- *Corresponding Author:
- S. Tiwari
Department of Pharmaceutics, Maliba Pharmacy College, Uka Tarasadia University, Bardoli-394 350, India
E-mail: sanjay.tiwari@utu.ac.in
| Date of Submission | 16 January 2015 |
| Date of Revision | 21 October 2014 |
| Date of Acceptance | 05 October 2013 |
| Indian J Pharm Sci,2015;77(1):55-61 |
Abstract
Fluconazole is a broad spectrum antifungal agent that has been extensively applied for the management of oral, pharyngeal and cutaneous candidiasis. Fluconazole has a high volume of distribution (0.55-0.65 l/kg) and has systemic toxicity due to high drug-drug interaction. The present study focuses on the formulation of bioadhesive film as a controlled release carrier for fluconazole. The formulation was intended to provide localized delivery of fluconazole exclusively at the site of infection, thereby reducing its total dose and hence, dose-related toxicities. Bioadhesive films were prepared by solvent casting method using sodium alginate and polyvinyl alcohol alone as well as in various combinations. Prepared films were evaluated for physical characteristics like, weight and content uniformity, film thickness, swelling index, microenvironment pH and folding endurance. In vitro drug release, in vitro and ex vivo residence time, bioadhesive strength and skin irritation were also studied. Accelerated stability study was conducted on the optimized formulation as per ICH guidelines. Weight of all the films were not more than 20 mg. Thickness of the films ranged between 0.09 to 0.15 mm whereas swelling indices showed a high extent of variation. Films composed of polyvinyl alcohol alone provided a swelling index of 6%. Bioadhesive strength was found to be more than 18 g. Microenvironment pH was near to 7.0 for most of the formulations. Ex vivo residence time of optimized batch was more than 5 h and it provided controlled drug release up to 8 h. As revealed in FT-IR and DSC studies, drug was found to be compatible with the excipients used in this study.
Keywords
Fluconzole, localized delivery, mucocutaneous candidiasis, bioadhesive film
Introduction
Fungal infection of skin is now a days one of the common dermatological problems. Mucocutaneous candidiasis refers to a heterogeneous group of diseases characterized by recurrent or persistent superficial infections of the skin, mucous membranes, nails and oropharynx with Candida organisms, usually Candida albicans. Fungal infections are one of the major causes of morbidity and mortality among cancer patients. Such infections, occurring especially among the surgical and high risk critically ill patients, are attributable to death rate estimated to be 38% [1,2]. Fluconazole (FLZ) is a bis-triazole antifungal agent used as the primary treatment option for almost all forms of Candida infections in both immune-competent and immune-compromised patients. Besides, treatment of mucocutaneous candidiasis with FLZ is even more effective as compared to other sites of infection [3,4].
Available marketed formulations of FLZ generally result into poor bioavailability due to its high volume of distribution [5]. Emergence of resistance as well as recurrence of infection has been reported in 33% patients population which could possibly be due to subtherapeutic drug concentrations [1]. Besides, intraoral administration results into disturbances in gastrointestinal tract (vomiting, bloating and abdominal discomfort) and causes irritation. Serious hepatotoxicity has been reported in the patients suffering from AIDS or malignancy [6,7]. Dose requirement of FLZ is also significantly high due to high volume of distribution (0.55-0.65 l/kg). Thus, chronic FLZ administration at high doses is undesirable for treatment of infections due to potential side effects. Therefore, to minimize these adverse effects and the risk of drug resistance and to provide localized delivery, topical therapy should be considered the first choice. However, topical delivery of FLZ with the formulation like creams, lotions and spray result into insufficient residence time, lack of accurate dosing and exhibit variability in their therapeutic performance. Such problems often create poor patient compliance and compromise the efficacy of the overall therapy.
In the present research work, we planned to design and evaluate FLZ-based bioadhesive films as a prolonged delivery vehicle for mucocutaneous candidiasis. It is expected that such a formulation approach would reduce the volume of distribution of FLZ by restricting it to the actual site of infection and thus, a significant dose reduction could be achieved. This, in turn, would reduce the incidences of dose-related side effects of FLZ. Sodium alginate (SA) was used as a bioadhesive component in polyvinyl alcohol (PVA) based films.
Materials and Methods
FLZ was obtained as a gift sample from Gufic Bioscience Ltd., Navsari, India. Sodium alginate and glycerine were procured from S. D. Fine Chem. Ltd., Mumbai, India. Polyvinyl alcohol was purchased from Burgoyne Burbridges and Co, Mumbai, India. All other reagents and chemicals were of analytical reagent grade and double distilled water was used throughout the study.
Preparation of bioadhesive films
Bioadhesive films were prepared by solvent casting method [8]. Formulation composition is shown in Table 1. Polymeric dispersion of SA was prepared by dissolving its required quantity in one third of the required volume of distilled water. PVA was dissolved in the other half of double distilled water by heating up to 90°. Both polymeric dispersions were mixed (Remi Instruments, India) and allowed to mix for 1 h. Finally, FLZ dissolved in a mixture of distilled water and 2% glycerine was added into prepared dispersion and allowed to mix. This mixture was kept overnight for deaeration. Then, it was casted in to petridish, which was pre-lubricated with glycerine. The dispersion was allowed to dry in hot air oven at 60-70° for 6-7 h. After drying, the film was removed by gentle peeling from the glass surface. It was cut into an appropriate size and packaged by wrapping in aluminium foil.
| Ingredients | F1 | F2 | F3 | F4 | F5 | F6 | F7 |
|---|---|---|---|---|---|---|---|
| Fluconazole (mg/cm2) | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| Sodium alginate (% w/v) | 3.0 | 2.5 | 2.0 | 1.5 | 1.0 | 0.5 | 0.0 |
| Polyvinyl alcohol (% w/v) | 0.0 | 0.5 | 1.0 | 1.5 | 2.0 | 2.5 | 3.0 |
| Glycerine (% v/v) | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| FLZ : Fluconazole |
FLZ : Fluconazole
Table 1: Formulation Composition Of Flz Bioadhesive Films
Drug excipient compatibility, differential scanning calorimetry
DSC thermograms of FLZ and its combination with excipient were recorded (DSC Shimadzu 60, Japan) with TDA trend line software. Thermal traces were obtained by heating from 50° to 300° at a rate of 10°/min under nitrogen atmosphere (20 ml/min) in open crucibles.
Physicochemical properties of prepared films
Films were evaluated for the parameters like, physical appearance and surface texture; thickness was measured at three different places using a digital Vernier callipers. Folding endurance was determined by the number of times films could be folded at the same place, without breaking [9]; weight uniformity was measured by weighing of 1 cm2 films using single pan electronic balance [10]; films were tested for drug content by UV spectrophotometric method (Shimadzu UV- 1800, Japan) [11]; and surface pH of all films were determined by cutting films with 1 cm2 area which were allowed to swell for 2 h on 2% w/v agar plate. Surface pH was measured by placing the tip of glass electrode close to the surface of the film and allowing it to equilibrate for 1 min prior to recording. Mean values and standard deviation for all the parameters were calculated and reported.
Swelling index
Swelling of polymer is essential for the relaxation and interpenetration of polymer chains. As films imbibe water, swelling and thereby bonding starts and finally adhesion occurs. Initially the bond formed remains weak but it increases with hydration. Swelling properties of films were determined by evaluating their percentage hydration [12].
Film of 1 cm2 area was cut and weighed (W1) and immersed in phosphate buffer (pH 6.8) for predetermined time and was taken out and wiped off to remove excess of surface water and weighed (W2) [13]. Swelling index was calculated using the Eqn., swelling index=(final weight-initial weight)/initial weight)×100.
Ex vivo residence time
Ex vivo residence time was determined using a modified IP disintegration apparatus [14]. Disintegrating medium was composed of 800 ml phosphate buffer (pH 6.8) maintained at 37° and filled in receptor compartment. Clean rat skin (3 cm long) was glued to the surface of a glass slab, vertically attached to the apparatus. The film was hydrated from one surface using phosphate buffer (pH 6.8) and then the hydrated surface was brought into contact with the skin. Glass slab was vertically fixed to the apparatus and allowed to move up and down such that the film was completely immersed in the buffer solution at the lowest point and was out at the highest point [15]. Time necessary for complete erosion or detachment of the film from the skin surface was recorded. Mean and standard deviation of three such readings were reported.
Mucoadhesive strength
A modified balance method was used for determining the mucoadhesive strength. Cellophane membrane, previously treated with 0.1 N NaOH, was cut into pieces. Two pieces of cellophane membrane were tied to the two wooden pieces separately. One wooden piece was fixed on the sieve and other piece was tied with the balance on right hand side. The right and left wooden pieces were balanced by adding extra weight on the left hand wooden piece.
Film was placed between these pieces, and extra weight from the left pan was removed to sandwich the two pieces of cellophane membrane. Gentle pressure was applied to remove the presence of air. After allowing a contact time of 5 min, water was added at 1ml/min to the left–hand pan until the film was detached from the membrane surface. Weight of water required to detach the film from the membrane surface provided the measure of mucoadhesive strength [16,17]. The mucoadhesive strength was calculated by using the Eqn., force of adhesion=mucoadhesive strength (g)×9.81/1000.
In vitro drug release
Determination of drug release rates from different film formulation was carried out using a diffusion cell. Complete media replacement method was used to maintain sink condition throughout the study period [18]. Egg shell membrane previously soaked for 24 h in phosphate buffer (pH 6.8) was stretched around one end of the tube. Donor compartment was assembled into a glass beaker (receptor compartment) with the membrane just touching the receptor medium. One cm2 film was introduced into the donor tube [19]. Receptor compartment contained 10 ml phosphate buffer (pH 6.8), which was thermostatically adjusted to 37±0.5° and stirred at 50 rpm. Dissolution media was replaced with fresh buffer medium at every specific predetermined time interval. Drug was analyzed spectrophotometrically using a UV spectrophotometer (Shimadzu 1800, Japan) at 261 nm. Cumulative percent of drug released was plotted against time for the different film formulations.
Scanning electron microscopy
Morphological structure of bioadhesive films was studied by SEM analysis. The dry films were gold coated to about 5 μm thickness using an IB-2 coater unit under a high vacuum [20]. After that, films were examined using SEM (JSM-5610 LV).
Accelerated stability study
Stability studies were done as per ICH guidelines. The formulated films were wrapped in aluminium foil and stored at 40±0.5° and 75±5% relative humidity for period of three months. After the period of three months, films were tested for their physico-chemical and in vitro release characteristics [21].
Results and Discussion
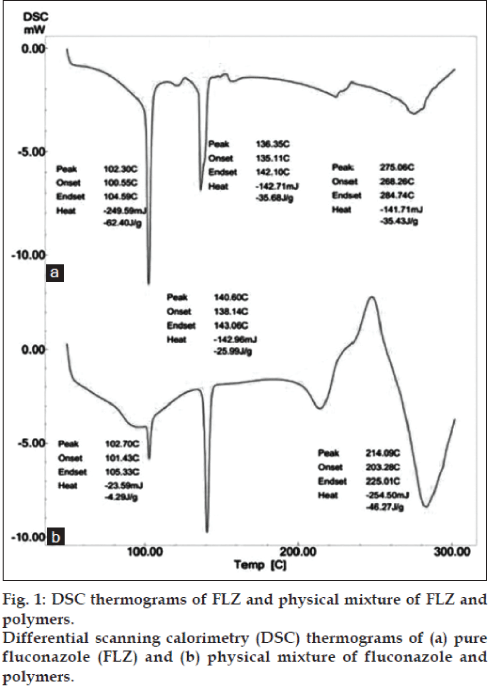
DSC thermograms revealed a sharp characteristic, endothermic melting peak of FLZ at 136.35° (fig. 1a), which was indicative of the pure state of the drug. Recrystallized forms of FLZ showed an additional endothermic peak at 102.30° which could be due to effect of bound solvent used during recrystallization. Certain amount of solvent remains as bound solvent within the crystal lattice which cannot be removed through normal drying procedure. In case of physical mixtures of drug and polymer (SA and PVA) a sharp endothermic peak of the drug was observed at the melting temperature 140.60° (fig. 1b). These results demonstrated that FLZ did not interact with the chosen additives [22].
Physical observation revealed that the films possessed smooth surface. They were transparent and elegant in appearance. Thickness of films ranged from 0.093±0.006 to 0.147±0.006 mm. Folding endurance was found optimum and the films exhibited good physical and mechanical properties. Weight of the film was in the range of 15.000±0.577 to 19.333±0.577 mg. Drug content was found to be in the range of 87.025%±3.072 to 102.775±3.929% suggesting that drug was uniformly dispersed throughout all prepared films.Significantly lower drug content was observed in the batches F6 and F7. Besides, they exhibited higher amount of surface-bound drug. This highlighted the fact that appropriate proportion of sodium alginate (more than 0.5% w/v) was essential into the films in order to contain the entire dose. Except batches F2 and F7, pH values were found to be close to 7. Films composed of solely PVA exhibited significantly lower pH. This could be attributed to the residual effects of acidic functional groups. So, it is expected that films will not cause any irritation upon their application. The results of all parameters have been given in Table 2. The standard deviation value calculated for all parameters is less which suggests that the results are reproducible and reflects the appropriateness of the method used to prepare the films.
| Formulation | Film thickness | Folding endurance | Weight uniformity | Surface pH | Drug content |
|---|---|---|---|---|---|
| code | (mm±SD) | (±SD) | (mg±SD) | (±SD) | (%±SD) |
| F1 | 0.093±0.06 | 262.33±6.50 | 19.33±0.57 | 7.63±0.03 | 100.35±1.60 |
| F2 | 0.117±0.01 | 263.33±4.04 | 17.33±1.15 | 6.33±0.02 | 97.85±2.03 |
| F3 | 0.117±0.00 | 282.33±4.50 | 17.00±1.00 | 6.99±0.03 | 95.35±1.77 |
| F4 | 0.117±0.01 | 309.66±5.50 | 17.66±0.57 | 7.67±0.08 | 102.77±3.92 |
| F5 | 0.097±0.01 | 318.66±8.02 | 15.66±0.57 | 6.81±0.06 | 99.85±2.75 |
| F6 | 0.147±0.00 | 301.33±4.50 | 15.00±1.00 | 7.62±0.04 | 93.35±3.12 |
| F7 | 0.103±0.01 | 304.33±10.59 | 18.000±1.00 | 6.11±0.04 | 87.02±3.07 |
| FLZ :Fluconazole. All estimations were performed in triplicate | |||||
Table 2: Evaluation Of Bioadhesive Films Of Flz
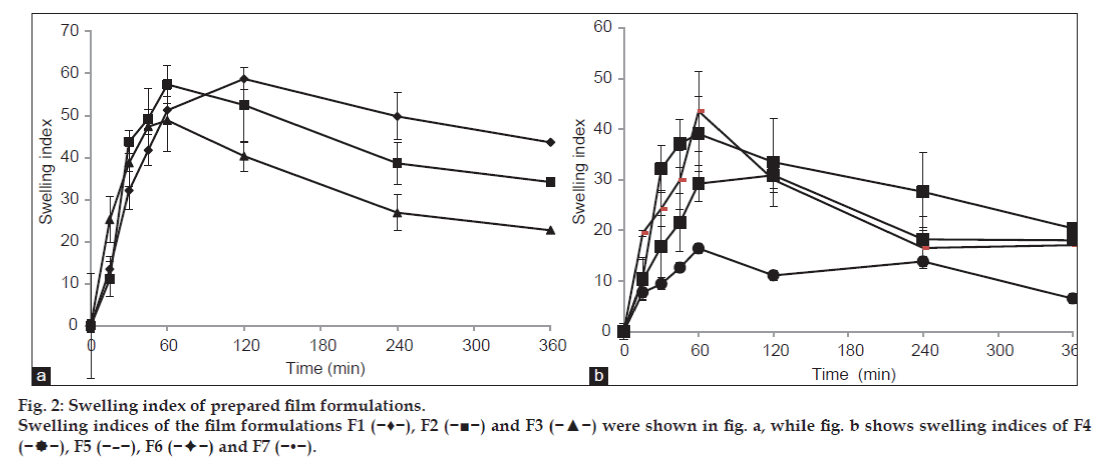
The degree of swelling of bioadhesive polymers is an important factor affecting adhesion. Adhesion occurs shortly after the beginning of swelling but the bond formed is not very strong [12]. Uptake of water results in relaxation of the originally stretched or entangled polymer chains. Consequently, bioadhesive sites within the polymer are exposed. Faster is the swelling of the polymer, faster is the initiation of diffusion and formation of adhesive bonds. In our study, the focus has been to achieve a quick rate of hydration with optimized swelling characteristics. An optimum combination of PVA and SA was selected to ensure a proper balance of hydration, swelling and mucoadhesive strength.
Maximum swelling was observed with the formulation F1 containing 3% SA (P<0.05, fig. 2). This may be due to high swelling nature of SA. Lowest swelling was observed with the formulation F7 containing 3% PVA (P<0.05; one way ANOVA). Initially swelling index of film increased but after certain time point swelling index decreased which could be due to dissolution of films in media. As a general trend, it was observed that as the concentration of SA increased, swelling index increased. Further, swelling index decreased with increase in PVA concentration.
The small swelling capacity of PVA film may be due to the crystalline nature of PVA which could possibly have decreased its hydration and subsequently, swelling rate. Addition of SA (bioadhesive polymer) greatly increased the swelling capacity of formulated films. Addition of SA initially increased the swelling, which led to arrival of a state of equilibrium. This was followed with a decrease in the swelling capacity. So, it was concluded that as the concentration of PVA increased in the films, their swelling capacity decreased.
Ex vivo residence time of all formulation batches was found to be in the range of 286.667±6.506 to 469.667±4.509 h and no significant difference was observed among various batches (P>0.05; one way ANOVA, Table 3). Batch F1 containing 3% SA showed highest residence time as compared to all other formulations due to its bioadhesive nature but it did not differ significantly. Films composed of 3% PVA showed lowest value of residence time. So, it was concluded that as the concentration of SA increased, ex vivo residence time of films also increased. Besides, ex vivo residence time decreased with increase in the concentration of PVA.
| Formulation | Ex vivoresidence | Mucoadhesive |
|---|---|---|
| code | time (h±SD) | strength (N±SD) |
| F1 | 469.66±4.50 | 0.275±0.02 |
| F2 | 348.33±6.02 | 0.376±0.01 |
| F3 | 325.33±6.50 | 0.370±0.00 |
| F4 | 378.66±7.66 | 0.193±0.08 |
| F5 | 403.33±5.50 | 0.297±0.01 |
| F6 | 301.33±10.01 | 0.191±0.00 |
| F7 | 286.66±6.50 | 0.185±0.01 |
| Mean±SD (n=3) |
Table 3: Ex Vivo Residence Time And Mucoadhesive Strength Of All Films
Mucoadhesive strength was found to be dependent on the polymer concentration (Table 3). For all the batches, it was found to be in the range of 0.185±0.011 to 0.376±0.016 N. It can be concluded that as the concentration of SA increased in the film, higher force was required to detach film from rat skin. As the concentration of PVA increased, mucoadhesive strength decreased. Maximum mucoadhesive strength was found to be with the formulation containing almost equal proportion of polymers and this value remained significantly higher as compared to other batches (P<0.05; one way ANOVA). Batches prepared from 3% PVA alone showed significantly lowest value of mucoadhesive strength as compared to other formulation batches (P<0.05; one way ANOVA).
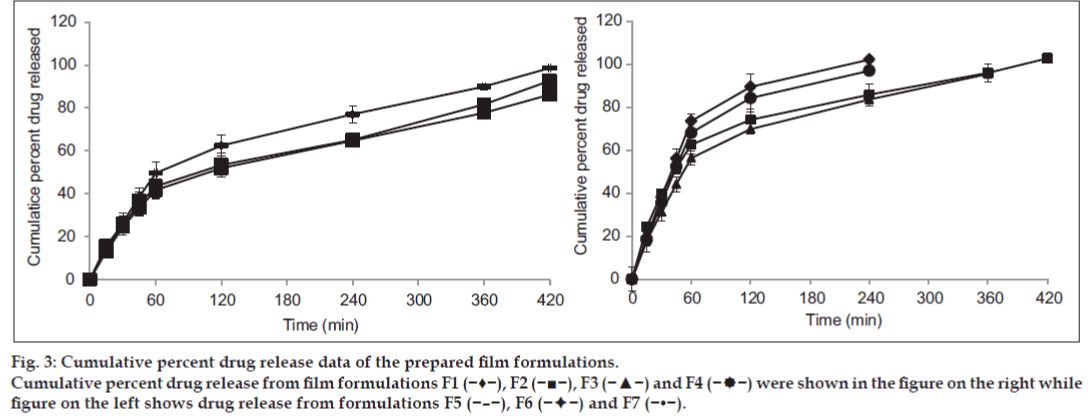
From the results, it was found that the drug release was dependent on the concentration of PVA and also on the viscosity of polymeric solution (fig. 3). With increase in PVA concentration, the drug release through films was decreased which may be due to the formation of dense network of the polymer. This in turn decreases the drug release rate from the film. Films composed of higher proportion of SA resulted into faster drug release which could be due to higher swelling and erosion tendency of the polymer.
Fig. 3: Cumulative percent drug release data of the prepared film formulations.
Cumulative percent drug release from film formulations F1 (−♦−), F2 (−■−), F3 (−▲−) and F4 (− −) were shown in the figure on the right while
figure on the left shows drug release from formulations F5 (−–−), F6 (−
−) were shown in the figure on the right while
figure on the left shows drug release from formulations F5 (−–−), F6 (− −) and F7 (−•−).
−) and F7 (−•−).
F1 and F2 batches showed complete drug release within 4 h due to the high concentration of SA. While F3 batch showed fairly controlled release rate which lasted up to 6 h. This might have happened due to decrease in the proportion of SA and increase in the proportion of PVA. From batch F4 to F7, the drug release was extended up to 8 h. F7 batch was prepared with 3% PVA. So drug release was found to be 86.20% within 8 h (P<0.05). So, it was concluded that due to PVA there was an extended drug release from the formulation.
To ascertain the drug release mechanism of the formulations, in vitro drug release data were subjected to kinetic analysis [23]. The data was plotted as Higuchi diffusion plots by taking cumulative percent drug release versus square root of time. The plots were found to be fairly linear and it was well supported by their regression coefficient values (Table 4). The formulations were tested for Peppas exponential plots by taking log percentage drug release versus log time. The correlation coefficient values (r2) indicated that the kinetics of drug release followed Higuchi model. The mechanism of drug release by Peppas model indicated non-Fickian transport.
| Formulation code | Zero order (r2) | First order (r2) | Higuchi plot (r2) | Hixon Crowell (r2) | Peppas plot (r2) | Mechanism of release | |
|---|---|---|---|---|---|---|---|
| n value | |||||||
| F1 | 0.7511 | 0.5755 | 0.8784 | 0.0009 | 0.8895 | 0.5921 | Non-Fickian transport |
| F2 | 0.7670 | 0.5877 | 0.8907 | 0.0335 | 0.8975 | 0.5963 | Non-Fickian transport |
| F3 | 0.8347 | 0.6474 | 0.9341 | 0.1129 | 0.9274 | 0.5526 | Non-Fickian transport |
| F4 | 0.8449 | 0.6820 | 0.9328 | 0.8540 | 0.9211 | 0.5077 | Non-Fickian transport |
| F5 | 0.8936 | 0.6899 | 0.9637 | 0.9607 | 0.9246 | 0.6052 | Non-Fickian transport |
| F6 | 0.9371 | 0.7726 | 0.9777 | 0.9645 | 0.9426 | 0.5066 | Non-Fickian transport |
| F7 | 0.9175 | 0.7228 | 0.9744 | 0.9711 | 0.9292 | 0.5624 | Non-Fickian transport |
Table 4: Kinetic Data Analysis Of Flz Bioadhesive Film
SEM analysis of the optimized batch was carried out using SEM (JSM-5610 LV). SEM micrograph indicated uniform dispersion of drug with polymer dispersion (fig. 4). Formulation appeared to be smooth with no roughness on its surface. This is conducive in terms of offering better appearance and therefore, patient acceptability.
Sample kept in stability chamber was evaluated for surface pH, drug content, swelling study and in vitro drug release. Surface pH was found to be nearly neutral. Percent drug content was found to be 94.858±1.422%. Force of adhesion was found to be 0.278±0.025 N, drug release was found to be 90.78±2.97% within 8 h and it followed Higuchi model. No significant changes were observed in the formulation for any of the parameters which were tested. So, it was concluded from the study that prepared formulations were stable under accelerated storage conditions.
In conclusion, bioadhesive films of FLZ were developed to overcome the problems of high dose requirement of FLZ which is primarily due to its high volume of distribution and first pass metabolism. Results showed that prepared films were uniform in weight and thickness. Optimized formulation (F5) exhibited no significant change in its characteristics under accelerated stability conditions. From the present research work, it can be concluded that bioadhesive films are one of the better alternatives to be considered for administration of FLZ with a low dosage requirement.
References
- Nairy HM, Charyulu NR, Shetty VA, Prabhakara P. A Pseudo-randomised clinical trial of in situ gels of fluconazole for the treatment of oropharngeal candidiasis. Trials 2011;12:12-99.
- Murray PA, Koletar SL, Mallegol I, Wu J, Moskovitz BL. Itraconazole oral solution versus clotrimazole troches for the treatment of oropharyngeal candidiasis in immunocompromised patients. ClinTher 1997;19:471-80.
- Bachhav YG, Patravale VB. Microemulsion-based Vaginal Gel of Fluconazole: Formulation, In vitro and In vivo Evaluation. Int J Pharm 2009;365:175-9.
- Meis J, Petrou M, Bille J, Ellis D, Gibbs D. A global evaluation of the susceptibility of candida species to fluconazole by disk diffusion. Diagn Microbiol Infect Dis 2000;36:215-23.
- Mohan H. Textbook of pathology. 5th ed. New Delhi: Jaypee Brothers, Medical Publishers; 2005. p. 188-90.
- Gupta M, Tiwari S, Vyas SP. Influence of Various Lipid Core on Characteristics of SLNs Designed for Topical Delivery of Fluconazole against Cutaneous Candidiasis. Pharm DevTechnol 2013;18:550-9.
- Gupta M, Goyal AK, Paliwal SR, Paliwal R, Mishra N, Vaidya B, et al. Development and Characterization of Effective Topical LiposomalSystem for Localized Treatment of Cutaneous Candidiasis. J Liposome Res 2010;20:341-50.
- Nesseem DI, Eid SF, El-Houseny SS. Development of noveltransdermal self-adhesive films for tenoxicam: An antiinflammatory drug. Life Sci 2011;89:430-8.
- Saroha K, Yadav B, Sharma B. Transdermal patch: A discrete dosage form. Int J Curr Pharm Res 2011;3:98-108.
- Patel HJ, Patel JS, Desai BG, Patel KD. Design and Evaluation of Amlodipine Besylate Transdermal Patches Containing Film Former. Int J Pharm Res Dev 2009;7:1-12.
- Sakalle P, Dwivedi S, Dwivedi A. Design, Evaluation, Parameters and Marketed Products of Transdermal Patches: A Review. J Pharm Res 2010;3:235-40.
- Yehia SA, El-Gazayerly ON, Basalious EB. Fluconazole MucoadhesiveBuccal Films: In vitro/in vivo Performance. Curr Drug Deliv 2009;6:17-27.
- Nair AB, Kumria R, Harsha S, Attimarad M, Al-Dhubiab B, Alhaider IA. In vitro Techniques to Evaluate Buccal Films. J Control Release 2013;166:10-21.
- Semalty M, Semalty A, Kumar G. Formulation and Characterization of MucoadhesiveBuccal Films of Glipizide. Indian J Pharm Sci 2008;70:43-8.
- Sudhakar Y, Kuotsu K, Bandyopadhyay AK. BuccalBioadhesive Drug Delivery - A Promising Option for Orally Less Efficient Drugs. J Control Release 2006;114:15-40.
- Koland M, Charyulu R, Prabhu P. Mucoadhesive films of losartan potassium for buccal delivery: Design and characterization. Indian J Pharm Educ Res 2010;44:315-23.
- Patel VM, Prajapati BG, Patel MM. Design and Characterization of Chitosan Containing MucoadhesiveBuccal Patches of Propranolol Hydrochloride. Acta Pharm 2007;57:61-72.
- D’Souza SS, DeLuca PP. Methods to Assess In vitro Drug Release from Injectable Polymeric Particulate Systems. Pharm Res 2006;23:460-74.
- Morales JO, McConville JT. Manufacture and Characterization of MucoadhesiveBuccal Films. Eur J Pharm Biopharm 2011;77:187-99.
- Guo R, Du X, Zhang R, Deng L, Dong A, Zhang J. Bioadhesive Film Formed from a Novel Organic–inorganic Hybrid Gel for Transdermal Drug Delivery System. Eur J Pharm Biopharm 2011;79:574-83.
- Doijad R, Manvi F, Rao M, Patel P. Buccoadhesive Drug Delivery System of IsosorbideDinitrate: Formulation and Evaluation. Indian J Pharm Sci 2006;68:744-8.
- Modha NB, Chotai NP, Patel VA, Patel BG. Preparation, Characterization and Evaluation of Fluconazole Polymorphs. Int J Res Pharm Biomed Sci 2010;1:124-7.
- Dash S, Murthy PN, Nath L, Chowdhury P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol Pharm 2010;67:217-23.