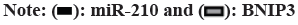- *Corresponding Author:
- Yongchao Zhang
Department of Cardiology, First Affiliated Hospital of Xi'an Jiaotong University, Shaanxi Province 710061, China
E-mail: wangkatg345482@163.com
| Date of Received | 05 November 2022 |
| Date of Revision | 14 October 2023 |
| Date of Acceptance | 07 May 2024 |
| Indian J Pharm Sci 2024;86(3):1156-1160 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To explore the role of steroid saponins from the flowers of Cestrum noctumum Linn on lung cancer cell epithelial-mesenchymal transition and the molecular mechanisms. Lung cancer cells (A549) were divided into normal control group, steroid saponins from the flowers of Cestrum noctumum Linn group, microRNA-181a-5p group, microRNA-control group, 40 μg/ml steroid saponins from the flowers of Cestrum noctumum Linn+anti-microRNA-181a-5p group and 40 μg/ml steroid saponins from the flowers of Cestrum noctumum Linn+anti-microRNA-control group. Reverse transcription-quantitative polymerase chain reaction was utilized for detecting ribonucleic acid expression. 3-(4, 5-dimethylthiazol-2-yl)-2, 5 diphenyl tetrazolium bromide, clone formation test and transwell assays were performed to analyze A549 lung cancer cell process. Western blot was conducted for detecting protein expression. Steroid saponins from the flowers of Cestrum noctumum Linn treatment inhibited A549 lung cancer cell proliferation and motility, accompanied by decrease of matrix metalloproteinase 2, matrix metalloproteinase 9, N-cadherin, vimentin as well as beta-catenin expression and increases of E-cadherin and microRNA-181a-5p expression. At the same time, elevating microRNA-181a-5p reduced the number of cell clones, repressed cell ability to motility and decreased matrix metalloproteinase 2, matrix metalloproteinase 9, N-Cadherin and vimentin expressions, whereas elevating microRNA-181a-5p increased E-cadherin expression. Inhibiting microRNA-181a-5p expression can rescue steroid saponins from the flowers of Cestrum noctumum Linn induced effects on epithelial-mesenchymal transition and beta-catenin expression in A549 cells. Steroid saponins from the flowers of Cestrum noctumum Linn inhibited epithelial-mesenchymal transition of lung cancer cells through regulation of microRNA-181a-5p/Wnt/β-catenin pathway.
Keywords
Myocardial ischemia, microRNA-210, adenovirus EIB interacting protein 3, apoptosis, autophagy signal
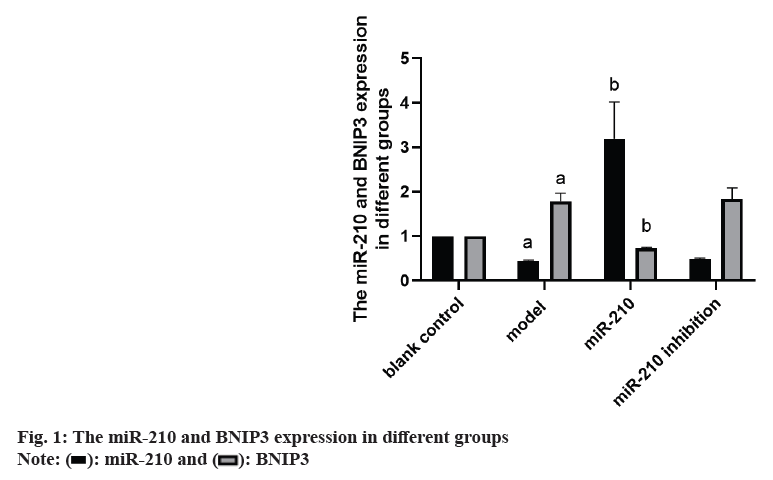
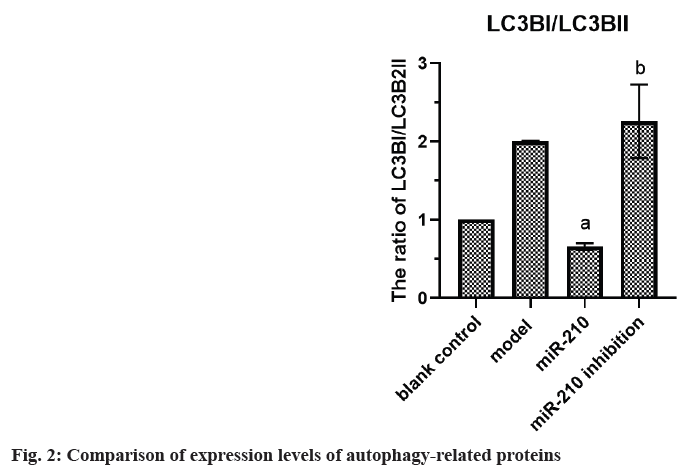
Cardiovascular Disease (CVD) is the main cause of human death worldwide, and ischemic injury is an important factor leading to CVD[1]. It has been reported that timely reperfusion is beneficial to promote the recovery of hypoxic tissue, but the longer the hypoxia time is, the more serious the injured tissue injury is, which is called ischemiareperfusion injury[2]. Ischemia-reperfusion injury is closely related to physiological and pathological processes such as apoptosis, autophagy and necrosis, in which autophagy has a certain regulatory function in CVD[3]. Therefore, exploring the possible regulatory factors of autophagy has been the focus in the field of cytology. microRNA-210 (miR-210), a member of the miRNAs family, has become a recognized hypoxia related miRNA and can play a stable biological effect in many cell lines[4]. MiR-210 can control many genes, but only some genes can confirm its regulatory function in CVD[5]. Adenovirus EIB Interacting Protein 3 (BNIP3) is a hypoxiaregulated protein with diverse functions in the process of apoptosis[6]. Clinical studies have shown that miR-210 can participate in a variety of cellular processes by regulating B-Cell Lymphoma 2 (Bcl- 2)/BNIP3, which can affect cell autophagy. Other studies have confirmed that BNIP3 can be highly expressed in CVD, and blocking the expression of this gene can alleviate the degree of myocardial damage, but its significance in the diagnosis and treatment of CVD is not clear[7,8]. Therefore, in this study, the rat cardiomyocyte H9c2 was used to establish the model of myocardial ischemia, and this study aimed to explore the mechanism of miR- 210's cytoprotective effect by inhibiting BNIP3 and then blocking apoptosis and autophagy signal pathway. Rat H9c2 cells were purchased from Wuxi Xinrun Biotechnology Co., Ltd., and all cells were incubated in high-glucose Dulbecco’s Modified Eagle Medium (DMEM) (containing 10 % Fetal Bovine Serum (FBS)) and incubated in Carbon dioxide (CO2) incubator (37°). After a series of operations such as resuscitation, passage and cryopreservation, they were cryopreserved. Rat H9c2 cells were randomly divided into blank control (group A), model (group B), miR-210 (group C) and miR-210 inhibition group (group D). Group B, C and D were cultured in hypoxia to establish myocardial ischemia model. After successful modeling, the group B did not do any treatment. Lentivirus transfection was used in group C and D to construct cells with high expression of miR-210 and low expression of miR- 210 inhibition. Western blotting was used to detect miR-210, BNIP3, autophagy related indexes (microtubule associated protein 1 Light Chain 3 (LC3B1), LC3B2), apoptosis related indexes (caspase-3), Bcl-2, Bcl-2-Associated protein X (BAX)) protein expression and LC3BI/LC3B2II ratio in H9c2 cells of rats in group A, B, C and D. The miR-210 and BNIP3 in each group were expressed by (x̄ ±s). T-test was used for comparison between the two groups, and multivariate analysis of variance was used for comparison between groups. All data in this study were analyzed by Statistical Package for the Social Sciences (SPSS) 19.0. p<0.05 was considered to be statistically significant and compared with the group A, ap<0.05 and compared with the group B, bp<0.05. The miR- 210 in the group B was reduced than the group A, while the BNIP3 protein in the group B was raised than the group A. The miR-210 in the group C was raised than the group B, while the BNIP3 protein was decreased than the group B (Table 1 and fig. 1). The LC3B1 and LC3B2 protein and the ratio of LC3BI/LC3B2II in the group B were raised than the group A, while these in group C were reduced than the group B, and these in group D were increased than the group B (Table 2 and fig. 2). The caspase-3 and BAX protein in the group B was raised than the group A, while the Bcl-2 protein was reduced than the group A. The protein caspase-3 and Bax in the group C was decreased than the group B, while the Bcl-2 protein was increased than the group B (Table 3 and fig. 3). MiR-210 has become the focus of current researchers, and the research on it is very extensive. Clinical studies have shown that Hypoxia- Inducible Factor 1-Alpha (HIF-1α) can downregulate the miR-210 in knockout mice. Through co-immunoprecipitation reaction, other scholars found that HIF-1α usually binds to the miR-210 promoter under hypoxia compared with cells with normal oxygen content[9]. Animal experiments have confirmed that some drugs, such as Huoxue Anxin recipe, can induce the up-regulation of miR-210 expression[10]. In addition, serum reactive factor can stimulate many miRNAs activation, which is an exogenous activator. MiR-210 can regulate the hypoxia response of cardiovascular system through apoptosis, autophagy, migration and other biological aspects, and play its protective role. The most common role of this gene is to block apoptosis and alleviate cell survival. A number of reports have shown that the increase of its expression level can significantly reduce the rate of apoptosis[11]. Other scholars have found that in hydrogen peroxide moral mouse cardiomyocyte model, miR-210 can block the expression of apoptosis-promoting gene caspase-8, and finally inhibit apoptosis[12]. Cells stimulated by hypoxia are mainly apoptotic, and the up-regulation of BNIP3 expression can promote apoptosis[13]. Some scholars found that hypertrophic HL-1 cardiomyocytes were used to establish hypoxia model, and found that BNIP3 accumulated obviously. In addition, it can be used as a key protein of hypoxic cell necrosis and apoptosis. Other studies found that mutants with Bcl-2 gene mutations could not bind to BNIP3, accompanied by the decrease of anti-apoptotic effect of Bcl-2. It is suggested that BNIP3 can bind to anti-apoptotic proteins to play a protective role[14]. Autophagy is a process involving the degradation of longevity proteins and organelles. Some scholars have established a malignant glioma model by ceramide stimulation. The results show that BNIP3 can play an autophagy control function in autophagy death[15]. Another study found that BINP3 can induce autophagy and protect hypertrophic HL-1 cardiomyocytes from ischemia-reperfusion injury[16]. Here, the ischemia model of cardiomyocytes was established by anoxic culture, and the high expression of miR-210 and low expression of miR-210 inhibition were constructed by lentivirus transfection, and the negative control group and group A were set up to explore whether miR-210 can protect cardiomyocytes by inhibiting BNIP3 from apoptosis and autophagy. First of all, we detected the expression of miR-210 and BNIP3 protein in the myocardial ischemia model. The miR-210 in the group B was reduced than the group A, while the BNIP3 protein in the group B was raised than the group A. The miR-210 in the group C was increased than the group B, while the BNIP3 protein was reduced than the group B. It is suggested that miR-210 and BNIP3 are involved in the whole process of cardiomyocyte ischemia, and the expression of miR-210 is low and BNIP3 is high in this disease. In addition, we further detected the expression level of autophagy-related proteins in each group. The LC3B1 and LC3B2 protein and the ratio of LC3BI/LC3B2II in the group B were raised than the group A. The LC3B1 and LC3B2 protein and the ratio of LC3BI/ LC3B2II in group C were reduced than the group B, while these in group D were increased than the group B. It is suggested that up-regulation of miR- 210 can block the LC3BI and LC3B2II, while down-regulation of miR-210 can prick them. Finally, we detected whether miR-210 can participate in the regulation of BNIP3 and then control apoptosis. The results showed that the caspase-3 and BAX protein in the group B was increased than the group A, while the Bcl-2 protein was decreased than the group A. The caspase-3 and Bax in the group C was reduced than the group B, while the Bcl-2 protein was raised than the group B. It is suggested that up-regulating of miR- 210 can inhibit the BNIP3 and further inhibit apoptosis, which has the function of cell protection. To sum up, the expression level of miR-210 decreased and the expression of BNIP3 increased in the model of cardiomyocyte ischemia. MiR-210 plays a protective role in the process of cardiomyocyte ischemia, which may be achieved by inhibiting BNIP3 and blocking apoptosis and autophagy signal pathway.
| Group | miR-210 | BNIP3 protein |
|---|---|---|
| A | 1.00 | 1.00 |
| B | 0.44±0.02a | 1.78±0.19a |
| C | 3.19±0.83b | 0.73±0.02b |
| D | 0.48±0.03 | 1.84±0.25 |
Note: Compared with the group B, ap<0.05 and compared with the group C, bp<0.05
Table 1: Comparison of miR-210 and BNIP3 in Expression in Different Groups (x̄±s)
| Group | LC3BI/LC3BII |
|---|---|
| A | 1.00 |
| B | 2.00±0.01 |
| C | 0.66±0.04a |
| D | 2.26±0.47ab |
Note: Compared with the group C, ap<0.05 and compared with the group D, bp<0.05
Table 2: Comparison of Expression Levels of Autophagy-Related Proteins (x̄±s)
| Group | Caspase-3 | Bcl-2 | BAX |
|---|---|---|---|
| A | 1.05±0.07 | 1.02±0.05 | 1.04±0.08 |
| B | 2.51±0.15 | 0.59±0.02 | 2.67±0.31 |
| C | 0.54±0.09a | 1.23±0.06ba | 0.49±0.04a |
| D | 2.86±0.23b | 0.51±0.01b | 2.88±0.38b |
Note: Compared with the group C, ap<0.05 and compared with the group D, bp<0.05
Table 3: Comparison of Expression Levels of Apoptosis-Related Proteins (x̄±s)
Conflict of interests:
The authors declared no conflict of interests.
References
- Ryom L, Lundgren JD, El-Sadr W, Reiss P, Kirk O, Law M, et al. Cardiovascular disease and use of contemporary protease inhibitors: The D: A: D international prospective multicohort study. Lancet HIV 2018;5(6):e291-300.
[Crossref] [Google Scholar] [PubMed]
- Chamberlain JJ, Johnson EL, Leal S, Rhinehart AS, Shubrook JH, Peterson L. Cardiovascular disease and risk management: Review of the American diabetes association standards of medical care in diabetes 2018. Ann Intern Med 2018;168(9):640-50.
[Crossref] [Google Scholar] [PubMed]
- Cheng D, Rui DU, Wu XY, Lin LI, Kui PE, Na L, et al. Serum uric acid is associated with the predicted risk of prevalent cardiovascular disease in a community-dwelling population without diabetes. Biomed Environ Sci 2018;31(2):106-14.
[Crossref] [Google Scholar] [PubMed]
- Guo W, Lian S, Zhen L, Zang S, Chen Y, Lang L, et al. The favored mechanism for coping with acute cold stress: Upregulation of miR-210 in rats. Cell Physiol Biochem 2018;46(5):2090-102.
[Crossref] [Google Scholar] [PubMed]
- Mazzone AL, Baker RA, McNicholas K, Woodman RJ, Michael MZ, Gleadle JM. Circulating and urinary miR-210 and miR-16 increase during cardiac surgery using cardiopulmonary bypass-A pilot study. J Extra Corporeal Technol 2018;50(1):19-29.
[Google Scholar] [PubMed]
- Romano E, Rufo N, Korf H, Mathieu C, Garg AD, Agostinis P. BNIP3 modulates the interface between B16-F10 melanoma cells and immune cells. Oncotarget 2018;9(25):17631.
[Crossref] [Google Scholar] [PubMed]
- Jiang Z, Yu F, Li M. Upregulation of BCL2 19 kD protein-Interacting Protein 3 (BNIP3) is predictive of unfavorable prognosis in uveal melanoma. Med Sci Monit 2018;24:4711-7.
[Crossref] [Google Scholar] [PubMed]
- Yu H, Chen B, Ren Q. Baicalin relieves hypoxia-aroused H9c2 cell apoptosis by activating Nrf2/HO-1-mediated HIF1α/BNIP3 pathway. Artif Cells Nanomed Biotechnol 2019;47(1):3657-63.
[Crossref] [Google Scholar] [PubMed]
- Huang X, Ding L, Bennewith KL, Tong RT, Welford SM, Ang KK, et al. Hypoxia-inducible miR-210 regulates normoxic gene expression involved in tumor initiation. Mol Cell 2009;35(6):856-67.
[Crossref] [Google Scholar] [PubMed]
- Puissegur MP, Mazure NM, Bertero T, Pradelli L, Grosso S, Robbe-Sermesant K, et al. miR-210 is overexpressed in late stages of lung cancer and mediates mitochondrial alterations associated with modulation of HIF-1 activity. Cell Death Differ 2011;18(3):465-78.
[Crossref] [Google Scholar] [PubMed]
- Yang W, Sun T, Cao J, Liu F, Tian Y, Zhu W. Downregulation of miR-210 expression inhibits proliferation, induces apoptosis and enhances radiosensitivity in hypoxic human hepatoma cells in vitro. Exp Cell Res 2012;318(8):944-54.
[Crossref] [Google Scholar] [PubMed]
- Lee DW, Futami M, Carroll M, Feng Y, Wang Z, Fernandez M, et al. Loss of SHIP-1 protein expression in high-risk myelodysplastic syndromes is associated with miR-210 and miR-155. Oncogene 2012;31(37):4085-94.
- Nakada C, Tsukamoto Y, Matsuura K, Nguyen TL, Hijiya N, Uchida T, et al. Overexpression of miR-210, a downstream target of HIF1α, causes centrosome amplification in renal carcinoma cells. J Pathol 2011;224(2):280-8.
[Crossref] [Google Scholar] [PubMed]
- Fu R, Deng Q, Zhang H, Hu X, Li Y, Liu Y, et al. A novel autophagy inhibitor berbamine blocks SNARE-mediated autophagosome-lysosome fusion through upregulation of BNIP3. Cell Death Dis 2018;9(2):243.
[Crossref] [Google Scholar] [PubMed]
- Rong J, Xu J, Liu Q, Wu Y, Guo H, Mu T, et al. Upregulation of long noncoding RNA RP4 exacerbates hypoxia injury in cardiomyocytes through regulating miR-939/BNIP3/Wnt/β-catenin pathway. Artif Cell Nanomed Biotechnol 2019;47(1):3013-20.
[Crossref] [Google Scholar] [PubMed]
- Guoyang Z, Ying W, Bo W, Donghua D. Effects of stress caused by iron excess on mitophagy and apoptosis of murine myoblast C2C12 cells. Chin J Exp 2019;36(1):24-6.