- *Corresponding Author:
- Kiranmai Mandava
Department of Pharmaceutical Chemistry, Bharat Institute of Technology, Ibrahimpatnam, Ranga Reddy-501 510, India
E-mail: gchaitra.kiran@gmail.com
| Date of Submission | 30 August 2016 |
| Date of Revision | 17 February 2017 |
| Date of Acceptance | 30 May 2017 |
| Indian J Pharm Sci 2017;79(4):501-512 |
Abstract
Nanotechnology in pharmacy and medicine is going to have a major impact on the survival of the human race. The unique optical, catalytic, electronic and physical properties (melting point) of metallic nanoparticles have made them potential candidates in the field of nanotechnology. The synthesis of metallic nanoparticles is being carried out by various methods. Method of synthesis is one of the important factors, which largely influences their biological effectiveness. Moreover, conventional physical and chemical processes involve the use of expensive chemicals and these methods are non-ecofriendly. The present review outlined different non-biological and biological methods of synthesizing metallic nanoparticles for therapeutic applications including a good emphasis on green expertise in this field. Updated tools of characterization and potential applications of metallic nanoparticles in the field of pharmacy are also reviewed.
Keywords
Metallic nanoparticles, green synthesis, characterization, silver nanoparticles, antimicrobial activity, pharmaceutical applications
“Nano” is the prefix word originating from the Latin word ‘nanus’ meaning literally ‘dwarf’ and according to an International System of units (SI) it is used to indicate a reduction factor of 109 times. So nanosized materials are typically measured in terms of nanometers (1 nm is 10-9 m). Nanoscience can be defined as a study of the phenomenon and size reduction of materials at atomic or molecular scales. Pharmaceutical nanotechnology embraces applications of nanoscience to pharmacy as nanomaterial’s and as devices like drug delivery, diagnostics, imaging and biosensors. The term nanomedicine refers to the application of nanotechnology to diagnosis, monitoring, control and treatment of diseases. Biologists are embracing nanotechnology as the engineering and manipulation of entities in 1-100 nm range and exploiting its potential to develop new therapeutics and diagnostics. Due to the advent of many modern and advanced analytical tools and capabilities to measure particle size in nanometres, particulate drug delivery systems research and development has been moving from macro to nano. It is an extremely large field ranging from in vivo and in vitro diagnostics to therapy, including targeted delivery and regenerative medicine [1]. Nanostructured materials brought about by the creation of functional materials with desired physicochemical properties.
Historically, India was probably the first country to maintain records of useful drugs. Charaka Samhita written by Acharya Charaka, the great Ayurvedic scholar of 1500 BC and later treatises describe the medicinal properties of various metals like gold, lead, mercury and silver. The metals used in Ayurvedic system of medicine includes copper, gold, iron, lead, mercury, silver and zinc [2-5]. The term metallic nanoparticles (MNPs) are used to describe nanosized metals with dimensions (length, width or thickness) with size ranges from 1-100 nm. The existence of MNPs in solution was first recognized by Faraday in 1857 and a qualitative explanation of their colour was given by Mie in 1908 [6,7]. The current research in the field of MNPs is largely studied due to their special properties. Especially, from the last two decades, considerable attention has been devoted to the synthesis of MNPs because of their unusual properties and potential applications in optical, electronic, catalytic, magnetic materials and in medicine [8-11].
This review outlined the various terminologies and preparative approaches of MNPs. It mainly draws attention to the advantages in synthesizing MNPs using a green approach, including its database and scope and pharmaceutical applications of MNPs.
Materials and Methods
Non-biological methods
Broadly speaking, there are two fundamental approaches to fabricate nanomaterial’s. The ‘top-down’ and ‘bottom-up’ approaches. The former method involves restructuring a bulk material in order to create a nanomaterial (larger size to nano level). The latter one represents the concept of constructing a nanomaterial from basic building blocks such as atoms or molecules. This approach illustrates the possibility of creating exact materials that are designed to have exactly the desired properties (atomic level) [12]. Top-down method is not very well suited to synthesize very-small sized particles. Bottom-up approaches are much better and suited for the generation of uniform particles often of distinct size, shape, and structure [13]. Various methods have been developed for the synthesis of metal nanoparticles includes chemical and physical methods. Chemical methods are based on the reduction of metal ions or decomposition of precursors to form atoms, followed by aggregation of atoms. MNPs produced by chemical methods usually have a narrow size distribution [14]. The mechanism involved in chemical synthesis of MNPs is reduction of metal ions with chemical reductants or decomposition of metal precursors with extra energy. In order to produce MNPs with a narrow size distribution, agents stabilizing colloidal dispersion of MNPs like sodium dodecyl sulphate (SDS), polyvinyl pyrrolidine (PVP), trisodium citrate and β-cyclodextrin are of vital importance. An explosion of reports and reviews appearing in the literature describing the chemical methods of synthesizing MNPs stand evidence to rising interest in this subject [15]. Some chemical agents can play a dual role as reducing and capping or stabilizing agents like plant constituents, β-cyclodextrin, dextrose and certain polymers. The physical methods are based on the subdivision of bulk metals including mechanical crushing or pulverization of bulk metal arc discharge between metal electrodes. MNPs produced by physical methods are usually larger in size and have wide size distribution (Table 1) [15-20].
| Chemical methods (bottom-up approach) | Physical methods (top-down approach) |
|---|---|
| Chemical reductants: Molecular hydrogen, alcohol, hydrazine, sodiumtetrahydroborate, lithium aluminum hydrate, citrate, polyols, N,N-dimethyl formamide, ethyleneglycol, β-cyclodextrin. Energy sources: Photoenergy like UV/Vis: light, γ-ray, electricity, thermal energy (heat), sonochemical energy. |
Vapor phase: Chemical vapor condensation, arc discharge, hydrogen plasma, laser pyrolysis. Liquid phase: Microemulsion, hydrothermal, solgel, sonochemical, microbial Solid phase: Ball mill |
Table 1: Various Non-Biological Preparative Methods for the Synthesis of MNPS[15-20]
Researchers in the field of nanotechnology are turning towards ‘nature’ to gain inspiration to develop novel innovative methods for nanoparticles synthesis. Currently used physical and chemical methods of synthesis use hazardous chemicals in their protocols [21].
Green synthesis of MNPs (biological/bioreduction)
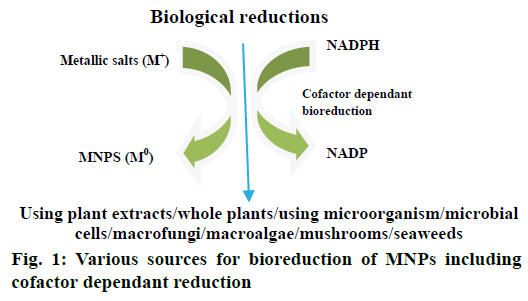
A lot of literature has been reported till date on the biological synthesis of MNPs using plants, microorganisms including bacteria and fungi because of their antioxidant/reducing properties typically responsible for reduction of metallic compounds to their respective MNPs (Figure 1). The ability of plant extracts to reduce MNPs has been known since early 19th century, although the nature of the reducing agents involved was not well understood [22]. In present days, green synthesis of MNPs has been an emerging research area in pharmacy. Advantages of this approach over physical and chemical methods are being eco-friendly, cost-effective, easy scale-up to synthesize large amounts of MNPs and devoid of using high temperature, pressure, energy and other toxic chemicals [23]. Plant extracts may act both as reducing and stabilizing agents in the synthesis of MNPs. The source of plant extract is known to influence the characteristics of nanoparticles (NPs) [24]. As different extracts contain different composition of phytoconstituents (organic reducing agents), the bioreduction process is relatively complex. Phytoconstituents like flavonoids, alkaloids, polyphenols, vitamins, catechins, and enzymes, functional group containing compounds, tannins, plant pigments and polysaccharides are responsible for the reduction of metal salts to MNPs [25]. Typically a plant extract mediated bioreduction of MNPs involves simple mixing of an aqueous solution of extract with an aqueous solution of metallic salt. The reaction takes place at room temperature and is generally completed within few minutes.
In the biological methods of synthesis of MNPs, the methods based on microorganisms (unicellular and multicellular) have been widely reported [26]. Microbial synthesis is considered as bottom-up approach where NP formation occurs due to reduction or oxidation of metal ions via biomolecules such as enzymes, sugars, proteins secreted by microorganisms [27]. However a complete mechanism is not well explored. This is due to the fact that the type of microorganism tends to behave and interacts differently with different metal ions. The interaction, biochemical processing activities of specific microorganism and the influence of environmental pH and temperature ultimately determines the formation of NP with a particular size and morphology [28,29]. Six main microbial routes employed for the synthesis of MNPs are actinomycetes [30-32], algae [33-35], bacteria [36-41], fungi [42-47], viruses [48-51] and yeast [52-56]. Microbial synthesis is of course readily scalable, environmentally benign and compatible with the use of product for medical applications but the production of microorganism is often more expensive than the production of plant extracts. Different plants used for the synthesis of MNPS of different size and shape are given in Table 2 [57-131].
| Plant | MNP | Size, nm | Shape | References |
|---|---|---|---|---|
| Vitex negundo | Ag;Au | 5; 10-30 | Spherical; face centered cubic | [57-58] |
| Melia dubia | Ag | 35 | Spherical | [59] |
| Portulaca oleracea | Ag | <60 | -- | [60] |
| Thevetia peruviana | Ag | 10-30 | Spherical | [61] |
| Pogostemon benghalensis | Ag | >80 | -- | [62] |
| Trachyspermum ammi | Ag | 87, 99.8 | -- | [63] |
| Swietenia mahogany | Ag | 50;100 at pH 12.5 |
spherical | [64] |
| Musa paradisiaca | Ag | 20 | -- | [65] |
| Moringa oleifera | Ag | 57 | -- | [66] |
| Garcinia mangostana | Ag | 35 | -- | [67] |
| Eclipta prostrate | Ag | 35-60 | Triangular, pentagon, hexagon | [68] |
| Nelumbo nucifera | Ag | 25-80 | Spherical, triangular | [69] |
| Acalypha indica | Ag | 25-80 | Spherical | [70] |
| Allium sativum | Ag | 4-22 | Spherical | [71] |
| Aloe vera | Ag, Au, In2O3 | 50-350; 5-50 | spherical, triangular | [72-73] |
| Citrus sinensis | Ag | 10-35 | Spherical | [74] |
| Eucalyptus hybrid | Ag | 50-150 | -- | [75] |
| Memecylon edule | Ag | 20-50 | Triangular, circular hexagon | [76] |
| Nelumbo nucifera | Ag | 25-80 | Spherical, triangular | [69] |
| Datura metel | Ag | 16-40 | Quasilinear suprastructures | [77] |
| Carica papaya | Ag | 25-50 | -- | [78] |
| Vitis vinifera | Ag | 30-40 | -- | [79] |
| Alternanthera dentate | Ag | 50-100 | Spherical | [80] |
| Acorus calamus | Ag | 31.83 | Spherical | [81] |
| Boerhaavia diffusa | Ag | 25 | Spherical | [82] |
| Tea extract | Ag | 20-90 | Spherical | [83] |
| Tribulus terrestris | Ag | 16-28 | Spherical | [84] |
| Cocous nucifera | Ag | 22 | Spherical | [85] |
| Abutilon indicum | Ag | 7-17 | Spherical | [86] |
| Pistacia atlantica | Ag | 10-50 | Spherical | [87] |
| Ziziphora tenuior | Ag | 8-40 | Spherical | [88] |
| Ficus carica | Ag | 13 | -- | [89] |
| Cymbopogan citrates | Ag | 32 | -- | [90] |
| Acalypha indica | Ag;Au | 0.5; 20-30 | Spherical | [70] |
| Premna herbacea | Ag | 10-30 | Spherical | [91] |
| Calotropis procera | Ag | 19-45 | Spherical | [92] |
| Centella asiatica | Ag | 30-50 | Spherical | [93] |
| Argyreia nervosa | Ag | 20-50 | -- | [94] |
| Psoralea corylifolia | Ag | 100-110 | -- | [95] |
| Brassica rapa | Ag | 16.4 | -- | [96] |
| Coccinia indica | Ag | 10-20 | -- | [97] |
| Alternanthera sessilis | Ag | 40 | Spherical | [98] |
| Andrographis paniculata | Ag | 67-88 | Spherical | [99] |
| Allium cepa | Ag | 33.6 | -- | [100] |
| Curcuma longa | Ag; Pd | 10-15 | Spherical | [101] |
| Withania somnifera leaves | Ag | 5-40 | Irregular, spherical | [102] |
| Ocimum sanctum L. | Ag | 4-30 | -- | [103] |
| Syzygium cumini L | Ag | 93 | -- | [104] |
| Euphorbiaceae latex | Ag; Cu | 18; 10.5 | -- | [105] |
| Trigonella-foenum graecum seed | Au | 15-25 | Spherical | [106] |
| Anacardium occidentale | Ag; Au | ~6 at 27°; 17 at 100° | -- | [107] |
| Apiin extracted from henna (Lawsonia inermis) leaves | Au | 7.5-65 | spherical, triangular, quasispherical | [108] |
| Azadirachta indica (neem) | Ag; Au | 50-100 | -- | [109] |
| Camelia sinensis (Green tea) | Ag; Au; ZnO | 30-40; 16 | hexagonal wurtzite structure for ZnO | [110-111] |
| Chenopodium album | Ag; Au | 10-30 | quasi-spherical shape | [112] |
| Cinnamomum camphora | Ag; Au; Pd | 55-80 | Cubic, hexagonal, crystalline | [113] |
| Cymbopogon sp. (lemon grass) | Au | 200-500 | spherical, triangular | [114] |
| Dioscorea bulbifera | Au | 11-30 | spheres | [115] |
| Emblica officinalis | Ag; Au | 10-20; 15-25 | -- | [116] |
| Geranium leaf | Au | 16-40 | -- | [117] |
| Memecylon edule | Au | 20-50 | triangular, circular, hexagonal | [118] |
| Mentha piperita (peppermint) | Ag; Au | 5-150 | spherical | [119] |
| Mucuna pruriens | Au | 6-17. 5 | spherical | [120] |
| Parthenium leaf | Au | 50 | face-centered cubic | [119] |
| Psidium guajava | Ag; Au | 25-30; 2-10 |
spherical | [121-122] |
| Tagetes erecta | Ag | 10-90 | - | [123] |
| Zingiber officinale Rosc. | 10 | -- | [124] | |
| Pyrus sp. (pear fruit extract) | Au | 200-500 | triangular, hexagonal | [125] |
| Rosa rugosa | Ag; Au | 30-60; 50-250 | -- | [126] |
| Swietenia mahogani (mahogany) | Au | 100 at pH 12.5 |
spherical | [64] |
| Tanacetum vulgare (tansy fruit) | Ag; Au | 16; 11 | -- | [127] |
| Terminalia catappa | Au | 10-35 | -- | [128] |
| Eucalyptus macrocarpa | Au | 20-100 | Spherical, triangular, hexagonal |
[129] |
| Diopyros kaki | Pt | 15-19 | -- | [130] |
| Jatropha curcas L. latex | Pb | 10-12 | -- | [131] |
Table 2: Green Synthesis of Metallic Nanoparticles from Plant Sources
Factors influencing biological synthesis of MNPs
During the course of biological synthesis of MNPs a number of controlling factors are involved in the nucleation and subsequent formation of stabilized NP. These factors include pH, reactants concentration, reaction time and temperature and details were given in Table 3 [132-136].
| Controlling factors | Influence on biological synthesis of MNPs | References |
|---|---|---|
| pH | Variability in size and shape | [132] |
| Reactants concentration | Variability in shape | [133] |
| Reaction time | Increase in reaction time increases the size of MNPs | [134] |
| Reaction temperature | Size, shape, yield and stability | [135-136] |
Table 3: Factors Influencing the Biological Synthesis of MNPs
Characterization of MNPs
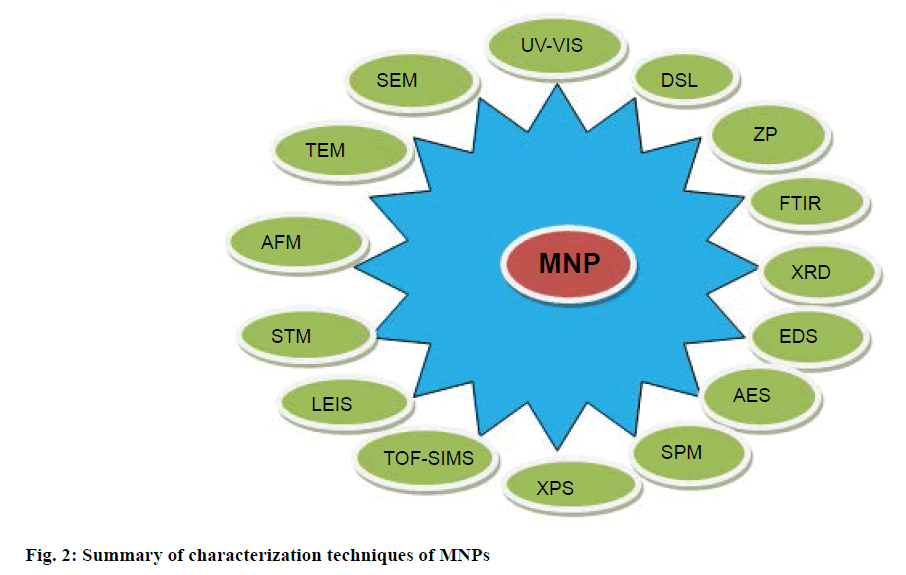
After green synthesis of NP, characterization is an important step to identify NP by their shape, size, surface area and dispersity [57]. For this purpose, various characterization techniques have been developed as analytical tools (Figure 2), like UV/Vis for identification, characterization and analysis; dynamic light scattering (DLS) for surface charge, size distribution and quality; scanning electron microscopy (SEM) and transmission electron microscopy (TEM) for surface and morphological characters; zeta potential (ZP) for indirect determination of surface charge; Fourier transform infrared spectroscopy (FTIR) for identification; X-ray diffraction (XRD) for crystal structure of NP; energy dispersive X-ray spectroscopy (EDS) for elemental composition; Auger electron microscopy (AEM), scanning probe electron microscopy (SPM), X-ray photo electron microscopy (XPS), time of flight-secondary ion mass spectroscopy (TOF-SIMS) for primary surface analysis; low energy ion scattering (LEIS) for identification of elements present in the outer most surface of the material under examination; scanning tunneling microscopy (STM), atomic force microscopy (AFM) for surface characterization at atomic scale; inductively coupled plasma-optical emission spectroscopy (ICP-OES) for optical properties; surface enhanced Raman scattering (SERS) for single molecular attachments to the surface of NP.
Pharmaceutical applications of MNPs
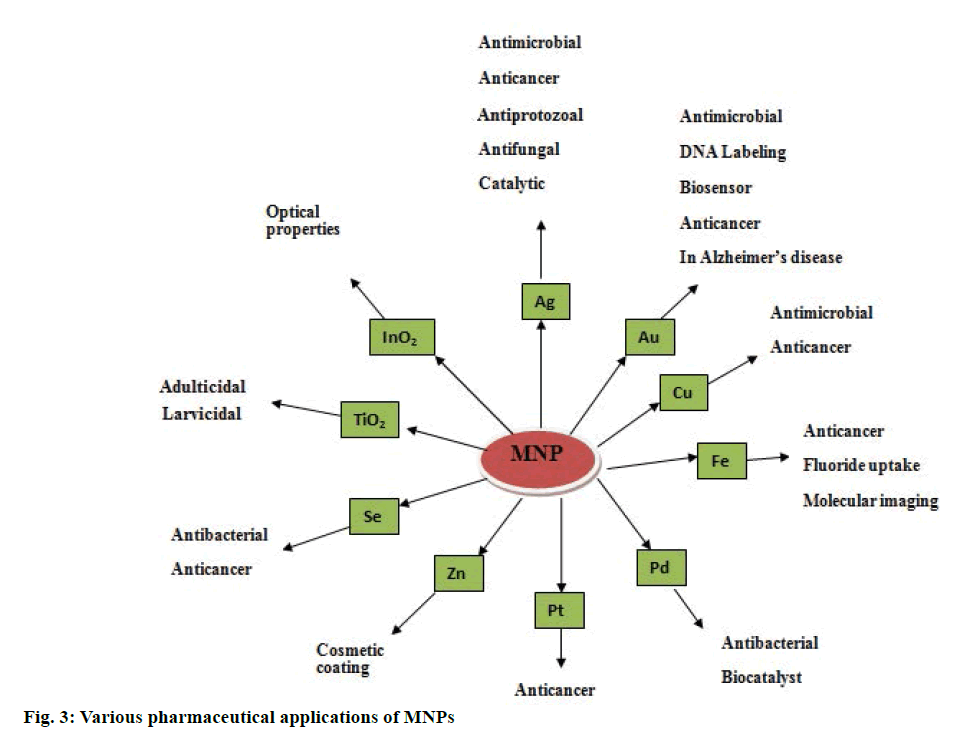
Various applications of MNPs are given in Figure 3. Antimicrobial activities of MNPs were well studied. Silver nanoparticles (AgNPs) and gold nanoparticles (AuNPs) have been reported to have a broad spectrum of antimicrobial activity against human and animal pathogens [137,138]. This is due to effective disruption of polymer subunits of cell membrane in a pathological organism, which in turn results in disturbance in bacterial system [139]. Membrane permeability of AgNPs can be increased by increasing the concentration and consequent rupture of cell wall can be achieved [140]. The interaction between silver and gold MNPs with cell membrane via binding to the active site is demonstrated [141]. Copper and copper oxide nanoparticles (CuNPs) were found to be effective antimicrobial agents [142]. CuNPs were found as strain specific antibacterial agents against Bacillus subtilis [143]. Antiinflammatory activity of AgNPs was explored previously and it was due to inhibition of interferon-γ, tumor necrosis factor alpha (TNF-α); reducing matrix metallo proteinase (MMP) and proinflammatory cytokines [144,145]. Biosynthesized MNPs was proved to be having wound healing and tissue regeneration activity in inflammatory mechanism [146]. AgNPs were demonstrated to have antifungal activity [147] and fungicidal properties were explored against Candida sp. and results were found to be significant to that of fluconazole and amphotericin. This activity is may be due to damage to fungal intracellular components [148]. As commercially available antifungal products are had various side effects, developing and exploring multifunctional MNPs to combat fungal infections is recommended. AgNPs were active against malarial vectors and reported to have a larvicidal effect [149,150]. Green synthesized MNPs of silver, platinum, palladium are effective in controlling malarial population. There is an urgent need to search for an alternative against vectors that are responsible for spreading the most common plasmodial diseases [151,152]. Recently, many investigators reported that green synthesized MNPs have potential to control tumor cell growth. This activity is due to presence of secondary metabolites in plant extracts [153,154]. AgNPs and AuNPs were found to be effective against malignant cells [155]. AntiHIV activity of AgNPs was studied at an early stage of reverse transcription mechanism [156]. Biosynthesized MNPs significantly reduce the level of hepatic enzymes like alanine transaminase, alkaline phophatase, serum creatinine and uric acid in diabetes-induced mice [157]. AgNPs were potent inhibitors of α-amylase at 140 mg/dl [158,159]. Secondary metabolites present in plant derived MNPs are responsible for antioxidant activity [160].
MNPs in drug delivery
In recent years, interest has been generated in the capability of MNPs to bind a wide range of organic molecules, their low toxicity and their strong and tunable absorption. Unique chemical, physical and photo-physical properties of MNPs paved innovative ways in drug delivery systems to achieve controlled transport, controlled release and specific targeting of drugs [161-164]. It has been shown that conjugates of MNPs with antibiotics provide promising results in antimicrobial therapy [163]. Combination of antibiotic with MNPs would be helpful to improve antibiotic efficacy. This conjugation can be via covalent, ionic or physical absorption [165,166]. Cisplatin conjugation to MNPs has shown a significant cytotoxic effect, which is seven times higher than that of cisplatin alone. Conjugation of methotrexate to AuNPs, which involves the interaction of carboxylic group of drug with the metal surface found to have high concentrations of drug in Lewis lung carcinoma cells [167]. Conjugation of tamoxifen and AuNPs has been reported [168]. MNPs surfaces have to be modified in order to avoid aggregation and to improve the efficiency of MNPs drug delivery systems [169]. Doxorubicin in combination with surface modified AuNPs reported to have significant cytotoxicity when compared to the free form of doxorubicin [170]. Polyethylene glycol (PEG) can be used to modify the surface of MNPs so that the cellular uptake of nanoparticles can be improved [171].
AntiHER antibody-targeted gold/silicon nanoparticles in the form of nanoshells to treat metastatic breast cancer [172], aminosilane coated iron oxide nanoparticles to treat brain tumors [173] and starch coated iron oxide nanoparticles in the form of magnetically guided mitoxantrone to treat tumor angiogenesis [174] have been reported. Calcium phosphate nanoparticles as vaccine adjuvant (Biovant) is developed by Biosante for subcutaneous administration has entered into phase I trials [175]. Author previously reported a simple, cost effective and eco-friendly method for the synthesis of water soluble AgNPs using root bark extract of Azadirachta indica (RBAI). Clinical ultrasound gel prepared from these NPs proved to be effective with significant antibacterial activity [176].
Drawbacks of MNPs
Few of the disadvantages of MNPs are the exact mechanism for synthesis of NPs needs to be elucidated, limitations to scale up production processes and reproducibility of the processes [177]. Chronic exposure to silver NPs causes adverse effects like argyria and argyrosis, soluble silver may cause organ damage [178].
Biological synthesis of MNPs has been always beneficial. Especially green synthesis of MNPs using plants and their extracts is more economical, energy efficient and eco-friendly approach, which is free of toxic contaminates as required in therapeutic applications. Microbes are regarded as potential biofactories for MNPs synthesis and serve a new generation antimicrobial agents with their unique physicochemical properties. The MNPs have found diverse applications in the field of pharmacy as direct therapeutic agents to treat ailments and also as carriers for drug delivery systems. In both the cases, stability and surface activity of MNPs are the vital areas where researchers have to concentrate. In particular, the development of rational protocol for green synthesis of MNPs in keeping view of the advantages of this approach; application of these particles to resolve problem of antibiotic resistance and in the target specific drug delivery systems to treat microbial infections including HIV and cancer should be highly focused in the future.
Acknowledgements
Author thanks Mr. Ch. Venugopal Reddy, Chairman, Bharat Institutions for his support and encouragement. This review did not receive any specific grant from funding agencies in the public, commercial, or not-forprofit sectors.
Conflict of interest
The authors report no declarations of interest.
Financial support and sponsorship
Nil.
References
- Thassu D, Deleers M, Pathak YV. Nanoparticulate Drug Delivery Systems. New York: Informa Health Care; 2009. p. 1-7.
- http://easyayurveda.com/2013/10/21/charaka-samhita-sutrasthana-chapter-1-quest-longevity/.
- Saper RB, Kales SN, Paquin J, Burns MJ, Eisenberg DM, Davis RB. Heavy metal content of ayurvedic herbal medicine products. JAMA 2004;292:2868-73.
- Saper RB, Phillips RS, Schgal A, Khouri N, Davis RB, Paquin J, et al. Lead, mercury and arsenic in US- and Indian-manufactured ayurvedic medicines sold via the internet. JAMA 2008;300:915-23.
- Kales SN, Saper RB. Ayurvedic lead poisoning: An under recognized international problem. Int J Med Sci 2009;63:379-81.
- Faraday M. Experimental relations of gold to light. London: Physiological Transactions of Royal Society; 1857. p. 1475.
- Mei G. Beiträge zur Optik trüber Medien, speziell kolloidaler Metallösungen. Ann Phys 1908;330:377-445.
- Beecroft LL, Ober CK. Nanocomposite material for optical applications. Chem Mater 1997;9:1302-17.
- Fendler JH. Atomic and molecular clusters in membrane mimetic chemistry. Chem Rev 1987;87:877-99.
- Gates BC. Supported metal clusters: synthesis, structure and catalysis. Chem Rev 1995;95:511-22.
- Kamat PV. Photochemistry on non-reactive and reactive (semiconductor) surfaces. Chem Rev 1993;93:267-300.
- Lane R, Craig B, Babcock W. Materials engineering with nature's building blocks. AMPTIAC News Lett 2002;6:31-7.
- Kenneth JK. Nanoscale materials in Chemistry. New York: Wiley; 2001.
- Liz-Marzán LM, Kamat PV. Nanoscaled materials. Boston: Kluwer Academic Publishers; 2003.
- Burda C, Chen X, Narayanan R, El Sayed MA. Chemistry and properties of nanocrystals of different shapes. Chem Rev 2005;105:1025-102.
- Gonsalves KE, Li H, Perez R, Santigo P, Joss-Yacaman M. Synthesis of nanostructured metals and metal alloys from organometallics. Coord Chem Rev 2000;206:607-30.
- Suslick KS, Price GJ. Applications of ultrasound to materials chemistry. Ann Rev Mater Sci 1999;29:295-326.
- Tjong SC, Chen H. Nano crystalline materials and coating. Mater Sci Eng 2004;45:1-88.
- Huber DL. Synthesis, properties and applications of iron nanoparticles. Small 2005;1:482-501.
- Daniel MC, Astruc D. Gold nanoparticles: assembly, supramolecular chemistry, quantum- size- related properties, and application towards biology, catalysis and nanotechnology. Chem Rev 2004;104:293-346.
- Guo R, Song Y, Wang G, Murray RW. Does core size matter in the kinetics of ligand exchanges of monolayer-protected Au clusters? J Am Chem Soc 2005;127:2752-7.
- Ankamwar B. Biosynthesis of gold nanoparticles (green-gold) using leaf extract of Terminalia catappa. Eur J Chem 2010;7:1334-9.
- Roy S, Das TK. Plant mediated green synthesis of silver nanoparticles-A review. Int J Plant Biol Res 2015;3:1044-55.
- Kumar V, Yadav SK. Plant‐mediated synthesis of silver and gold nanoparticles and their applications. J Chem Technol Biotechnol 2009;84:151-7.
- Mukunthan K, Balaji S. Cashew apple juice (Anacardium occidentale L.) speeds up the synthesis of silver nanoparticles. Int J Green Nanotechnol 2012;4:71-9.
- Amit Kumar M, Yusuf C, Uttam CB. Synthesis of metallic nanoparticles using plant extracts. Biotech Adv 2013;31:346-56.
- Prabhu S, Poulose EK. Silver nanoparticles: Mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int Nano Lett 2012; 2:1-10.
- Lengke M, Southam G. Bioaccumulation of gold by sulphate-reducing bacteria cultured in the presence of gold (I)-thiosulfate complex. Acta 2006;70:3646-61.
- Makarov VV, Love AJ, Sinitsyna OV, Makarova SS, Yaminsky IV, Taliansky ME, et al. “Green” nanotechnologies: synthesis of metal nanoparticles using plants. Acta Naturae 2014;6:35-44.
- Abdeen S, Geo S, Sukanya S, Praseetha PK, Dhanya RP. Biosynthesis of silver nanoparticles from Actinomycetes for therapeutic applications. Int J Nano Dimens 2014;5:155-62.
- Karthik L, Kumar G, Vishnu-Kirthi A, Rahuman AA, Rao VB. Streptomycessp. LK3 mediated synthesis of silver nanoparticles and its biomedical application. Bioprocess Biosyst Eng 2014;37:261-7.
- Golinska P, Wypij M, Ingle AP, Gupta I, Dahm H, Rai M. Biogenic synthesis of metal nanoparticles from actinomycetes: Biomedical applications and cytotoxicity. Appl Microbiol Biotechnol 2014;98:8083-97.
- Govindaraju K, Kiruthiga V, Kumar VG, Singaravelu G. Extracellular synthesis of silver nanoparticles by a marine alga, Sargassum wightii Grevilli and their antibacterial effects. J Nanosci Nanotechnol 2009;9:5497-501.
- Rajasulochana P, Dhamotharan R, Murugakoothan P, Murugesan S, Krishnamoorthy P. Biosynthesisand characterization of gold nanoparticles using the alga Kappaphycus alvarezii. Int J Nanosci 2010;9:511-6.
- Mata YN, Blázquez ML, Ballester A, González F, Muñoz JA. Gold biosorption and bioreduction with brown alga Fucus vesiculosus. J Hazard Mater 2009;166:612-8.
- Dhillon GG, Brar SK, Kaur S, Verma M. Green approach for nanoparticle biosynthesis by fungi: current trends and applications. Crit Rev Biotechnol 2012;32:49-73.
- Iravani S. Green synthesis of metal nanoparticles using plants. Green Chem 2011;13:2638-50.
- Sunkar S, Nachiyar CV. Biogenesis of antibacterial silver nanoparticles using the endophytic bacterium Bacillus cereus isolated from Garcinia xanthochymu. Asian Pac J Trop Biomed 2012;12:953-9.
- Tollamadugu NVKV, Prasad T, Kambala VSR, Naidu R. A critical review on biogenic silver nanoparticles and their antimicrobial activity. Curr Nanosci 2011;7:531-44.
- Liu YY, Fu JK, Chen P, Yu X, Yang P. Studies on biosorption of Au by Bacillus megaterium. Acta Microbiol Sin 2000;40:425-9.
- Sneha K, Sathishkumar M, Mao J, Kwak IS, Yun YS. Corynebacterium glutamicum-mediated crystallization of silver ions through sorption and reduction processes. Chem Eng J 2010;162:989-96.
- Vigneshwaran N, Ashtaputre NM, Varadarajan PV, Nachane RP, Paralikar KM, Balasubramanya RH. Biological synthesis of silver nanoparticles using the fungus Aspergillus flavus. Mater Lett 2007;61:1413-8.
- Shankar SS, Ahmad A, Pasricha R, Sastry M. Bioreduction of chloroaurate ions by geranium leaves and its endophytic fungus yields gold nanoparticles of different shapes. J Mater Chem 2003;13:1822-6.
- Mukherjee P, Senapati S, Mandal D, Ahmad A, Khan MI, Kumar R, et al. Extracellular synthesis of gold nanoparticles by the fungus Fusarium oxysporum. Chem Bio Chem 2002;3:461-3.
- Philip D. Biosynthesis of Au, Ag and Au-Ag nanoparticles using edible mushroom extract. Spectrochim Acta A Mol Biomol Spectrosc 2009;73:374-81.
- Kathiresan K, Manivannan S, Nabeel MA, Dhivya B. Studies on silver nanoparticles synthesized by a marine fungus, Penicillium fellutanum isolated from coastal mangrove sediment. Colloids Surf B Biointerfaces 2009;71:133-7.
- Gade AK, Bonde P, Ingle AP, Marcato PD, Duran N, Rai MK. Exploitation of Aspergillus niger for synthesis of silver nanoparticles. J Biobased Mater Bioenergy 2008;2:243-7.
- Royston E, Ghosh A, Kofinas P, Harris MT, Culver JN. Self-assembly of virus-structured high surface area nanomaterials and their application as battery electrodes. Langmuir 2008;24:906-12.
- Aljabali AAA, Barclay JE, Lomonossoff GP, Evans DJ. Virus templated metallic nanoparticles. Nanoscale 2010;2:2596-600.
- Gorzny ML, Walton AS, Evans SD. Synthesis of high-surface-area platinum nanotubes using a viral template. Adv Funct Mater 2010;20:1295-300.
- Kobayashi M, Tomita S, Sawada K, Shiba K, Yanagi H, Yamashita I, et al. Chiral meta-molecules consisting of gold nanoparticles and genetically engineered tobacco mosaic virus. Opt Express 2012;20:24856-63.
- Kowshik M, Arhtaputre S, Kharrazi S, Vogel W, Urban J, Kulkarni SK, et al. Extracellular synthesis of silver nanoparticles by a silver-tolerant yeast strain MKY3. Nanotechnology 2003;14:95-100.
- Kowshik M, Deshmukh N, Vogel W, Urban J, Kulkarni SK, Paknikar KM. Microbial synthesis of semiconductor CdS nanoparticles, their characterization, and their use in the fabrication of an ideal diode. Biotechnol Bioeng 2002;78:583-8.
- Kowshik M, Vogel W, Urban J, Kulkarni SK, Paknikar KM. Microbial synthesis of semiconductor PbS nanocrystallites. Adv Mater 2002;14:815-8.
- Reese RN, Winge DR. Sulfide stabilization of the cadmium-glutamyl peptide complex of Schizosaccharomyces pombe. J Biol Chem 1998;263:12832-5.
- Agnihotri M, Joshi S, Kumar AR, Zinjarde SS, Kulkarni SK. Biosynthesis of gold nanoparticles by the tropical marine yeast Yarrowia lipolytica. Mater Lett 2009;63:1231-4.
- Jiang J, Oberdorster G, Biswas P. Characterization of size, surface charge and agglomeration state of nanoparticle dispersions for toxicological studies. Nanopart Res 2009;11:77-89.
- Zargar M, Hamid AA, Bakar FA, Shamsudin MN, Shameli K, Jahanshiri F. Green synthesis and antibacterial effect of silver nanoparticles using Vitex negundo L. Molecules 2011;16:6667-76.
- Kathiravan V, Ravi S, Kumar SA. Synthesis of silver nanoparticles from Melia dubia leaf extract and their in vitro anticancer activity. Spectrochim Acta A Mol Biomol Spectrosc 2014;130:116- 21.
- Firdhouse MJ, Lalitha P. Green synthesis of silver nanoparticles using the aqueous extract of Portulaca oleracea (L). Asian J Pharm Clin Res 2012;6:92-4.
- Rupiasih NN, Aher A, Gosavi S, Vidyasagar PB. Green synthesis of silver nanoparticles using latex extract of Thevetia peruviana: a novel approach towards poisonous plant utilization. J Phys Conf Ser 2013;423:1-8.
- Gogoi SJ. Green synthesis of silver nanoparticles from leaves extract of ethnomedicinal plants Pogostemonbenghalensis (B) O. Ktz Adv Appl Sci Res 2013;4:274-8.
- Vijayaraghavan K, Nalini S, Prakash NU, Madhankumar D. One step green synthesis of silver nano/microparticles using extracts of Trachyspermum ammi and Papaver somniferum. Colloid Surf B Biointerfaces 2012;94:114-7.
- Mondal S, Roy N, Laskar RA, Sk I, Basu S, Mandal D. Biogenic synthesis of Ag, Au and bimetallic Au/Ag alloy nanoparticles using aqueous extract of mahogany (Swietenia mahogani JACQ) leaves. Colloids Surf B Biointerfaces 2011;82:497-504.
- Bankar A, Joshi B, Kumar AR, Zinjarde S. Banana peel extract mediated novel route for the synthesis of silver nanoparticles. Colloids Surf A 2010;368:58-63.
- Prasad TNVKV, Elumalai E. Biofabrication of Ag nanoparticles using Moringa oleifera leaf extract and their antimicrobial activity. Asian Pac J Trop Biomed 2011;1:439-42.
- Veerasamy R, Xin TZ, Gunasagaran S, Xiang TFW, Yang EFC, Jeyakumar N. Biosynthesis of silver nanoparticles using mangosteen leaf extract and evaluation of their antimicrobial activities. J Saudi Chem Soc 2010;15:113-20.
- Rajakumar G, Abdul Rahuman A. Larvicidal activity of synthesized silver nanoparticles using Eclipta prostrata leaf extract against filariasis and malaria vectors. Acta Trop 2011;118:196-203.
- Santhoshkumar T, Rahuman AA, Rajakumar G, Marimuthu S, Bagavan A, Jayaseelan C. Synthesis of silver nanoparticles using Nelumbo nucifera leaf extract and its larvicidal activity against malaria and filariasis vectors. Parasitol Res 2011;108:693-702.
- Krishnaraj C, Jagan E, Rajasekar S, Selvakumar P, Kalaichelvan P, Mohan N. Synthesis of silver nanoparticles using Acalypha indica leaf extracts and its antibacterial activity against water borne pathogens. Colloids Surf B Biointerfaces 2010;76:50-6.
- Ahamed M, Khan M, Siddiqui M, AlSalhi MS, Alrokayan SA. Green synthesis, characterization and evaluation of biocompatibility of silver nanoparticles. Phys E Low Dimens Syst Nanostruct 2011;43:1266-71.
- Chandran SP, Chaudhary M, Pasricha R, Ahmad A, Sastry M. Synthesis of gold nanotriangles and silver nanoparticles using Aloe vera plant extract. Biotechnol Prog 2006;22:577-83.
- Maensiri S, Laokul P, Klinkaewnarong J, Phokha S, Promarak V, Seraphin S. Indium oxide (In2O3) nanoparticles using Aloe vera plant extract: synthesis and optical properties. J Optoelectron Adv Mater 2008;10:161-5.
- Kaviya S, Santhanalakshmi J, Viswanathan B, Muthumary J, Srinivasan K. Biosynthesis of silver nanoparticles using Citrus sinensis peel extract and its antibacterial activity. Spectrochem Acta A Mol Biomol Spectrosc 2011;79:594-8.
- Dubey M, Bhadauria S, Kushwah B. Green synthesis of nanosilver particles from extract of Eucalyptus hybrida (Safeda) leaf. Dig J Nanomater Biostruct 2009;4:537-43.
- Elavazhagan T, Arunachalam KD. Memecylon edule leaf extract mediated green synthesis of silver and gold nanoparticles. Int J Nanomed 2011;6:1265-78.
- Kesharwani J, Yoon KY, Hwang J, Rai M. Phytofabrication of silver nanoparticles by leaf extract of Datura metel: hypothetical mechanism involved in synthesis. J Bionanosci 2009;3:39-44.
- Jain D, Daima HK, Kachhwaha S, Kothari S. Synthesis of plant-mediated silver nanoparticles using papaya fruit extract and evaluation of their antimicrobial activities. Dig J Nanomater Biostruct 2009;4:557-63.
- Gnanajobitha G, Paulkumar K, Vanaja M, Rajeshkumar S, Malarkodi C, Annadurai G, et al. Fruit-mediated synthesis of silver nanoparticles using Vitis vinifera and evaluation of their antimicrobial efficacy. J Nanostruct Chem 2013;3:1-6.
- Kumar DA, Palanichamy V, Roopan SM. Green synthesis of silver nanoparticles using Alternanthera dentata leaf extract at room temperature and their antimicrobial activity. Spectrochim Acta A Mol Biomol Spectrosc 2014;127:168-71.
- Nakkala JR, Mata R, Kumar Gupta A, Rani Sadras S. Biological activities of green silver nanoparticles synthesized with Acorous calamus rhizome extract. Eur J Med Chem 2014;85:784-94.
- Nakkala JR, Mata R, Gupta AK, Sadras SR. Green synthesis and characterization of silver nanoparticles using Boerhaavia diffusa plant extract and their antibacterial activity. Indus Crop Prod 2014;52:562-6.
- Suna Q, Cai X, Li J, Zheng M, Chenb Z, Yu CP. Green synthesis of silver nanoparticles using tea leaf extract and evaluation of their stability and antibacterial activity. Colloid Surf A Physicochem Eng Aspects 2014;444:226-31.
- Gopinatha V, Ali MD, Priyadarshini S, Priyadharsshini NM, Thajuddinb N, Velusamy P. Biosynthesis of silver nanoparticles from Tribulus terrestris and its antimicrobial activity: a novel biological approach. Colloid Surf B Biointerfaces 2012;96:69-74.
- Mariselvam R, Ranjitsingh AJA, Usha Raja Nanthini A, Kalirajan K, Padmalatha C, Mosae Selvakumar P. Green synthesis of silver nanoparticles from the extract of the inflorescence of Cocos nucifera (Family: Arecaceae) for enhanced antibacterial activity. Spectrochim Acta A Mol Biomol Spectrosc 2014;129:537-41.
- Ramesh B, Rajeshwari R. Anticancer activity of green synthesized silver nanopartcles of Abutilon indicum L. leaf extract. Asian J Phy Med Clin Res 2015;3:124-31.
- Sadeghi B, Rostami A, Momeni SS. Facile green synthesis of silver nanoparticles using seed aqueous extract of Pistacia atlantica and its antibacterial activity. Spectrochim Acta A Mol Biomol Spectrosc 2015;134:326-32.
- Sadeghi B, Gholamhoseinpoor F. A study on the stability and green synthesis of silver nanoparticles using Ziziphora tenuior (Zt) extract at room temperature. Spectrochim Acta A Mol Biomol Spectrosc 2015;134:310-5.
- Ulug B, HalukTurkdemir M, Cicek A, Mete A. Role of irradiation in the green synthesis of silver nanoparticles mediated by fig (Ficus carica) leaf extract. Spectrochim Acta A Mol Biomol Spectrosc 2015;135:153-61.
- Masurkar SA, Chaudhari PR, Shidore VB, Kamble SP. Rapid biosynthesis of silver nanoparticles using Cymbopogan citratus (lemongrass) and its antimicrobial activity. Nano-Micro Lett 2011;3:189-94.
- Kumar S, Daimary RM, Swargiary M, Brahma A, Kumar S, Singh M. Biosynthesis of silver nanoparticles using Premna herbacea leaf extract and evaluation of its antimicrobial activity against bacteria causing dysentery. Int J Pharm Biol Sci 2013;4:378-84.
- Gondwal M, Pant GJN. Biological evaluation and green synthesis of silver nanoparticles using aqueous extract of Calotropis procera. Int J Pharm Biol Sci 2013;4:635-43.
- Rout A, Jena PK, Parida UK, Bindhani BK. Green synthesis of silver nanoparticles using leaves extract of Centella asiatica L. For studies against human pathogens. Int J Pharm Biol Sci 2013;4:661-74.
- Thombre R, Parekh F, Patil N. Green synthesis of silver nanoparticles using seed extract of Argyreia nervosa. Int J Pharm Biol Sci 2014;5:114-9.
- Sunita D, Tambhale D, Parag V, Adhyapak A. Facile green synthesis of silver nanoparticles using Psoralea corylifolia. Seed extract and their in vitro antimicrobial activities. Int J Pharm Biol Sci 2014;5:457-67.
- Narayanan KB, Park HH. Antifungal activity of silver nanoparticles synthesized using turnip leaf extract (Brassica rapa L.) against wood rotting pathogens. Eur J Plant Pathol 2014;140:185-92.
- Kumar AS, Ravi S, Kathiravan V. Green synthesis of silver nanoparticles and their structural and optical properties. Int J Curr Res 2013;5:3238-40.
- Niraimathi KL, Sudha V, Lavanya R, Brindha P. Biosynthesis of silver nanoparticles using Alternanthera sessilis (Linn.) extract and their antimicrobial, antioxidant activities. Colloids Surf B Biointerfaces 2013;102:288-91.
- Sulochana S, Krishnamoorthy P, Sivaranjani K. Synthesis of silver nanoparticles using leaf extract of Andrographis paniculata. J Pharmacol Toxicol 2012;7:251-8.
- Saxena A, Tripathi RM, Singh RP. Biological synthesis of silver nanoparticles by using onion (Allium cepa) extract and their antibacterial activity. Dig J Nanomater Bios 2010;5:427-32.
- Sathishkumar M, Sneha K, Yun YS. Immobilization of silver nanoparticles synthesized using Curcuma longa tuber powder and extract on cotton cloth for bactericidal activity. Biores Technol 2010;101:7958-65.
- Nagati VB, Alwala J, Koyyati R, Donda MR, Banala R, Padigya PRM. Green Synthesis of plant-mediated silver nanoparticles using Withania somnifera leaf extract and evaluation of their antimicrobial activity. Asian Pac J Trop Biomed 2012;2:1-5.
- Ramteke C, Chakrabarti T, Sarangi BK, Pandey R. Synthesis of silver nanoparticles from the aqueous extract of leaves of Ocimum sanctum for enhanced antibacterial activity. J Chem 2013;2013:278925.
- Banerjee J, Narendhirakannan RT. Biosynthesis of silver nanoparticles from Syzygium cumini (L.) seed extract and evaluation of their in vitro antioxidant activities. Dig J Nanomater Bios 2011;6:961-8.
- Patil SV, Borase HP, Patil CD, Salunke BK. Biosynthesis of silver nanoparticles using latex from few Euphorbian plants and their antimicrobial potential. Appl Biochem Biotechnol 2012;167:776-90.
- Aswathy Aromal S, Philip D. Green synthesis of gold nanoparticles using Trigonella foenum-graecum and its size dependent catalytic activity. Spectrochim Acta A Mol Biomol Spectrosc 2012;97:1-5.
- Sheny D, Mathew J, Philip D. Phytosynthesis of Au, Ag and Au-Ag bimetallic nanoparticles using aqueous extract and dried leaf of Anacardium occidentale. Spectrochim Acta A Mol Biomol Spectrosc 2011;79:254-62.
- Kasthuri J, Veerapandian S, Rajendiran N. Biological synthesis of silver and gold nanoparticles using apiin as reducing agent. Colloids Surf B Biointerfaces 2009;68:55-60.
- Shankar SS, Rai A, Ahmad A, Sastry M. Rapid synthesis of Au, Ag, and bimetallic Au core-Ag shell nanoparticles using neem (Azadirachta indica) leaf broth. J Colloid Interface Sci 2004;275:496-502.
- Vilchis-Nestor AR, Sánchez-Mendieta V, Camacho-López MA, Gómez-Espinosa RM, Arenas-Alatorre JA. Solventless synthesis and optical properties of Au and Ag nanoparticles using Camellia sinensis extract. Mater Lett 2008;62:3103-05.
- Kumar V, Yadav SK. Plant-mediated synthesis of silver and gold nanoparticles and their applications. J Chem Technol Biotechnol 2009;84:151-7.
- Dwivedi AD, Gopal K. Plant-mediated biosynthesis of silver and gold nanoparticles. J Biomed Nanotechnol 2011;7:163-4.
- Huang JL, Li QB, Sun DH, Lu YH, Su YB, Yang X, et al. Biosynthesis of silver and gold nanoparticles by novel sundried Cinnamomum camphora leaf. Nanotechnology 2007;18:1-11.
- Shankar SS, Rai A, Ahmad A, Sastry M. Controlling the optical properties of lemongrass extract synthesized gold nanotriangles and potential application in infrared-absorbing optical coatings. Chem Mater 2005;17:566-72.
- Ghosh S, Patil S, Ahire M, Kitture R, Jabgunde A, Kale S, et al. Synthesis of gold nano-anisotrops using Dioscorea bulbifera tuber extract. J Nanomater 2011;2011:354793.
- Ankamwar B, Damle C, Ahmad A, Sastry M. Biosynthesis of gold and silver nanoparticles using Emblica officinalis fruit extract, their phase transfer and transmetallation in an organic solution. J Nanosci Nanotechnol 2005;5:1665-71.
- Shankar SS, Ahmad A, Sastry M. Geranium leaf assisted biosynthesis of silver nanoparticles. Biotechnol Prog 2003;19:1627-31.
- Elavazhagan T, Arunachalam KD. Memecylon edule leaf extract mediated green synthesis of silver and gold nanoparticles. Int J Nanomedicine 2011;6:1265-78.
- Parashar V, Parashar R, Sharma B, Pandey AC. Parthenium leaf extract mediated synthesis of silver nanoparticles: a novel approach towards weed utilization. Dig J Nanomater Biostruct 2009;4:45-50.
- Arulkumar S, Sabesan M. Biosynthesis and characterization of gold nanoparticle using antiparkinsonian drug Mucuna pruriens plant extract. Int Res Pharm Sci 2010;1:417-20.
- Raghunandan D, Basavaraja S, Mahesh B, Balaji S, Manjunath SY, Venkataraman A. Biosynthesis of stable poly shaped gold nanoparticles from microwave-exposed aqueous extracellular anti-malignant guava (Psidium guajava) leaf extract. NanoBiotechnology 2009;5:34-41.
- Lokina S, Stephen A, Kaviyarasan V, Arulvasu C, Narayanan V. Cytotoxicity and antimicrobial studies of silver nanoparticles synthesized using Psidium guajava L. extract. Synth React Inorg Met-Org Nano-Metal Chem 2015;45:426-32.
- Padalia H, Moteriya P, Chanda S. Green synthesis of silver nanoparticles from marigold flower and its synergistic antimicrobial potential. Arab J Chem 2014;8:732-41.
- Singh C, Sharma V, Naik KRP, Khandelwal V, Singh H. A green biogenic approach for synthesis of gold and silver nanoparticles using Zingiber officinale. Dig J Nanomater Bios 2011;6:535-42.
- Ghodake G, Deshpande N, Lee Y, Jin E. Pear fruit extract-assisted room-temperature biosynthesis of gold nanoplates. Colloids Surf B Biointerfaces 2010;75:584-9.
- Dubey SP, Lahtinen M, Sillanpaa M. Green synthesis and characterizations of silver and gold nanoparticles using leaf extract of Rosa rugosa. Colloids Surf A Physicochem Eng Aspects 2010;364:34-41.
- Dubey SP, Lahtinen M, Sillanpää M. Tansy fruit mediated greener synthesis of silver and gold nanoparticles. Process Biochem 2010;45:1065-71.
- Ankamwar B. Biosynthesis of gold nanoparticles (green-gold) using leaf extract of Terminalia catappa. Eur J Chem 2010;7:1334-9.
- Poinern GEJ, Le X, Chapman P, Fawcett D. Green biosynthesis of gold nanometre scale plate using the leaf extracts from an indigenous Australian plant Eucalyptus macrocarpa. Gold Bull 2013;46:165-73.
- Song JY, Kim BS. Biological synthesis of bimetallic Au/Ag nanoparticles using Persimmon (Diospyros kaki) leaf extract. Korean J Chem Eng 2009;25:808-11.
- Joglekar S, Kodam K, Dhaygude M, Hudlikar M. Novel route for rapid biosynthesis of lead nanoparticles using aqueous extract of Jatropha curcas L. latex. Mater Lett 2011;65:3170-2.
- Gardea-Torresdey JL, Tiemann KJ, Gamez G, Dokken K, Tehuacamanero S, Jose-Yacaman M. Gold nanoparticles obtained by bio-precipitation from gold (III) solutions. J Nanopart Res 1999;1:397-404.
- Huang J, Li Q, Sun D, Lu Y, Su Y, Yang X, et al. Biosynthesis of silver and gold nanoparticles by novel sundried Cinnamomum camphora leaf. Nanotech 2007;18:1-11.
- Ahmad N, Sharma S. Green synthesis of silver nanoparticles using extracts of Ananas comosus. Gr Sustainable Chem 2012;2:141-7.
- Sathishkumar M, Krishnamurthy S, Yun YS. Immobilization of silver nanoparticles synthesized using the Curcuma longa tuber powder extract on cotton cloth for bactericidal activity. Biores Technol 2010;101:7958-65.
- Song JY, Jang HK, Kim BS. Biological synthesis of gold nanoparticles using Magnolia kobus and Diopyros kaki leaf extracts. Process Biochem 2009;44:113-38.
- Jain D, Daima HK, Kachhwaha S, Kothari S. Synthesis of plant-mediated silver nanoparticles using papaya fruit extract and evaluation of their antimicrobial activities. Dig J Nanomater Biostruct 2009;4:557-63.
- Krishnaraj C, Jagan E, Rajasekar S, Selvakumar P, Kalaichelvan P, Mohan N. Synthesis of silver nanoparticles using Acalypha indica leaf extracts and its antibacterial activity against water borne pathogens. Colloids Surf B Biointerfaces 2010;76:50-6.
- Sondi I, Salopek-Sondi B. Silver nanoparticles as antimicrobial agent:a case study on E. coli as a model for Gram-negative bacteria. J Colloid Interface Sci 2004;275:177-82.
- Kasthuri J, Kathiravan K, Rajendiran N. Phyllanthin assisted biosynthesis of silver and gold nanoparticles: a novel biological approach. J Nanopart Res 2009;11:1075-85.
- Kim JS, Kuk E, Yu KN, Jong-Ho K, Park SJ, Lee HJ, et al. Antimicrobial effects of silver nanoparticles. Nanomedicine 2007;3:95-101.
- Ren G, Hu D, Cheng EW, Vargas-Reus MA, Reip P, Allaker RP. Characterization of copper oxide nanoparticles for antimicrobial application. Int J Antimicrob Agents 2009;33:587-90.
- Ruparelia JP, Chatterjee AK, Duttagupta SP, Mukherji S. Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomater 2008;4:707-16.
- Kirsner R, Orsted H, Wright B. Matrix metalloproteinase in normal and impaired wound healing:a potential role of monocrystalline silver. Wounds 2001;13:5-10.
- Tian J, Wong KK, Ho CM, Lok CM, Yu WY, Che CM, et al. Tropical delivery of silver nanoparticles promotes wound healing. Chem Med Chem 2007;2:129-36.
- Gurunathan S, Kyung-Jin L, Kalishwaralal K, Sheikpranbabu, Vaidyanathan R, Eom SH. Antiangiogenic properties of silver nanoparticles. Biomaterials 2009;30:6341-50.
- Vivek M, Kumar PS, Steffi S, Sudha S. Biogenic silver nanoparticles by Gelidiella acerosa extract and their antifungal effects. Avicenna J Med Biotechnol 2011;3:143-8.
- Logeswari P, Silambarasa, S, Abraham J. Synthesis of silver nanoparticles using plant extracts and analysis of their antimicrobial activity. J Saudi Chem Soc 2012;4:23-45.
- Rajakumar G, Abdul Rahuman A. Larvicidal activity of synthesized silver nanoparticles using Eclipta prostrata leaf extract against filariasis and malaria vectors. Acta Trop 2011;118:196-203.
- Santhoshkumar T, Rahuman AA, Rajakumar G, Marimuthu S, Bagavan A, Jayaseelan C. Synthesis of silver nanoparticles using Nelumbo nucifera leaf extract and its larvicidal activity against malaria and filariasis vectors. Parasitol Res 2011;108:693-702.
- Gnanadesigan M, Anand, M, Ravikumar S, Maruthupandy M, Vijayakumar V. Biosynthesis of silver nanoparticles by using mangrove plant extract and their potential mosquito larvicidal property. Asian Pac J Trop Med 2011;3:799-803.
- Jayaseelan C, Rahuman AA, Rajakumar G, Kirthi AV, Santhoshkumar T, Marimuthu S. Synthesis of pediculocidal and larvicidal silver nanoparticles by leaf extract from heart leaf moon seed plant Tinospora cordifolia Miers. Parasitol Res 2011;109:185-94.
- Raghunandan D, Ravishankar B, Sharanbasava G, Mahesh DB, Harsoor V, Yalagatti MS, et al. Anti-cancer studies of noble metal nanoparticles synthesized using different plant extracts. Cancer Nanotechnol 2011;2:57-65.
- Das S, Das J, Samadder A, Bhattacharyya SS, Das D, Khuda-Bukhsh AR. Biosynthesized silver nanoparticles by ethanolic extracts of Phytolacca decandra, Gelsemium sempervirens, Hydrastis canadensis and Thuja occidentalis induce differential cytotoxicity through G(2/M arrest in A375 cells. Colloids Surf B Biointerfaces 2013;101:325-36.
- Dipankar C, Murugan S. The green synthesis, characterization and evaluation of the biological activities of silver nanoparticles synthesized from Iresine herbstii leaf aqueous extracts. Colloids Surf B Biointerfaces 2012;98:112-9.
- Suriyakalaa U, Antony JJ, Suganya S, Siva D, Sukirtha R, Kamalakkannan S, et al. Hepatocurative activity of biosynthesized silver nanoparticles fabricated using Andrographis paniculata. Colloids Surf B Biointerfaces 2013;102:189-94.
- Swarnalatha C, Rachela S, Ranjan P, Baradwaj P. Evaluation of in vitro antidiabetic activity of Sphaeranthus amaranthoides silver nanoparticles. Int J Nanomat Biostr 2012;2:25-29.
- Pickup JC, Zhi ZL, Khan F, Saxl T, Birch DJ, Nanomedicine and its potential in diabetes research and practice. Diab Met Res 2008;24:604-10.
- Mary EJ, Inbathamizh L. Green synthesis and characterization of nano silver using leaf extract of Morinda pubescens. Asian J Pharm Clin Res 2012;5:159-62.
- Reichelt KV, Hoffmann-Lucke P, Hartmann B, Weber B, Ley JP, Krammer GE, et al. Phytochemical characterization of South African bush tea (Athrixia phylicoides DC). South Afr J Bot 2012;83:1-8.
- Anil Kumar S, Khan MI. Hetero functional nanomaterials: fabrication, properties and applications in nanobiotechnology. J Nanosci Nanotechnol 2010;10:4124.
- Skirtach AG, Javier AM, Kreft O, Köhler K, Alberola AP, Möhwald H, et al. Laser-induced release of encapsulated materials inside living cells. Angew Chem 2006;118:4728.
- Sershen SR, Westcott SL, Halas NJ, West JL. Temperature-sensitive polymer-nanoshell composites for photothermally modulated drug delivery. J Biomed Mater Res 2000;51:293.
- Gu H, Ho PL, Tong E, Wang L, Xu B. Presenting vancomycin on nanoparticles to enhance antimicrobial activities. Nano Lett 2003;3:1261.
- Estella-Hermoso de Mendoza A, Campanero MA, Mollinedo F, Blanco-Prieto MJ. Lipid nanomedicines for anticancer drug therapy. J Biomed Nanotechnol 2009;5:323.
- Saravanakumar G, Kim K, Park JH, Rhee K, Kwon IC. Current status of nanoparticle based imaging agents for early diagnosis of cancer and atherosclerosis. J Biomed Nanotechnol 2009;5:20.
- Chen YH, Tsai CY, Huang PY, Chang MY, Cheng PC, Chou CH, et al. Methotrexate conjugated to gold nanoparticles inhibits tumor growth in a syngeneic lung tumor model. Mol Pharm 2007;4:713.
- Tseng YC, Huang L. Self-assembled lipid nanomedicines for siRNA tumor targeting. J Biomed Nanotechnol 2009;5:351.
- Andersen AJ, Hashemi SH, Andresen TL, Hunter AC, Moghimi SM. Complement: Alive and kicking nanomedicines. J Biomed Nanotechnol 2009;5:364.
- Asadishad B, Vossoughi M, Alemzadeh I. Folate-receptor-targeted delivery of doxorubicin using polyethylene glycol-functionalized gold nanoparticles. Ind Eng Chem Res 2010;49:1958.
- Erdogan S. Liposomal nanocarriers for tumor imaging. J Biomed Nanotechnol 2009;5:141.
- Hirsch LR, Stafford RJ, Bankson JA, Sershen SR, Rivera B, Price RE, et al. Nanoshell-mediated near-IR thermal therapy of tumors under magnetic resonance guidance. Proc Natl Acad Sci USA 2003;100:13549-54.
- Jordan A, Scholz R, Maier-Hauff K, van Landeghem FK, Waldoefner N, Teichgraeber U, et al. The effect of thermotherapy using magnetic nanopartcles on rat malignant glioma. J Neurooncol 2006;78:7-14.
- Jurgons R , Seliger C, Hilpert A, Trahms L, Odenbach S, Alexiou C. Drug loaded magnetic nanopartcles for cancer therapy. J Phys Condens Matter 2006;18:S2893-S902.
- Zhang L, Gu FX, Chan JM, Wang AZ, Langer RS, Farokhzad OC. Nanoparticles in medicine: Therapeutic applications and developments. Clin Pharmacol Ther 2008;83:761-9.
- Aravind K, Tasneem M, Kiranmai M. Rapid green synthesis of silver nanopartcles from root bark extract of Azadirachta indica A. Juss and their application in clinical ultra sound gel. Ann Drug disc Biomed Res 2014;1:144-54.
- Seabra AB, Duran N. Nanotoxicity of metal oxide nanoparticles. Metals 2015;5:934-75.
- Reddy PN, Eladia MP, Havel J. Silver or silver nanoparticles: a hazardous threat to the environment and human health? J Appl Biomed 2008;6:117-29.