili S, Proietti M, Perticone F, Corazza GR, Violi F. Atrial fibrillation is not associated with increased risk of venous thromboembolism: Results from ARAPACIS study. Thromb Haemost 2015;114(9):655.
[Crossref] [Google Scholar] [PubMed]
- Watson T, Shantsila E, Lip GY. Mechanisms of thrombogenesis in atrial fibrillation: Virchow's triad revisited. Lancet 2009;373(9658):155-66.
[Crossref] [Google Scholar] [PubMed]
- Wang CC, Lin CL, Wang GJ, Chang CT, Sung FC, Kao CH. Atrial fibrillation associated with increased risk of venous thromboembolism. Thromb Haemost 2015;113(1):185-92.
[Crossref] [Google Scholar] [PubMed]
- Miao L, Shi J, Yu H, Song L, Zhu C, Shi D, et al. Studies on atrial fibrillation and venous thromboembolism in the past 20 years: A bibliometric analysis via citespace and VOSviewer. J Am Heart Assoc 2023;12(17):e029810.
[Crossref] [Google Scholar] [PubMed]