- *Corresponding Author:
- Reng Ren
Department of Neurointensive Care, Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, Zhengjiang Province 310009, China
E-mail: renreng1203@zju.edu.cn
| This article was originally published in a special issue, “Emerging Therapeutic Interventions of Biopharmaceutical Sciences” |
| Indian J Pharm Sci 2024:86(3) Spl Issue “33-38” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Using the Cancer Genome Atlas database, we downloaded sequencing ribonucleic acid data from low-grade glioma tissues and normal tissues and analyzed them further. First, we analyzed the exposure of I kappa B kinase interacting protein in pan-cancer. We further analyzed the differential representation of I kappa B kinase interacting protein in normal tissues and low-grade gliomas. Survival analysis showed the connection between I kappa B kinase interacting protein exposure and clinical survival. In addition, we performed I kappa B kinase interacting protein correlation analysis and selected the fifty genes with the highest relevance for heat mapping. Gene set enrichment analysis illustrated the pathways associated with I kappa B kinase interacting protein. Finally, we analyzed the immune microenvironment of the tumor and drew a lollipop map. Pan-cancer analysis showed that I kappa B kinase interacting protein was differentially expressed in 25 tumors, including adrenocortical carcinoma and bladder urothelial carcinoma. I kappa B kinase interacting protein was highly expressed in low-grade gliomas. Survival analysis showed that I kappa B kinase interacting protein high expression group performed poorly in terms of overall survival, disease-specific survival and progression-free interval. Gene set enrichment analysis revealed that I kappa B kinase interacting protein was expressed in Reactome signaling of Ras homologous GTPase and Reactome signaling of interleukins. Immune microenvironment analysis showed that immune cells such as Th2 cells, T helper cells and DC were distinctly distinguished between the high and low-I kappa B kinase interacting protein-expressing groups. I kappa B kinase interacting protein is highly expressed in low-grade gliomas and affects the prognosis and immunity of low-grade gliomas.
Keywords
Gliomas, immune microenvironment, low-grade gliomas, immune cells, adrenocortical carcinoma
Gliomas are those tumors that arise from the neuroepithelium, which are collectively referred to as gliomas. They constitute 40 %-50 % of intracranial tumors and represent the majority of the most common type of primary intracranial tumors. The annual incidence rate is 3 to 8 per 100 000 population. The signs and symptoms caused by gliomas depend on their occupational effects and the function of the brain regions affected. Gliomas can cause headaches, nausea and vomiting, seizures, and blurred vision due to their occupying effect in space. In addition, they can also cause other symptoms due to their impact on local brain tissue function. For example, glioma of the optic nerve can cause loss of vision; glioma of the spinal cord can cause pain, numbness, and weakness of the limbs; glioma of the central region can cause motor and sensory impairment; glioma of the speech area can cause difficulty in speech and understanding. The rate of symptoms varies depending on the degree of malignancy of the glioma. For example, people with lower-grade gliomas often present with a history of months or even years, while people with higher-grade gliomas often present with a history of weeks to months. Based on the patient’s history, symptoms, and signs, an initial inference can be made about the location of the lesion and the degree of malignancy. Gliomas can be graded in two ways[1]; according to the malignancy of tumor cells, they can be divided into Low-Grade Gliomas (LGG), which are World Health Organization (WHO) grade I-II gliomas with good differentiation and relatively low malignancy. It can also be divided into high-grade gliomas, i.e. WHO grade III-IV gliomas, which are relatively poorly differentiated and have a high degree of malignancy; the grading system developed by WHO, gliomas can be divided into grade I-IV. Grade I is generally dominated by benign hairy cell type glial astrocytomas, which can account for about 5 % of gliomas, and most of them can be cured clinically. Grade II is general astrocytoma or astrocytic oligodendroglia, which accounts for 30 %-40 % of gliomas, and the prognosis can achieve the survival of >5 y. Grade III is a mesenchymal type of astrocytoma, accounting for 15 %-25 % of gliomas, and generally evolves from grade II gliomas, with an average survival of 2 y-3 y. Grade IV is malignant glioblastoma, the most malignant, accounting for about 1/3rd of gliomas, with an average survival of only about 6 mo to 2 y.
LGG are an infrequent group of primary tumors of the Central Nervous System (CNS) that are categorized as grade I and II by the WHO. They are usually inactive, but many eventually become fatal high-grade gliomas. Because of the long asymptomatic natural history of these tumors, there is uncertainty as to whether treatment should be aggressive or delayed for those people with finite, asymptomatic lesions; and the timing of postoperative radio and chemo-therapy. Overall, the causative factors of LGG are unclear. Although alterations in genetic pathways have been postulated to be involved in the formation of low-grade and high-grade tumors, mutations in most genes remain a mystery. Low-grade astrocytomas are often associated with Von Recklinghausen disease (neurofibromatosis type I), suggesting deletion or mutation of a tumor suppressor gene on chromosome 17q. Gliomas can also be associated with neurofibromatosis type II, particularly in the spinal cord, reflecting a functional deletion of the tumor suppressor gene on chromosome 22. Tuberous sclerosis is thought to be linked to germ cell mutations in the tumor suppressor genes Tuberous Sclerosis Complex (TSC)-1 or TSC2. It is often directly related to an uncommon pathological type of LGG and shy; D subventricular canalicular giant cell astrocytoma. Astrocytic glioma can also be associated with Li-Fraumeni syndrome due to mutations in the TP53 gene.
Histologically, LGG was once considered to be a relatively homogeneous tumor with a benign or well-developed natural course. In fact, however, it contains many different types of tumors in the CNS, and its prognosis depends on the anatomic site, pathological type, and treatment of the tumor. Hairy cell astrocytomas occur most often in the cerebellum (85 % of primary tumors in the cerebellum), but can also be seen in the cerebral hemispheres. These tumors are often cystic in nature and are more common in children and young patients. Although some features suggest a malignant tendency (enhancement on imaging, microscopic nuclear schizophrenia, and microvascular hyperplasia), the presence of biphasic cell populations composed of dense bipolar cells and Rosenthal fibers, multipolar cells with microcyst formation, and granulosa identifies a hairy cell astrocytoma. In addition, there are some uncommon subtypes of LGG, such as Pleomorphic Yellow Astrocytomas (PXAs), sub ventricular ventricular meningiomas, and sub ventricular giant cell astrocytomas. As with hairy cell astrocytomas, these tumors are usually well-defined, slow-growing, and rarely malignant. Broadly speaking, most LGG in adults can be divided into three groups; astrocytomas, oligodendrogliomas, and mixed oligodendroglial astrocytomas. The typical low-grade astrocytoma has an indistinct border, infiltrates and spreads to adjacent brain tissue, and is either parenchymal or cystic in nature. Histologically, low-grade astrocytomas exhibit increased cell numbers and microcyst formation without the features of homogeneous heterogeneity, necrosis, and endothelial cell proliferation. Differentiation from reactive gliosis is sometimes difficult, especially in the case of small tissue specimens. The tumor has several histologic subtypes, with the obese cell type more often undergoing early transformation to high-grade glioma (mesenchymal astrocytoma or glioblastoma).
I Kappa B Kinase Interacting Protein (IKBIP), localized on chromosome 12 plays a role in promoting apoptosis[2]. In addition, it has been found that IKBIP also seems to play an important role in inflammation occurrence[3]. However, little is known about IKBIP. Our article focuses on the role of IKBIP in LGG using bioinformatics and its impact on prognosis. In addition, we analyzed the pathways of IKBIP enrichment. Finally, we investigated the impact of IKBIP expression on the immune microenvironment.
Materials and Methods
Data collection and analysis:
We collected and analyzed gene expression and clinical data from various tumors in the-The Cancer Genome Atlas (TCGA) database[4].
Survival analysis:
We divided IKBIP into two groups according to expression status, high and low expression, and studied the survival of both groups. We analyzed the Overall Survival (OS), disease-specific survival, and progress-free interval separately using the R language.
Correlation and enrichment analysis:
Genome Enrichment Analysis (GSEA) is a method to analyze genome-wide exposure spectral microarray data. We used GSEA analysis to find the pathways enriched by IKBIP and mapped the mountains.
Immune cell infiltration:
Tumor immune cell infiltration is the isolation of immune cells that infiltrate from tumor tissue as they move from the bloodstream and begin to act. Immune cell infiltration in tumors is strongly associated with clinic results, and immune cells invading tumors have been shown to be the most likely pharmaceutical targets to enhance patient outcomes. We divided the IKBIP into high and low-groups based on their moderate exposure, and analyzed the degree of infiltration of immune cells in the high- and low-groups, respectively.
Results and Discussion
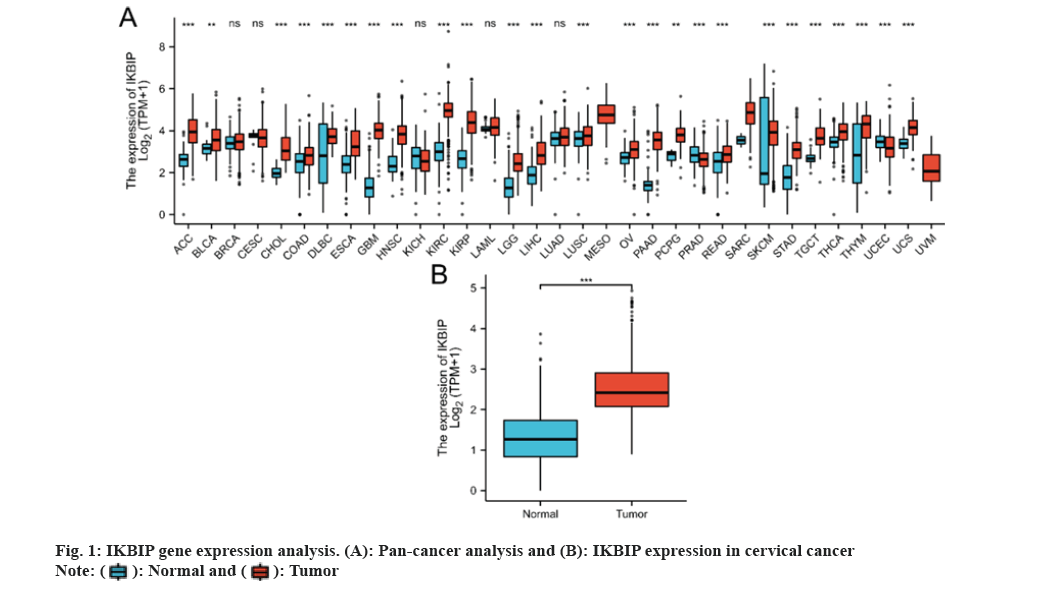
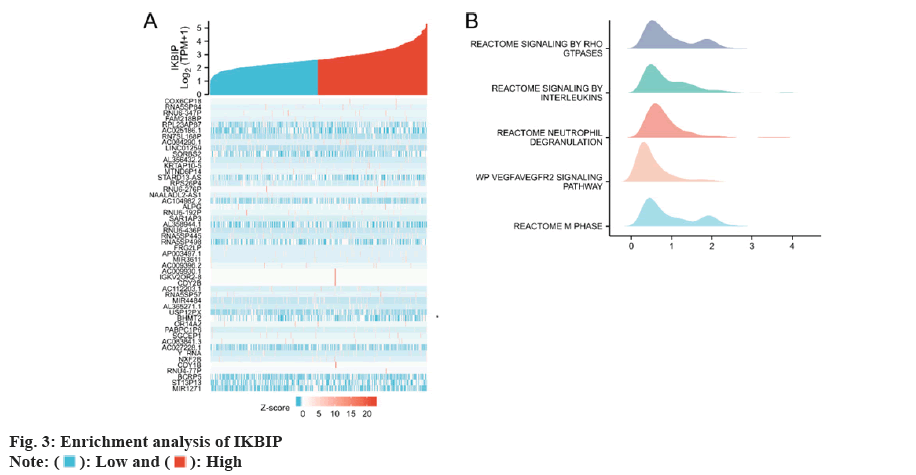
We reviewed the exposure to the IKBIP gene in 33 tumors and noted a significant difference in the exposure of IKBIP in 25 cancers compared to normal tissues. IKBIP was highly expressed in LGG (fig. 1).
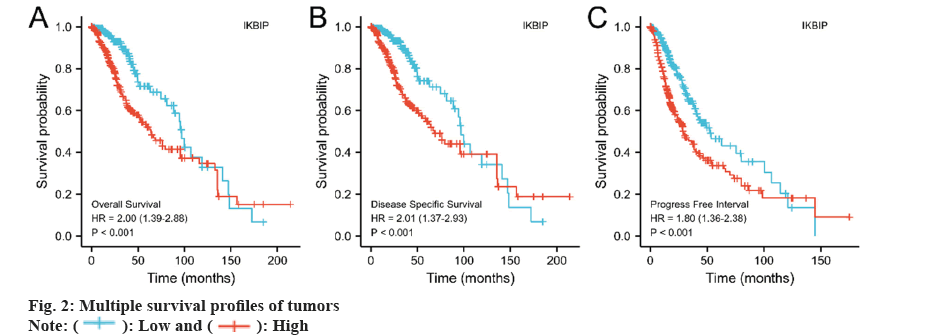
We also investigated the association between IKBIP exposure and patient survival. The results told us that the IKBIP high expression group had shorter survival times in OS, disease-specific survival, and progress-free interval (fig. 2) (p<0.001).
We first selected the genes most closely related to IKBIP for enrichment analysis and selected the top 30 for heat map analysis. Then we performed a GSEA analysis and found that IKBIP was enriched in Reactome signaling by RHO GTPases and Reactome signaling by Interleukin (IL), etc. (fig. 3).
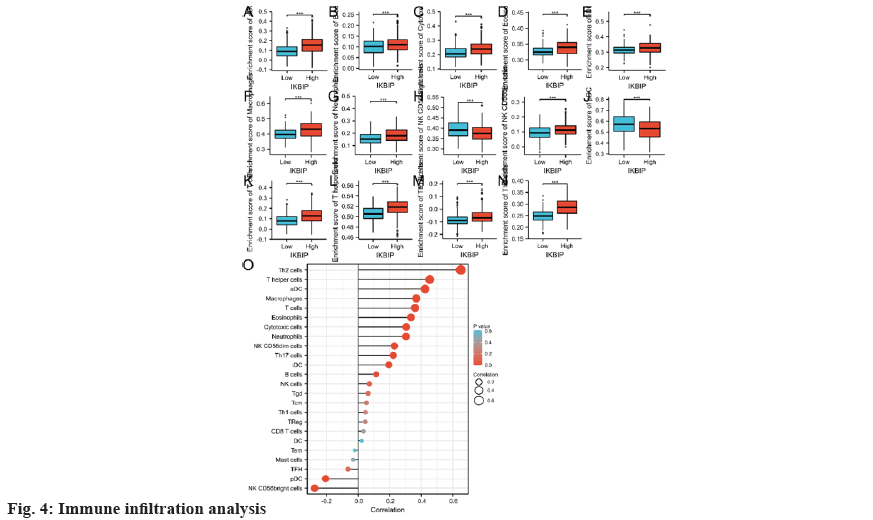
Finally, we analyzed the immune infiltration of LGG patients. In the IKBIP high expression group, the infiltration of Th2 cells and T helper cells, etc., was increased, while the infiltration of Natural Killer (NK) Clusters of Differentiation (CD)-56 bright cells, plasmacytoid Dendritic Cells (pDC), etc., was significantly reduced (fig. 4). Together, these findings indicate that IKBIP exposure may affect the immune cell infiltration in cervical cancer and thus the prognosis of the tumor.
To explore the link of IKBIP exposure and LGG, we conducted a bioinformatics profiling using the TCGA database. First, we evaluated the exposure of IKBIP in pan-cancer and revealed significant differences in the expression of IKBIP in 25 cancers including LGG. Then, to examine the link between IKBIP and prognosis of LGG patients, we analyzed the TCGA clinical data again. The results revealed that high IKBIP expression significantly reduced the survival time of patients. In addition, we performed related gene expression analysis and pathway enrichment analysis of IKBIP and further investigated the mechanism of action of IKBIP that may be related to causing an inflammatory response and secreting cytokines. It is well-known that the tumor immune microenvironment serves an essential role in tumorigenesis and development. Thus, we investigated the link of IKBIP exposure with immune cells. It was revealed that the infiltration of Th2 cells and T helper cells, etc., was increased in the high IKBIP expression group, while the infiltration of NK CD56 bright cells, pDC, etc. was significantly reduced.
LGGs are a diverse group of tumors that typically occur in young adults[5], and LGGs are characterized by a longer survival period compared to high-grade gliomas[6]. The common age of onset of LGG is 2 y-40 y. LGG mainly causes hydrocephalus[7]. In addition, most patients also cause seizures. Malignant transformation is a common form of progression of LGG and leads to treatment failure. Recurrence of LGG after multiple surgeries often leads to transformation to a mesenchymal tumor. Several recurrences can lead to glioblastoma. The time frame for transformation from LGG to mesenchymal glioma is large, averaging 4 y-5 y, while progression from mesenchymal glioma to glioblastoma is usually rapid (1 y-2 y).
Tumor tissue is not only composed of tumor cells alone but also includes surrounding immune cells and extracellular matrix[8]. Cancer cells alter the surrounding environment of cancer cells by secreting cytokines that interact with other normal cells. These play a very important role in tumors[9]. Among the adaptive immune cells of tumors, we already have a good understanding of T cell infiltration, including CD4+ T cells, CD8+ T cells, and effector T cells, among others[10-12]. NKT cells are specialized T cells that are able to identify CD1 d-related lipid antigens and react to the inflammatory factors despite the lack of T cell receptor signaling, thus giving these cells the profile of both innate and adaptive cells[13]. DC are key specialized antigen-presenting cells that form the basis for T cell activation and differentiation[14]. DC cells consist of specific subpopulations of DC with different functions, morphology, phenotype, and distribution, including typical type 1 DC (cDC1), typical type 2 DC (cDC2), pDC and monocyte-DC (mDC). Different subpopulations are associated with different prognosis and functional statuses (e.g. mature and immature). CD208+ DC is essential for generating cytotoxic T cytokine-dependent tumor-targeted inflammatory responses that translate toward clinical gain in patients with High Grade Serous Ovarian Cancer (HGSOC). Macrophages are distinguished tissue-resident monocytes whose engulfment activity is usually classified into M1 and M2 subtypes according to their differentiation status and function role. M1 macrophages have their pro-inflammatory properties (e.g. production of IL-12 and reactive oxygen species), thus facilitating anti-tumor Th1-type responses; M2 macrophages have anti-inflammatory properties and secrete IL-10, Transforming Growth Factor-Beta (TGF-β) and other micro environmental mediators that facilitate the establishment of tolerance, and as well as pro-angiogenic factors. The use of CD68 as a prototypical macrophage signifier does not distinguish between M1 and M2 macrophage types. Furthermore, the typing of M1 and M2 types is overly simplistic and polarizing. It is widely believed that macrophages represent a multitude of cell types that have a series of regulatory functional roles in equilibrium and pathology. This functional diversity is regulated by developmental origins, resident tissues, and acute micro environmental stimuli. Tumor-Associated Macrophages (TAMs) are currently referred to as tumor-recruited[15], usually differentiated monocytes of the original tumor population with an M2-like phenotype, and higher invasion of TAMs is associated with lower OS levels in many kinds of cancers. Due to their ability to produce antibodies, B cells are key cells of humoral adaptive immunity, important immune cells of the Tumor Microenvironment (TME), and Tertiary Lymphoid Structures (TLS) that form the tumor localization, especially in CD8+, CD20+ tumors[16]. B cells are found in TLS in most gastric cancers and they are linked to a better clinic outcome. The presence of both CD8+ T cells and CD20+ B cells in the tumor was linked to longer survival in migratory melanoma, independent of additional clinical covariates. The vast majority of studies have identified B cells, including Breg, based on CD20 expression. Breg cells generate anti-inflammatory mediators, like IL-10, and acquire an immunosuppressive phenotype in tumor beds. The absence of a single surface-based marker to profile Breg cells makes their recognition in TME still difficult. Neutrophils are the most abundant circulating leukocytes, with engulfment and anti-microbial capacity. In cancer, increased neutrophil numbers are linked to poor OS. Poor OS, Progression Free Survival (PFS), Disease-Free Survival (DFS) and cancer-specific survival are observed in diseases with a neutrophil-to-lymphocyte ratio >4.
LGG is a relatively common tumor in the pediatric and young adult populations. The relationship between IKBIP expression and LGG is still unclear. Our study showed that IKBIP is highly expressed in LGG tissues. In addition, high IKBIP expression can affect patient prognosis and the immune microenvironment. Therefore, IKBIP is expected to be a predictor of poor prognosis in LGG and a guiding gene for immunotherapy. However, our study is still inadequate; the effect mechanism of IKBIP is not clear and needs further study. In addition, a number of drugs targeting IKBIP overexpression and thus treating LGG remain to be developed.
All in all, our study found that IKBIP expression can serve as a biomarker for LGG and can predict prognosis and guide subsequent treatment.
Conflict of interests:
The authors declared no conflict of interests.
References
- Chen R, Smith-Cohn M, Cohen AL, Colman H. Glioma subclassifications and their clinical significance. Neurotherapeutics 2017;14(2):284-97.
[Crossref] [Google Scholar] [PubMed]
- Hofer-Warbinek R, Schmid JA, Mayer H, Winsauer G, Orel L, Mueller B, et al. A highly conserved proapoptotic gene, IKIP, located next to the APAF1 gene locus, is regulated by p53. Cell Death Differ 2004;11(12):1317-25.
[Crossref] [Google Scholar] [PubMed]
- Wu H, Liu H, Zhao X, Zheng Y, Liu B, Zhang L, et al. IKIP negatively regulates NF-κB activation and inflammation through inhibition of IKKα/β phosphorylation. J Immunol 2020;204(2):418-27.
[Crossref] [Google Scholar] [PubMed]
- Blum A, Wang P, Zenklusen JC. SnapShot: TCGA-analyzed tumors. Cell 2018;173(2):530.
[Google Scholar] [PubMed]
- Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 2007;114:97-109.
[Google Scholar] [PubMed]
- Forst DA, Nahed BV, Loeffler JS, Batchelor TT. Low-grade gliomas. Oncologist 2014;19(4):403-13.
- Pouratian N, Asthagiri A, Jagannathan J, Shaffrey ME, Schiff D. Surgery insight: The role of surgery in the management of low-grade gliomas. Nat Clin Pract Neurol 2007;3(11):628-39.
[Crossref] [Google Scholar] [PubMed]
- Sumpter TL, Dangi A, Matta BM, Huang C, Stolz DB, Vodovotz Y, et al. Hepatic stellate cells undermine the allostimulatory function of liver myeloid dendritic cells via STAT3-dependent induction of IDO. J Immunol 2012;189(8):3848-58.
[Crossref] [Google Scholar] [PubMed]
- Hinshaw DC, Shevde LA. The tumor microenvironment innately modulates cancer progression. Cancer Res 2019;79(18):4557-66.
[Crossref] [Google Scholar] [PubMed]
- Basit F, Mathan T, Sancho D, de Vries IJ. Human dendritic cell subsets undergo distinct metabolic reprogramming for immune response. Front Immunol 2018;9:412397.
[Crossref] [Google Scholar] [PubMed]
- Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, et al. Polarization of tumor-associated neutrophil phenotype by TGF-β: “N1” vs. “N2” TAN. Cancer Cell 2009;16(3):183-94.
[Crossref] [Google Scholar] [PubMed]
- Chaput N, Conforti R, Viaud S, Spatz A, Zitvogel L. The Janus face of dendritic cells in cancer. Oncogene 2008;27(45):5920-31.
[Crossref] [Google Scholar] [PubMed]
- Nair S, Dhodapkar MV. Natural killer T cells in cancer immunotherapy. Front Immunol 2017;8:293921.
- Lee YS, Radford KJ. The role of dendritic cells in cancer. Int Rev Cell Mol Biol 2019;348:123-78.
[Crossref] [Google Scholar] [PubMed]
- Ngambenjawong C, Gustafson HH, Pun SH. Progress in tumor-associated macrophage (TAM)-targeted therapeutics. Adv Drug Deliv Rev 2017;114:206-21.
[Crossref] [Google Scholar] [PubMed]
- Liu Y, Zhou X, Wang X. Targeting the tumor microenvironment in B-cell lymphoma: Challenges and opportunities. J Hematol Oncol 2021;14(1):125.
[Crossref] [Google Scholar] [PubMed]
 .
.
 .
.
 .
.