- *Corresponding Author:
- Chandi Vishala Thonangi
Pharmacology Department, Andhra University College of Pharmaceutical Sciences, Andhra University, Visakhapatnam, Andhra Pradesh 530003, India
E-mail: chandivishala@yahoo.com
| Date of Received | 20 May 2021 |
| Date of Revision | 14 December 2021 |
| Date of Acceptance | 20 July 2022 |
| Indian J Pharm Sci 2022;84(4):902-909 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The present study is aimed to evaluate the antioxidant and hepatoprotective properties of selected methanolic fractions of Polyalthia longifolia (Sonn.) Thwaite seeds in ethanol-induced oxidative stress in rats. Initially, methanolic extract of Polyalthia longifolia seeds was fractionated using column chromatography. The preliminary antioxidant screening of these fractions identified two main bioactive fractions (F3 and F5), which were found to have significant radical scavenging and metal ion chelation properties compared with ascorbic acid. Based on the antioxidant profile, F3 and F5 were evaluated for hepatoprotective activity in ethanol-intoxicated rats. The Wistar rats were grouped (n=6) and treated with F3 and F5 (200 and 400 mg/kg), ethanol (5 g/kg, 20 % w/v) and silymarin (100 mg/kg) orally for 28 d. The outcomes of the study found that chronic administration of ethanol significantly (p<0.0001) altered the liver parameters and oxidative stress markers (malondialdehyde, superoxide dismutase and catalase). The co-administration of F5 prominently ameliorated the oxidative stress induced by ethanol compared to F3. Histopathological studies further supported the significant protective action of F5. The present study demonstrates that the Polyalthia longifolia seeds possess significant antioxidant properties by augmenting the magnitude of the antioxidant enzymes superoxide dismutase and catalase and further reducing malondialdehyde levels.
Keywords
Antioxidant, alcohol-induced hepatotoxicity, hepatoprotective, oxidative stress, Polyalthia longifolia
The liver is the major organ of the body, which plays an essential role in the metabolism of carbohydrates, proteins and lipids. It is the major site for the detoxification of various toxic substances[1]. The alteration in the liver’s normal functioning led to abnormal metabolism and decreased the elimination of toxic metabolites that alters the homeostasis. The main factors causing liver damage are disorders/diseases, drugs, chronic alcoholism and various poisonous substances[2].
Day by day, alcohol consumption is increasing in all countries across the world. According to the World Health Organization, 3.3 million deaths related to chronic alcoholism have been recorded every year globally[3]. Generally, 80 % of consumed alcohol is metabolized by cytochrome P450 enzyme resulting in the generation of the toxic metabolite named acetaldehyde. The accumulated acetaldehyde leads to the production of Reactive Oxygen Species (ROS) free radicals, which ultimately culminates in oxidative stress and tissue inflammation[3,4].
The exact mechanism of alcohol-induced hepatotoxicity is complicated and unclear. But, it is proposed by few researchers that the metabolites of alcohol alters the Nicotinamide Adenine Dinucleotide (NAD)/beta (β)-Nicotinamide Adenine Dinucleotide 3-Phosphate (NADPH) ratio[5], increased generation of ROS, damaging the Deoxyribonucleic Acid (DNA), proteins and mitochondrial respiratory chain[6], oxidation of lipids, activation of kupffer cells and metabolism of iron[7]. Hence, there is a need to search for multitargeted therapy against alcohol-induced intoxication.
A tall evergreen plant Polyalthia longifolia (Sonn.) Thwaites (P. longifolia) (family: Annonaceae) is widely distributed in India and Srilanka[8]. In the folklore, Polyalthia has been used in the treatment of rheumatism and febrile reaction[9]. The chemical examination of P. longifolia reported to have alkaloids and diterpenoids as major constituents[10].
The biological investigation on various parts of this plant was found to have antimicrobial[11], antioxidant[12], cytotoxic[13], anti-inflammation[14], antihyperglycemic[15], hypotensive[16] and anti-ulcer activities[17]. In addition, three groups have tested the hepatotoxicity of methanolic extracts of leaves of P. longifolia using different inducing agents such as diclofenac sodium[15], paracetamol[18] and carbon tetrachloride[19]. These studies justify that methanolic extracts of P. longifolia have potent hepatoprotective activity in both in vitro and in vivo models. The preliminary studies on P. longifolia seed extracts also proven that methanolic extract possess good antioxidant properties[12]. Taken together, the present study aimed to evaluate the antioxidant and hepatoprotective activities of bioactive fractions of methanol extract of P. longifolia seeds against chronic ethanol-induced intoxication in Wistar albino rats.
Materials and Methods
Chemicals:
1,1-Diphenyl-2-Picrylhydrazyl (DPPH), NADPH, Silymarin, 2-Thiobarbituric Acid (TBA), Nitro Blue Tetrazolium (NBT), phenazine methosulphate, sodium dodecyl sulfate were purchased from Sigma Aldrich Co., St. Louis, Missouri, United States of America (USA). Hydrogen peroxide, ethanol and acetic acid were purchased from Fisher Scientific, Mumbai, India. All other chemicals used in the study were of analytical grade and were obtained commercially.
Plant material:
The seeds of P. longifolia were collected from Tirumala hills, Andhra Pradesh, India, in August 2018. The material was authenticated by the Faculty of Botany, Sri Venkateswara University (SVU), Andhra Pradesh, India and a voucher specimen (No. 782) was deposited in the herbarium of SVU.
Extraction and fractionation:
The shade dried seeds of P. longifolia were ground into a coarse powder using an electrical blender. By hot continuous percolation technique[20], the powdered material (1 kg) was extracted with methanol for 48 h using a Soxhlet apparatus[21]. The obtained solvent mixture was concentrated under reduced pressure using Rotavapor (Buchi R-210 Rotavapor, Marshall Scientific, USA) yielded methanolic extract of seeds of P. longifolia (PLS, 45 g, 4.5 % w/w) as dark black solid. Using column chromatography of mesh size 100- 200, PLS (20 g) was fractionated[22] using a hexane/ ethyl acetate solvent system (step gradient flow from 100:0, 95:5, 90:10, …, 5:95, 0:100), which yielded five fractions (F1 (6 g), F2 (5 g), F3 (3 g), F4 (4 g) and F5 (2 g)). These fractions were subjected to antioxidant activity[23,24] (as per the procedure mentioned below) and noticed that only two of them, namely F3 (3 g) and F5 (2 g), were biologically more active (Table 1).
| Sample | Percentage inhibition (%) at 1 mg/ml* | |
|---|---|---|
| DPPH radicals | Ferric ions | |
| Ascorbic acid | 92.61±3.25 | 90.06±6.24 |
| PLS | 81.71±3.10 | 68.58±1.82 |
| F1 | 2.19±0.30 | 2.73±0.57 |
| F2 | 0.69±0.10 | 1.31±0.12 |
| F3 | 66.65±6.90 | 80.53±5.88 |
| F4 | 18.85±2.81 | 22.13±2.19 |
| F5 | 71.22±5.19 | 82.19±7.23 |
Note: *Mean±Standard Deviation (SD) (n=3)
Table 1: Primary screening of antioxidant activity of PLS and its fractions
In vitro antioxidant activity:
Evaluation of DPPH radical scavenging activity: By employing the DPPH (Sigma Aldrich Co., USA) assay[23,24] in triplicate, the F1-5 and PLS were evaluated for antioxidant activity. To the known concentrations of F1-5 and PLS, added 0.004 % DPPH and incubated for 30 min at 37°. Later, absorbance was measured at 517 nm against the blank. Ascorbic acid was used as a standard drug. The percentage (%) of inhibition was calculated by the following equation and the half maximal Inhibitory Concentration (IC50) values were calculated via logistic regression analysis[25].
% inhibition =A0-A1/A0×100
Evaluation of ferric ion reducing power assay: The ferric ion reducing power assay was determined in triplicate by the modified method of Haritha et al.[26]. To 2.5 ml of potassium ferricyanide added various concentrations of F1-5 and PLS and incubated at 50° for 20 min. To it, 0.5 ml of ferric chloride (0.1 %) and 2.5 ml trichloroacetic acid (10 %) were added, and the absorbance was noted at 700 nm. Ascorbic acid was used as a standard drug. The percentage of inhibition was calculated by the following equation and the IC50 values were calculated via logistic regression analysis[25].
% inhibition =A0-A1/A0×100
Total phenol and flavonoid contents
The total flavonoid and phenolic content of the extract were evaluated by using aluminum chloride[27] and Folin-Ciocalteu reagent[28], respectively. The total flavonoid and phenolic content of the F3 and F5 (1 mg/ ml) were expressed as rutin and gallic acid equivalent, respectively.
Animals:
Adult Wistar albino rats (weighing 190±10 g, age 6-8 w) of either sex were used in this study. The animals were given food and water ad libitum and were housed in the Animal House of the Andhra University of Pharmacy under the standard condition with a temperature of 25°±2°, the relative humidity (50 %±10 %), and a 12 h light/12 h dark cycle. This study was approved by the Institutional Animal Ethics Committee (Code: 516/ PO/c/01/IAEC).
Acute toxicity studies:
The Organisation for Economic Co-operation and Development (OECD) main test 420 was utilized for acute toxicity studies. Rats were randomly divided into two groups (five males and five females) and dosed with 2000 mg/kg body weight (b.w) of PLS suspended in gum acacia and Tween-80. The test animals have undergone fasting overnight before administering the PLS using oral gavage. The testing was ended until the last three animals survived the upper bound dose and all of the test animals were observed up to 14 d[29,30].
Experimental protocol:
At the beginning of the experiment, rats were randomly divided into seven groups (six rats in each group). In group 1 (normal control), rats were administered orally with only 0.2 ml of the vehicle for 28 d. In group 2 (toxic control), rats were dosed orally with ethanol (5 g/kg, 20 % w/v) for 28 d. In group 3 (standard), rats were received 100 mg/kg b.w of silymarin and ethanol (5 g/kg, 20 % w/v) orally for 28 d. Rats in groups 4 and 5 received 200 mg/kg b.w (as a low dose) and 400 mg/ kg b.w (as a high dose) of F3 and ethanol (5 g/kg, 20 % w/v), respectively, orally for 28 d. Rats in groups 6 and 7 received 200 mg/kg b.w (as a low dose) and 400 mg/ kg b.w (as a high dose) of F5 and ethanol (5 g/kg, 20 % w/v), respectively, orally for 28 d.
Collection of serum samples:
The blood samples were obtained from the portal vein, 0.5 ml of blood samples were transferred into laboratory tubes containing pre-autoclaved nutrient broth medium (Sigma-Aldrich, Germany) and put in an incubator at 37°. The remaining blood samples decanted gently into collection plastic tubes, centrifuged at 4000 rpm for 5 min. Then serum was obtained, aliquoted into microtubes and stored at -80° for biochemical analysis.
Tissue homogenization:
At the end of the study, by cervical dislocation, all the rats were sacrificed. The livers were removed, weigh up and washed thoroughly. Some portion of tissue was stored immediately in buffered formalin (10 %) for histopathological studies and the remaining tissue was processed. In 0.05 M of ice-cold phosphate buffer saline (pH 7), the tissue was minced into small pieces and homogenized with a homogenizer (Remi Homogenizer, Mumbai, India) to obtain 10 % whole homogenate. To the homogenate, an equal volume of trichloroacetic acid (10 %) was mixed and centrifuged (Sigma-3-30 KS, USA) for 10 min at 5000 rpm.
Assessment of oxidative stress parameters:
The above-obtained supernatant was subjected to estimate the oxidative stress parameters, namely Malondialdehyde (MDA) levels[31], Superoxide Dismutase (SOD) levels[32] and Catalase (CAT) activity[33] using the established procedure.
Assessment of serum biochemical parameters:
The serum biochemical parameters such as Alkaline Phosphatase (ALP)[34], Serum Glutamic Pyruvate Transaminase (SGPT)[35], bilirubin[36] and Serum Glutamic Oxaloacetate Transaminase (SGOT)[35] were estimated as per the standard method described in the Excel kit leaflet (Excel Pvt. Ltd., India).
Histopathological studies:
The thin sections of formalin-fixed liver tissues were made using paraffin blocks and stained by hematoxylin and eosin stain. These stained sections were inspected under a light microscope.
Statistical analysis:
Data was expressed as mean±Standard Error of Mean (SEM) where n=6. All data were analysed by one way Analysis of Variance (ANOVA) with Dunnett’s multiple comparison test, when compared to toxic control group by using Graphpad Prism software (5.0 version). p values less than 0.05 were considered as statistically significant.
Results and Discussion
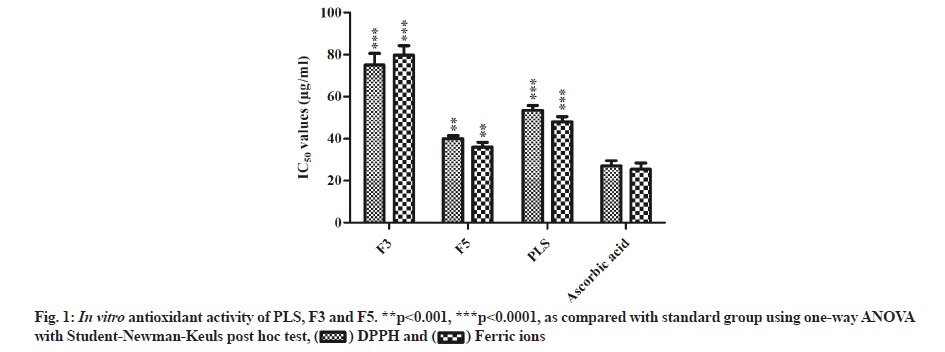
The antioxidant activity of F3, F5 and PLS was evaluated by DPPH free radical scavenging activity and ferric ion reducing power assays. All the tested samples exhibited a considerable antioxidant activity compared with ascorbic acid, F5 and PLS (p<0.0001) showed significant activity when compared with F3 (p<0.001) (fig. 1).
The total flavonoid value of F3 and F5 was equivalent to 2.86±0.10 and 6.2±0.12 mg/g rutin, respectively, while the total phenolic value was equal to 114±1.7 and 146.5±2.4 mg/g of gallic acid, respectively.
In the toxic control group, the chronic administration of ethanol (p<0.0001) significantly elevated the serum SGOT, SGPT, ALP and bilirubin levels compared with the normal control group (Table 2). The animal group co-administered with F3 did not show significant variation in SGPT and bilirubin levels at 200 mg/kg dose. In contrast, the rat group co-administered with F5 offered significant dose-dependent protection and significantly reduced the elevated levels of liver parameters at both low and high doses (Table 2). Moreover, co-administration of 400 mg/kg dose of F5 (p<0.0001) offered almost equivalent protection in the liver parameters compared with silymarin (100 mg/kg) (Table 2).
| Groups | SGOT (IU/l) | SGPT (IU/l) | ALP (IU/l) | Bilirubin (µmol/l) |
|---|---|---|---|---|
| Normal control | 58.16±4.79 | 31.16±3.06 | 194.33±6.37 | 0.29±0.12 |
| Toxic control | 125.83±6.08*** | 70.33±5.50*** | 332.16±6.30*** | 1.55±0.41*** |
| Silymarin (100 mg/kg) | 65.66±4.76### | 47.16±5.56### | 235.83±4.99### | 0.47±0.05### |
| F3 (200 mg/kg) | 119.66±5.39### | 65.16±3.71ns | 326.16±3.97# | 1.36±0.27ns |
| F3 (400 mg/kg) | 103.83±5.77### | 59.5±4.27## | 305.83±8.44### | 1.21±0.11# |
| F5 (200 mg/kg) | 74.66±4.63### | 53.66±6.88### | 268.66±3.82### | 0.53±0.06### |
| F5 (400 mg/kg) | 61.5±4.84### | 44.66±5.53### | 210.5±8.01### | 0.38±0.05### |
Note: All the values were expressed as mean±SEM (n=6) where, ***p<0.0001 as compared with normal control and #p<0.05, ##p<0.001, ###p<0.0001 as compared with toxic control was considered as statistically significant using one-way ANOVA with Dunnett’s multiple comparison test, while p>0.05 was considered as statistically non-significant (ns)
Table 2: Effect of F3 and F5 on Liver function tests
The ethanol-treated group (p<0.0001) significantly reduced the endogenous antioxidant enzymes, namely SOD and CAT, compared with the normal control group. The animal group co-administered with both the fractions (F3 and F5) offered significant protection against the ethanol-toxicity (Table 3). The high dose of F3 significantly elevated the SOD and CAT levels compared with its low dose and toxic control group (Table 3). On the other hand, co-administered F5 offered dose-dependent protection and the high dose of F5 (p<0.0001) showed potent elevation of SOD and CAT levels similar to that of silymarin (Table 3).
| Groups | MDA (n mol/g tissue) | SOD (U/mg protein) | CAT (n mol H2O2/mg protein/min) | |
|---|---|---|---|---|
| Normal control | 2.68±0.12 | 25.6±0.16 | 56.8±0.45 |
|
| Toxic control | 12.12±0.43*** | 7.8±0.66*** | 3.42±0.24*** |
|
| Silymarin (100 mg/kg) | 2.82±0.50### | 28.4±1.94### | 53.67±2.19### |
|
| F3 (200 mg/kg) | 9.43±0.15ns | 12.12±0.43ns | 13.14±0.34# |
|
| F3 (400 mg/kg) | 6.28±0.52# | 17.65±1.64## | 20.68±1.21### |
|
| F5 (200 mg/kg) | 5.34±0.28## | 19.84±2.32### | 28.90±1.41### |
|
| F5 (400 mg/kg) | 3.28±0.64### | 26.89±2.54### | 52.20±1.21### |
|
Note: All the values were expressed as mean±SEM (n=6). Where, ***p<0.0001 as compared with normal control and #p<0.05, ##p<0.001, ###p<0.0001 as compared with toxic control was considered as statistically significant using one-way ANOVA with Dunnett’s multiple comparison test, while p>0.05 was considered as statistically non-significant (ns)
Table 3: Effect of F3 and F5 on tissue oxidative stress markers
Further, the toxic control group (p<0.0001) significantly elevated the serum levels of MDA compared with the normal control group (Table 3). The co-administration of F3 and F5 significantly depleted the elevated MDA levels compared with the ethanol-intoxicated group (Table 3). At 400 mg/kg dose of F3 (p<0.05) significantly decreased the MDA levels, while 400 mg/kg of F3 was non-significant in controlling MDA levels. F5 markedly diminished the MDA levels in a dose-dependent manner and the effects at a higher dose (400 mg/kg) were comparable with silymarin (Table 3).
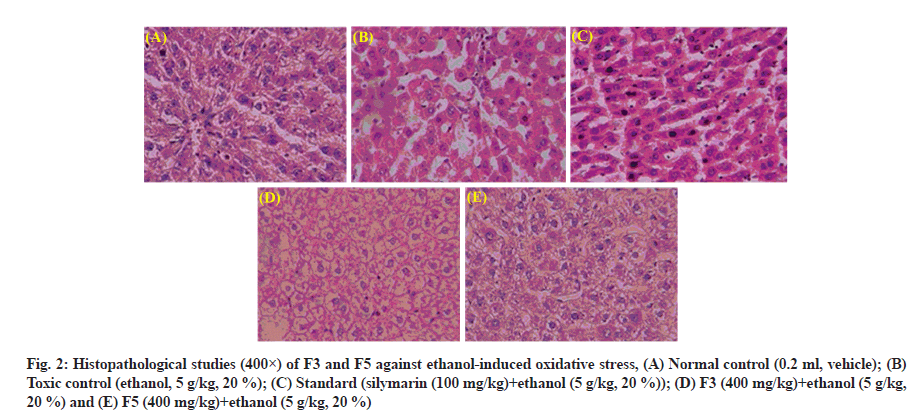
The liver’s architecture is clear and the hepatocytes were arranged clearly in normal control (fig. 2A). In contrast, the ethanol administration results in disruption of the architecture, infiltration of inflammatory cells, vacuolated hepatocytes deposition of lipids and degenerated nuclei (fig. 2B). At a high dose, coadministration F5 remarkably reversed the alterations induced by ethanol than F3, supporting the research findings of biochemical studies (fig. 2C-fig. 2E).
Fig. 2: Histopathological studies (400×) of F3 and F5 against ethanol-induced oxidative stress, (A) Normal control (0.2 ml, vehicle); (B) Toxic control (ethanol, 5 g/kg, 20 %); (C) Standard (silymarin (100 mg/kg)+ethanol (5 g/kg, 20 %)); (D) F3 (400 mg/kg)+ethanol (5 g/kg, 20 %) and (E) F5 (400 mg/kg)+ethanol (5 g/kg, 20 %)
Since ancient times, plants and their parts are being used to treat hepatotoxicity both in Ayurveda and Unani systems[37,38]. Based on this evidence, the search for new lead molecules for liver disorders from natural sources is a crucial point of interest. As a result, many plants and their phytochemicals were tested for antioxidant and hepatoprotective potentialities[39]. Thus, few groups have tested the hepatotoxicity of a folklore medicinal plant P. longifolia using different induced models[15,18,19] and found that methanolic extracts of P. longifolia have potent hepatoprotective activity. Therefore, in the present study, the seeds of P. longifolia were extracted with methanol and fractionated. The two obtained fractions (F3 and F5) exhibited significant DPPH free radical scavenging activity and ferric ion reducing power capabilities attributed to the substantial quantity of phenol and flavonoid compounds[40] (fig. 1).
As mentioned earlier, acetaldehyde accumulation in the liver inhibits the transport pumps present on the hepatocytes membrane, thereby outflow the liver enzymes such as SGOT, SGPT, ALP and bilirubin into serum results in hepatotoxicity[41]. In the current investigation, similar observations were noticed in the toxic control group. Co-administration of F3 and F5 significantly reduced the elevated levels of SGOT, SGPT, ALP and bilirubin (Table 2). Notably, co-administration of a higher dose of F5 displayed equivalent protection in the liver parameters compared with silymarin (Table 2).
Generally, oxidative stress is the pathogenesis of numerous chronic diseases that affects the imbalance between the antioxidants and ROS[42,43]. The endogenous antioxidant enzymes like SOD and CAT play a vital role in catalyzing the Hydrogen peroxide (H2O2) into the water, the major precursor of ROS. The depletion of SOD and CAT results in oxidative stress in surrounding tissues and causes tissue and organ necrosis[44]. Moreover, the excess generation of ROS plays a vital role in hepatotoxicity’s etiology[45]. The chronic administration of ethanol activates the kupffer cells, thereby generates ROS and pro-inflammatory substances[46]. These excess ROS covalently binds to the hepatocytes membrane and results in oxidation of polyunsaturated lipids that markedly elevate the MDA levels[47]. Further, excess accumulation of both ROS and MDA levels together causes oxidative stress in hepatocytes[48].
The present study’s findings indicate that ethanol administration markedly elevated the MDA levels by depleting the endogenous antioxidants (SOD and CAT) (Table 3). In contrast, the administration of fractions significantly antagonized the oxidative stress induced by ethanol by enhancing the SOD and CAT levels and simultaneously depleting the MDA levels in a dose-dependent manner when comparing with toxic control (Table 3). The co-administration of 400 mg/kg of F5 exhibited marked protection of tissue oxidative stress markers similar to silymarin. Moreover, our outcomes are identical to the previous study models of hepatotoxicity[15,18,19].
The histopathological studies are in support of the hepatoprotective activity of F3 and F5. The chronic administration of alcohol causes disruption of hepatocytes, infiltration of cells and ballooning of cells[49], whereas both fractions co-administration reversed the damage effects caused by alcohol. Among both, F5 maintained the better structural integrity of hepatocytes and antagonized ethanol-induced toxicity than F3. These histopathological results support the hepatoprotective potentiality of seeds of P. longifolia against ethanol-intoxicated rats.
To conclude, the present study showed that fractions of P. longifolia have a good amount of total flavonoid and phenolic contents with potent antioxidant activity. Additionally, F3 and F5 unexpectedly improved serum liver parameters and oxidative stress markers in rats induced with ethanol, which is attributed to viz., radical scavenging and antioxidant activities. Also, the co-administration of these fractions showed hepatoprotective effects in ethanol-induced Wistar albino rats. Hence, the seeds of P. longifolia could be used in the treatment of different liver ailments.
Acknowledgements
The authors sincerely thank the Department of Science and Technology (DST) for funding the project with the grant number (DST/DISHA/SoRF-PM/032/2015/01/G) and Andhra University for bestowing me with the entire necessary infrastructure for completion of my research work.
Conflict of interests:
The authors declared that they have no conflict of interest.
References
- Abou Seif HS. Ameliorative effect of pumpkin oil (Cucurbita pepo L.) against alcohol-induced hepatotoxicity and oxidative stress in albino rats. Beni-suef Univ J Basic Appl Sci 2014;3(3):178-85.
- Zhou X, Deng Q, Chen H, Hu E, Zhao C, Gong X. Characterizations and hepatoprotective effect of polysaccharides from Mori Fructus in rats with alcoholic-induced liver injury. Int J Biol Macromol 2017;102:60-7.
[Crossref] [Google Scholar] [PubMed]
- Zhao H, Cheng N, He L, Peng G, Xue X, Wu L, et al. Antioxidant and hepatoprotective effects of A. cerana honey against acute alcohol-induced liver damage in mice. Food Res Int 2017;101:35-44.
[Crossref] [Google Scholar] [PubMed]
- Bhujanga Rao C, Babu DC, Bharadwaj TV, Srikanth D, Vardhan KS, Raju TV, et al. Isolation, structural assignment and synthesis of (SE)-2-methyloctyl 3-(4-methoxyphenyl) propenoate from the marine soft coral Sarcophyton ehrenbergi. Nat Prod Res 2015;29(1):70-6.
[Crossref] [Google Scholar] [PubMed]
- Nagy LE. Molecular aspects of alcohol metabolism: Transcription factors involved in early ethanol-induced liver injury. Annu Rev Nutr 2004;24:55-78.
[Crossref] [Google Scholar] [PubMed]
- Cederbaum AI, Lu Y, Wu D. Role of oxidative stress in alcohol-induced liver injury. Arch Toxicol 2009;83(6):519-48.
[Crossref] [Google Scholar] [PubMed]
- Koch OR, Pani G, Borrello S, Colavitti R, Cravero A, Farrè S, et al. Oxidative stress and antioxidant defenses in ethanol-induced cell injury. Mol Aspects Med 2004;25(1-2):191-8.
[Crossref] [Google Scholar] [PubMed]
- Moniruzzaman M, Ferdous A, Bokul FW. Evaluation of antinociceptive activity of ethanol extract of bark of Polyalthia longifolia. J Ethnopharmacol 2015;172:364-7.
[Crossref] [Google Scholar] [PubMed]
- Annan K, Dickson RA, Sarpong K, Asare C, Amponsah K, Woode E. Antipyretic activity of Polyalthia longifolia Benth. & Hook. F. var. pendula (Annonaceae), on lipopolysaccharide-induced fever in rats. J Med Biomed Sci 2013;2(1):8-12.
- Wu YC, Duh CY, Wang SK, Chen KS, Yang TH. Two new natural azafluorene alkaloids and a cytotoxic aporphine alkaloid from Polyalthia longifolia. J Nat Prod 1990;53(5):1327-31.
[Crossref] [Google Scholar] [PubMed]
- Murthy MM, Subramanyam M, Bindu MH, Annapurna J. Antimicrobial activity of clerodane diterpenoids from Polyalthia longifolia seeds. Fitoterapia 2005;76(3-4):336-9.
- Thonangi CV, Akula A. In vitro antioxidant and anti-inflammatory activity of Polyalthia longifolia (Sonn.) Thwaite seeds. Int J Pharm Sci Res 2018;9(9):3774-80.
- Manjula SN, Kenganora M, Parihar VK, Kumar S, Nayak PG, Kumar N, et al. Antitumor and antioxidant activity of Polyalthia longifolia stem bark ethanol extract. Pharm Biol 2010;48(6):690-6.
[Crossref] [Google Scholar] [PubMed]
- Nguyen HT, Vu TY, Chandi V, Polimati H, Tatipamula VB. Dual COX and 5-LOX inhibition by clerodane diterpenes from seeds of Polyalthia longifolia (Sonn.) Thwaites. Sci Rep 2020;10(1):15965.
- Tanna A, Nair R, Chanda S. Assessment of anti-inflammatory and hepatoprotective potency of Polyalthia longifolia var. pendula leaf in Wistar albino rats. J Nat Med 2009;63(1):80-5.
[Crossref] [Google Scholar] [PubMed]
- Saleem R, Ahmed M, Ahmed SI, Azeem M, Khan RA, Rasool N, et al. Hypotensive activity and toxicology of constituents from root bark of Polyalthia longifolia var. pendula. Phytother Res 2005;19(10):881-4.
[Crossref] [Google Scholar] [PubMed]
- Malairajan P, Gopalakrishnan G, Narasimhan S, Veni KJ. Evalution of anti-ulcer activity of Polyalthia longifolia (Sonn.) Thwaites in experimental animals. Indian J Pharmacol 2008;40(3):126-8.
[Crossref] [Google Scholar] [PubMed]
- Jothy SL, Aziz A, Chen Y, Sasidharan S. Antioxidant activity and hepatoprotective potential of Polyalthia longifolia and Cassia spectabilis leaves against paracetamol-induced liver injury. Evid Based Complement Alternat Med 2012;2012:561284.
[Crossref] [Google Scholar] [PubMed]
- Rajangam J, Christina AJ. Evaluation of Hepatoprotective and antioxidant potential of methanolic extract of Polyalthiya longifolia fruits: An In vitro and In vivo approach. J Appl Pharm Sci 2013;3(2):69-76.
- Tatipamula VB, Vedula GS, Sastry AV. Chemical and pharmacological evaluation of manglicolous lichen Roccella montagnei Bel em. DD Awasthi. Future J Pharm Sci 2019;5(1):1-9.
- Tatipamula VB, Vedula GS. Fibrinolytic, anti-inflammatory and cytotoxic potentialities of extracts and chemical constituents of manglicolous lichen, Graphis ajarekarii Patw. & CR Kulk. Nat Prod J 2020;10(1):87-93.
- Tatipamula VB, Vedula GS, Sastry AV. Antarvediside AB from manglicolous lichen Dirinaria consimilis (Stirton) DD Awasthi and their pharmacological profile. Asian J Chem 2019;31(4):805-12.
- Tatipamula VB, Haritha P, Rao GS, Ketha A, Yejella PR. Isolation and Characterization of metabolites from Clathria procera Ridley extract and Evaluation of its antidiabetic effects in Streptozotocin-induced diabetic rats. J Exp Appl Anim Sci 2019;3(1):35-56.
- Hieu HV, Tatipamula VB, Killari KN, Koneru ST, Srilakshmi N, Ranajith SK. HPTLC analysis, antioxidant and antidiabetic activities of ethanol extract of moss Fissidens grandiflora. Indian J Pharm Sci 2020;82(3):449-55.
- Nguyen HT, Vu TY, Kumar AV, Hoang VN, My PT, Mandal PS, et al. N-Aryl iminochromenes inhibit cyclooxygenase enzymes via π–π stacking interactions and present a novel class of anti-inflammatory drugs. RSC Adv 2021;11(47):29385-93.
- Haritha P, Patnaik SK, Tatipamula VB. Chemical and pharmacological evaluation of Manglicolous lichen Graphis ajarekarii PATW. & CR KULK. Vietnam J Sci Technol 2019;57(3):300-38.
- Tatipamula VB, Vedula GS, Rathod BB, Shetty PR, Sastry AV. Study of phytochemical analysis, total flavonoid and phenolic content, antimicrobial properties and chemical constituents of two manglicolous lichens extracts. Invent Rapid Planta Act 2018;2018(2):129-34.
- Tatipamula VB, Killari KN, Gopaiah KV, Ketha A. GC-MS analysis of ethanol extract of Taxithelium napalense (Schwaerg) Broth along with its alpha-glucosidase inhibitory activity. Indian J Pharm Sci 2019;81(3):569-74.
- Tatipamula VB, Kolli MK, Lagu SB, Paidi KR, Reddy P R, Yejella RP. Novel indolizine derivatives lowers blood glucose levels in Streptozotocin-induced diabetic rats: A histopathological approach. Pharmacol Rep 2019;71(2):233-42.
[Crossref] [Google Scholar] [PubMed]
- Tatipamula VB, Annam SS, Nguyen HT, Polimati H, Yejella RP. Sekikaic acid modulates pancreatic β-cells in streptozotocin-induced type 2 diabetic rats by inhibiting digestive enzymes. Nat Prod Res 2021;35(23):5420-4.
[Crossref] [Google Scholar] [PubMed]
- Mollazadeh H, Sadeghnia HR, Hoseini A, Farzadnia M, Boroushaki MT. Effects of pomegranate seed oil on oxidative stress markers, serum biochemical parameters and pathological findings in kidney and heart of streptozotocin-induced diabetic rats. Ren Fail 2016;38(8):1256-66.
[Crossref] [Google Scholar] [PubMed]
- Tatipamula VB, Kukavica B. Protective effects of extracts of lichen Dirinaria consimilis (Stirton) DD Awasthi in bifenthrin-and diazinon-induced oxidative stress in rat erythrocytes in vitro. Drug Chem Toxicol 2022;45(2):680-7.
[Crossref] [Google Scholar] [PubMed]
- Zhang J, Chen R, Yu Z, Xue L. Superoxide dismutase (SOD) and catalase (CAT) activity assay protocols for Caenorhabditis elegans. Bio Protoc 2017;7(16):e2505.
[Crossref] [Google Scholar] [PubMed]
- Prakash S, Verma AK. Effect of arsenic on serum biochemical parameters of a fresh water cat fish, Mystus vittatus. Int J Biol Innov 2020;2(1):11-9.
- Kumar S, Raman RP, Prasad KP, Srivastava PP, Kumar S, Rajendran KV. Effects on haematological and serum biochemical parameters of Pangasianodon hypophthalmus to an experimental infection of Thaparocleidus sp.(Monogenea: Dactylogyridae). Exp Parasitol 2018;188:1-7.
[Crossref] [Google Scholar] [PubMed]
- Moolchandani K, Priyadarssini M, Rajappa M, Parameswaran S, Revathy G. Serum bilirubin: A simple routine surrogate marker of the progression of chronic kidney disease. Br J Biomed Sci 2016;73(4):188-93.
[Crossref] [Google Scholar] [PubMed]
- Sastry AV, Vedula GS, Tatipamula VB. In vitro biological profile of mangrove associated lichen, Roccella montagnei extracts. Inven Rapid Ethnopharmacol 2018;2018(3):153-8.
- Tatipamula VB, Polimati H, Gopaiah KV, Babu AK, Vantaku S, Rao PR, et al. Bioactive metabolites from Manglicolous lichen Ramalina leiodea (Nyl.) Nyl. Indian J Pharm Sci 2020;82(2):379-84.
- Kumaran A, Karunakaran RJ. In vitroantioxidant activities of methanol extracts of five Phyllanthus species from India. LWT Food Sci Technol 2007;40(2):344-52.
- Tatipamula VB, Ketha A. Manglicolous lichen Parmotrema tinctorum (Despr. ex Nyl.) Hale: Isolation, characterization and biological evaluation. Indian J Chem 2020;59:856-61.
- Lodhi P, Tandan N, Singh N, Kumar D, Kumar M. Camellia sinensis (L.) Kuntze extract ameliorates chronic ethanol-induced hepatotoxicity in albino rats. Evid Based Complement Alternat Med 2014;2014:1-7.
[Crossref] [Google Scholar] [PubMed]
- Oktay M, Gülçin ?, Küfrevio?lu Ö?. Determination of in vitro antioxidant activity of fennel (Foeniculum vulgare) seed extracts. LWT Food Sci Technol 2003;36(2):263-71.
- Tatipamula VB, Killari KN, Prasad K, Rao GS, Talluri MR, Vantaku S, et al. Cytotoxicity studies of the chemical constituents from marine algae Chara baltica. Indian J Pharm Sci 2019;81(5):815-23.
- Abdellatief SA, Galal AA, Farouk SM, Abdel-Daim MM. Ameliorative effect of parsley oil on cisplatin-induced hepato-cardiotoxicity: A biochemical, histopathological, and immunohistochemical study. Biomed Pharmacother 2017;86:482-91.
[Crossref] [Google Scholar] [PubMed]
- Yan SL, Wang ZH, Yen HF, Lee YJ, Yin MC. Reversal of ethanol-induced hepatotoxicity by cinnamic and syringic acids in mice. Food Chem Toxicol 2016;98:119-26.
[Crossref] [Google Scholar] [PubMed]
- Dixon LJ, Barnes M, Tang H, Pritchard MT, Nagy LE. Kupffer cells in the liver. Compr Physiol 2013;3(2):785-97.
[Crossref] [Google Scholar] [PubMed]
- Ilaiyaraja N, Khanum F. Amelioration of alcohol-induced hepatotoxicity and oxidative stress in rats by Acorus calamus. J Diet Suppl 2011;8(4):331-45.
[Crossref] [Google Scholar] [PubMed]
- Alsaif MA. Effect of thymoquinone on ethanol-induced hepatotoxicity in Wistar rats. J Med Sci 2007;7(7):1164-70.
- Padmanabhan P, Jangle SN. Hepatoprotective activity of herbal preparation (HP-4) against alcohol induced hepatotoxicity in mice. Int J Appl Sci Biotechnol 2014;2(1):50-8.
 DPPH and
DPPH and  Ferric ions
Ferric ions