- *Corresponding Author:
- K. N. Killari
A. U. College of Pharmaceutical Sciences, Andhra University, Visakhapatnam-530 003, India
E-mail: kishorenaidu.killari@gmail.com
| Date of Received | 30 Septemebr 2019 |
| Date of Revision | 10 January 2020 |
| Date of Acceptance | 28 February 2020 |
| Indian J Pharm Sci 2020;82(2):379-384 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The chemical investigation of acetone extract of manglicolous lichen Ramalina leiodea yielded three known metabolites, methyl 2,6-dihydroxy-4-methyl benzoate (1), haematommic acid (2) and ethyl haematommate (3), which are reported for the first time in this species. The acetone extract and the metabolites (1-3) were screened for antioxidant activity in α,α-diphenyl-β-picrylhydrazyl, 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) and superoxide free radical assays, for antiinflammatory activity in pretein denaturation assay and for anticancer activity in sulforhodamine B assay on lung, head and neck, and cervical cancer cells. The results showed that compounds 2 and 3 depicted inhibitory profiles against 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) free radical with an IC50 of 40.0 and 40.5 µg/ml, respectively and caused protein denaturation with an IC50 of 435 and 403 µg/ml, respectively. Furthermore, compounds 2 and 3 exhibited a significant degree of specificity against cervical, head and neck, and lung cancer cells, while these compounds showed little toxicity against normal human mammary epithelial cell line. In summary the manglicolous lichen Ramalina leiodea possessed free radical scavenging, antiinflammatory, and anticancer activities and the main metabolites responsible for these activities could be compounds 2 and 3.
Keywords
Antioxidant; protein denaturation; antiinflammatory, anticancera
Lichens is a symbiotic organism belongs to bryophytes that have an ability to persevere on any geographical region or any substratum[1]. Due to their unique survival and mutualistic characteristics, lichens and their secondary metabolites are used to treat several infections and diseases[2]. The lichens that particularly associated with mangroves or mangals are termed as manglicolous lichens[3]. Lichen and their secondary metabolites exert a varied range of biological actions that include analgesic, antibiotic, antiinflammatory, antimycotic, antipyretic, antiviral, and cytotoxicity[2,4,5]. Especially, manglicolous lichens show a difference in their biological components and actions compared to normal lichens due to their physiological adaptation towards the intertidal zone i.e., having both the marine and freshwater environments[6]. As mangals persist in a stressful environment such as high concentration of` moisture and salt, low and high tidal water, lichens habituated on these plants also exposed to these stress conditions. As a result, they show a difference in phytochemical constituents than normal lichens due to stressed physiological adaptations[2,6,7]. Besides, there are very few chemical and pharmacological reports that exist on manglicolous lichens due to their slow growth (1 cm/y) and difficulty in collecting a good amount of specimen from mangrove regions[8-10]. Ramalina genus has about 246 species distributed around the world, of which only 118 species were investigated for their chemical and biological properties[11,12]. The diverse secondary metabolites isolated include usnic acid derivatives, depsides, depsidones, fatty acids, sterols, and monocyclic aromatic compounds[11-19]. Moreover, biological screening of this genus resulted in the identification of antibiotics, antimutagenic, antiHIV, enzyme inhibitory, antioxidant, antiinflammatory, anticancer, antimicrobial activities[5,11,14-21]. Ramalina leiodea is a corticolous fruticose lichen that belongs to the Ramalina genus. Earlier, our group reported the phytochemical analysis, antimicrobial, antimycobacterial, and antiinflammatory activities of various extracts of R. leiodea[5,11]. In continuation of the reported work, the present study was taken up to identify the bioactive constituents present in R. leiodea.
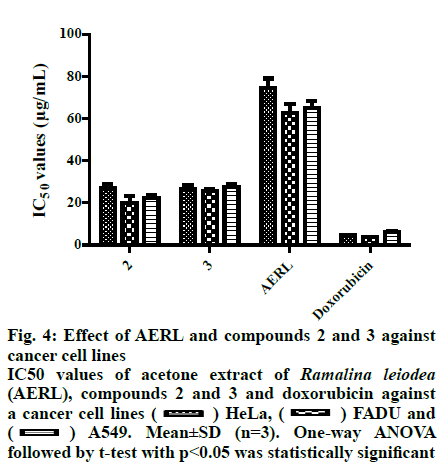
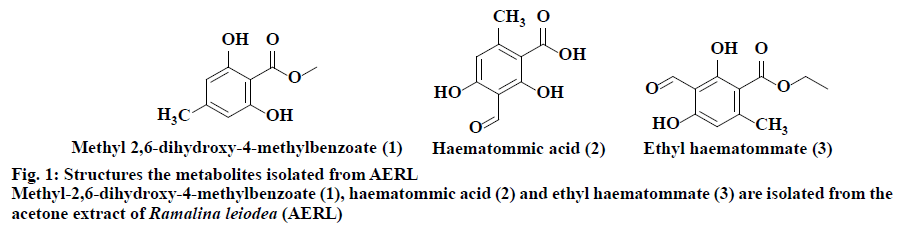
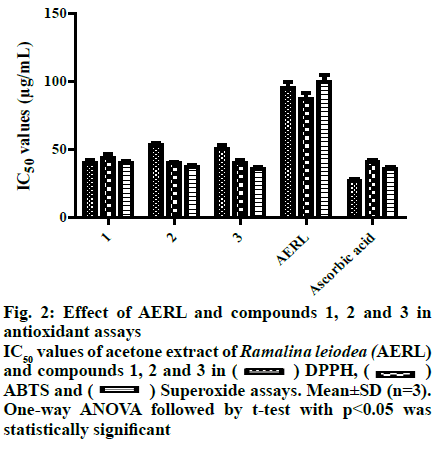
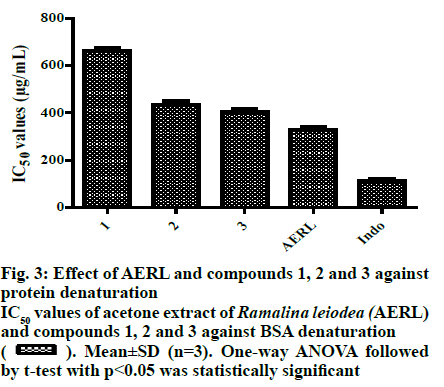
From the twigs of the mangrove plant, Excoecaria agallocha specimens of manglicolous Ramalina leiodea Bel em. D. D. Awasthi was collected from Bhitharkanika Island, Rajnagar, Orissa, India (20º74’N and 86º87’E at 0 m elevation) in April 2019. The species was determined and a voucher specimen (16-027175) was deposited at the Lucknow Lichen herbarium, National Botanical Research Institute, Lucknow, India[3]. The collected manglicolous lichen Ramalina leiodea was shade dried, and about 100 g of dried lichen material was exhaustively extracted with acetone. The acetone extract of Ramalina leiodea (AERL) obtained, 4.6 g, 4.6 % based on total lichen material was subjected to column chromatography (CC) (#230-400) using n-hexane in ethyl acetate (EA) (increasing polarity) as eluent, which eventually resulted in 3 fractions. Fraction I was further subjected to CC (#230-400) using n-hexane in EA (increasing polarity) as eluent, yielded metabolite 1 (2.4 g, 2.4 % based on total lichen material) as pale yellow crystals and metabolite 2 (150 mg, 0.15%) as pale yellow needles. Fraction II was retreated with CC (#230-400) using dichloromethane in EA (increasing polarity) as eluent, yielded metabolite 3 (90 mg, 0.09 %) as a greenish solid. The metabolites (1-3) and AERL were tested in the α,α-diphenyl-2- picrylhydrazyl (DPPH) assay in triplicate and results were reported as the % inhibition of DPPH free radical[22,23]. Initially, to a known concentrations of the sample 0.004 % DPPH dissolved in methanol was added and incubated for 30 min at 37º. Using UV/Vis spectrophotometry (Spectra MAX plus 384, USA), the absorbance of all samples was measured at 517 nm against the blank. Plotting concentration against % inhibition determined IC50 values of the metabolites (1-3) and AERL. The metabolites (1-3) and AERL were tested in the 2,2’-azino-bis(3-ethylbenzothiazoline-6- sulphonic acid) (ABTS) assay in triplicate and results were reported as % inhibition of ABTS free radical[23]. To 7 mM ABTS+?, 2.45 mM potassium persulfate was added at room temperature and standardized. Samples were added to 1 ml of above-standardized solution and incubated for 30 min and the absorbance of all samples was measured at 750 nm against a blankand the IC50 values of the metabolites (1-3) and AERL were determined. The metabolites (1-3) and AERL were further subjected to superoxide radical scavenging assay in triplicate, and results were reported as % inhibition of superoxide free radicals[24]. NADH (73 μM) was added to 15 μM PMS and 50 μM NBT in 20 mM phosphate buffer (pH 7.4) and standardized. Then know concentrations of sample was added to 1 ml of the above-standardized solution and incubated for 30 min. After incubation, the absorbance of all the samples were measured at 562 nm against the blank and IC50 values of the metabolites (1-3) and AERL were calculated. In vitro antiinflammatory activity of metabolites (1-3) and AERL was evaluated using the protein denaturation method[2,5] in triplicate and results were expressed as % inhibition of protein denaturation against blank. Bovine serum albumin (BSA, 1%) was dissolved in sodium phosphate buffer (50 mM, pH 6.4) and know concentrations of sample were added 0.2 ml of and makeup to 5 ml with sodium phosphate buffer and incubated for 20 min at 37º. Later, all the samples were boiled for 20 min in a steam bath at 95° and set to room temperature. After incubation, all the samples were observed absorbance at 562 nm against the blank. Plotting concentrations against % inhibition determined IC50 values of the metabolites (1-3) and AERL. All in vitro assay test results were expressed as mean±SD. Using one-way analysis of variance (ANOVA) followed by the t-test test with p<0.05 statistically significantresults were determined. Lung cancer cells (A549), cervical (HeLa) and head and neck (FADU) cancer cells and normal human mammary epithelial (NHME) cell lines were obtained from the National Centre for Cell Science, Pune. All the cancer cell lines were preserved in minimal essential medium (MEM), which contained 5% mixture of streptomycin (100 μg/ ml) and penicillin (100 units), fetal calf serum (10 %) in presence of CO2 (5%) with 90 % humidity for 72 h at 37º. Three days earlier to assay, selected cancer cell lines were maintained in MEM and grown on 10 % FBS supplemented with trypsin (0.25%). In a sterilized polypropylene tube, the final suspension of cancer cells was taken, and the concentration of the cells in each well was calculated using a 0.4% trypan blue solution in a hemocytochameter chamber under a microscope. The minimal concentration of 1×104 cells per well was used as the nominal seed density. The samples were dissolved in dimethyl sulfoxide (DMSO), which is used as a control and doxorubicin as the standard. Primary screening of the samples was performed at 100 μg/ml for AERL, 30 μg/ml for compounds 1-3 and 10 μg/ml for doxorubicin. Active samples were further screened at different concentrations (25, 50, 75 and 100 μg/ml) for AERL, 5, 10, 20 and 30 μg/ml for compounds 1-3 and 2.5, 5.0, 7.5 and 10 μg/ml for doxorubicin against particular cancer cells. The anticancer activity of AERL and compounds 1-3 was determined using the SRB assay in triplicate[4,26]. In a 96-well plate, 190 μl screened ideal cancer cells suspension and test samples were added and incubated under 90 % relative humidity, 5% CO2 for 3 h at 37º. Formerly to each well 100 μl of cold TCA was added and incubated for 1 h at 4º. After that the 96-well plate was gently washed with water and airdried at 25º. Next, to each well, 100 μl of 0.057% SRB solution was added, incubated for 30 min and stained with CH3COOH (1%). Then 200 μl of Tris base (10 mM, pH 10.5) was added to each well, agitated for a few minutes and the optical density was measured at 510 nm. The control contained only cancer cells, whereas blank contained only MEM medium. The % growth inhibition was determined using the formula, % growth inhibition=100-(absorbance of sample/ absorbance of control)×100. Chemical investigation of AERL yielded compounds 1-3, which are illustrated in fig. 1. The chemical structures of metabolites 1-3 were characterized by elemental analysis, 1H and 13C NMR, and mass spectral data, and competing with the existing literature data[27]. Methyl-2,6-dihydroxy-4-methylbenzoate (1), pale yellow crystals, Rf: 0.6 (1:1 hexane:EA), mp: 138-139º, UV (λmax): 219.5 nm in methanol, molecular formula: C9H10O4; 1H NMR (400 MHz, DMSO-d6): δ 2.23 (s, 3H), 3.75 (s, 3H), 6.12 (d, 2H, J= 1.2 Hz), 9.93 (s, 1H), 10.65 (s, 1H). 13C NMR (400 MHz, DMSO-d6): δ 22.46 (C-9), 52.16 (C-8), 100.83 (C-1), 107.93 (C-5), 110.58 (C-3), 141.15 (C-4), 161.46 (C-2/C-6), 170.59 (C-7). Elemental analysis: found C-59.66, H-5.62(%), calcd. C, 59.34, H, 5.53(%). ESI-MS negative mode: m/z 183.0 ([M-H+], 68.81 %). Haematommic acid (2), pale yellow needles, Rf: 0.4 (1:1 hexane:EA), m.p: 172-173º, UV (λmax): 219.5 nm in ethanol, molecular formula: C9H8O5; 1H NMR (400 MHz, DMSO-d6): δ 2.54 (s, 3H), 6.42 (s, 1H), 9.68 (s, 1H), 10.59 (s, 1H), 11.46 (s, 1H), 13.75 (s, 1H) (fig. S4). 13C NMR (400 MHz, DMSO-d6): δ 17.10 (C-9), 105.25 (C-1), 109.34 (C-3/C-5), 155.22 (C-6), 163.90 (C-4), 167.04 (C-2), 173.42 (C-7), 191.73 (C-8). Elemental analysis: found C-55.64, H-4.52(%), calcd. C-55.11, H-4.11(%). ESI-MS negative mode: m/z 198.3 ([M-H+], 5.64 %). Ethyl haematommate (3), greenish solid, Rf: 0.6 (7:3 DCM:E), m.p: 112-113º, UV (λmax): 209.5 nm in methanol, molecular formula: C11H12O5; 1H NMR (400 MHz, DMSO-d6): δ 0.93-0.97 (t, 3H), 1.59-1.65 (m, 2H), 2.54 (s, 3H), 6.42 (s, 1H), 9.68 (s, 1H), 10.59 (s, 1H), 11.46 (s, 1H), 13.75 (s, 1H). 13C NMR (400 MHz, DMSO-d6): δ 14.28 (C-11), 20.14 (C-9), 68.63 (C-8), 106.38 (C-1), 109.34 (C-5), 114.78 (C-3), 155.22 (C-6), 163.90 (C-4), 167.04 (C-2), 173.42 (C-7), 191.73 (C-8). Elemental analysis: found C-58.64, H-5.52(%), calcd. C-58.93, H-5.39(%). ESI-MS negative mode: m/z 224.9 ([M-H+], 31.36%). In DPPH assay, reduction the DPPH free radicals to a DPPH-H (non-radical) form by antioxidant substance has taken place[28]. As shown in fig. 2, IC50 value of ascorbic acid on DPPH free radicals was 27.0 μg/ml. Furthermore, the IC50 values of 1, 2, 3 and AERL were determined to be 40.0, 53.0, 50.5 and 95.0 μg/ml, respectively. In ABTS radical assay, radical cation ABTS?+ is decoyed[28]. As shown in fig. 2, the IC50 value of ascorbic acid on ABTS free radicals was 41.0 μg/ml. Among all samples, 2 and 3 showed better IC50 than that of the standard. The IC50 values against ABTS radical were, 40.0 μg/ml (2)>40.5 μg/ml (3)> 43.5 μg/ml(1)>87.0 μg/ml(AERL). Generally, the superoxide free radicals are generated from biological metabolisms interrelate with chemical species, i.e. substrates in the occurrence of metallic or enzymatic catalyzed routes to produce 1O2 and OH radical[26,29]. These superoxide radicals influence oxidative impairment in lipids, DNA, as well as proteins. The superoxide free radical assay of all the prepared samples was presented in Table S3. As shown in fig. 2, the concentration of 1, 2, 3 and AERL required for 50% inhibition of superoxide free radicals were found to be 40.0, 37.50, 36.0 and 99.50 μg/ml, respectively, whereas the standard was 35.5 μg/ml. The root cause for inflammation is biological protein denaturation, which occurs by alkaline/acidic/radiation reactions and heat treatment[2,5]. Therefore, in the current work, AERL and compounds 1-3 from Ramalina leiodea were tested for the inhibition of albumin protein denaturation by heat. The results of in vitro antiinflammatory assay were presented in fig. 3, which indicated that all isolates showed significant antiinflammatory activity. The IC50 values of 1, 2, 3 and AERL on protein denaturation were determined to be 664, 435, 403 and 330 μg/ml, respectively, whereas indomethacin was 110 μg/ml (fig. 3). In general, chronic inflammation is the root cause for numerous lethal disorders and diseases, including cancer. Additionally, compounds (1-3) displayed better antiinflammatory activities, hence compounds 1-3 and AERL were screened for anticancer activity and the IC50 value were presented in fig. 4. The lower IC50 value indicate improved inhibitory activity against cancer cells. From the primary evaluation, among the metabolites of AERL, only compounds 2 and 3 displayed a reasonable degree of specificity towards cancer cells tested. Moreover, AERL and its metabolites showed very little effect on normal NHME cell lines indicating lack of cytotoxicity. It was evident that AERL showed a more pronounced degree of specificity against HeLa, FADU, and A549 with IC50 values of 74.5, 62.5, and 64.9 μg/ml, respectively. Further screening of the isolates obtained from this extract showed a significant inhibitory profile against all the experimented cancer cells. Compounds 2, 3 and standard yielded IC50 values of 27.0, 26.5 and 4.5 μg/ml on HeLa, 20.0, 25.5 and 3.8 μg/ml on FADU and 22.5, 27.5 and 6.3 μg/ml on A549, respectively. In the present study, chromatographic examination of AERL yielded three monoaromatic compounds (1-3) substituted with a hydroxyl group(s), which are confirmed by UV, NMR, Mass spectral and elemental analysis (fig. 1). All the isolated metabolites and AERL were screened for antioxidant activity using DPPH, superoxide and ABTS free radicals assays, in vitro antiinflammatory activity in the protein denaturation assay, and anticancer activity using the SRB assay. From the antioxidant and in vitro antiinflammatory outcomes it could be concluded that AERL and samples showed prominent inhibitory activities against DPPH, superoxide and ABTS free radicals, and protein denaturation, which could be probably due to the presence of phenolics, carboxylic acids (figs. 2 and 3). The results of the current research indicated that AERL exhibited antiinflammatory capability. Therefore, outcomes of current research explained the application of AERL in folklore medicine for managing both acute and chronic inflammation. It is likely that AERL could have the ability to block the biosynthesis of thromboxane (TXA2), prostanoids (PGE2, PGF2α, PGD2, PGI2), and Interluekin-8[2,5]. Metabolites (1-3) and AERL were screened for anticancer activity on HeLa, FADU and A549 cancer cells and NHME cells. The results indicated that AERL and compounds 2 and 3 exhibited anticancer activity. As the redox reactions leading to free radical production and chronic inflammation could be involved in the pathogenesis of cancer, and since the metabolites from AERL exhibited antioxidant and antiinflammatory activity, it is evident that the anticancer activity of these compounds could be attributed to these activities. Moreover, AERL and its metabolites showed a very little degree toxicity against normal cell line tested. Therefore, metabolites (1-3) could provide useful leads to design effective anticancer agents. The present research work is a preliminary study of chemical and biological evaluation of manglicolous lichen R. leiodea. Chemical investigation of acetone extract of R. leiodea resulted in the isolation of three known metabolites, methyl 2,6-dihydroxy-4-methyl benzoate (1), haematommic acid (2) and ethyl haematommate (3), whose structures were confirmed by spectral data. The pharmacological evaluation revealed the inhibitory capabilities of R. leiodea against DPPH free radicals, superoxide free radicals, ABTS free radicals, albumin protein denaturation and antiproliferative activity on HeLa, FADU and A549. Moreover, compounds 2 and 3 appear to be responsible for the activities observed.
Fig. 4: Effect of AERL and compounds 2 and 3 against cancer cell lines
IC50 values of acetone extract of Ramalina leiodea (AERL), compounds 2 and 3 and doxorubicin against a cancer cell lines ( ) HeLa, (
) HeLa, ( ) FADU and (
) FADU and ( ) A549. Mean±SD (n=3). One-way ANOVA followed by t-test with p<0.05 was statistically significant
) A549. Mean±SD (n=3). One-way ANOVA followed by t-test with p<0.05 was statistically significant
Conflict of interest
The authors declare that they have no competing interests.
Acknowledgements
The authors thank the authorities of K L College of Pharmacy, K L Deemed to be University, Koneru Lakshmaiah Educational Foundation, Guntur, India for providing the necessary facilities to complete present work. The authors also acknowledge the support of Dr. D. K. Upreti, Lucknow Lichen herbarium, National Botanical Research Institute, Lucknow, India for determining the species of the lichens used in the study.
References
- Awasthi DD. A compendium of the Macro lichens from India, Nepal and Sri Lanka. Bishen Singh Mahendra Pal Singh, Dehra Dun; 2007.
- Tatipamula VB, Vedula GS. Fibrinolytic, anti inflammatory and anticancer potentialities of extracts and chemical constituents of manglicolous lichen, Graphis ajarekarii Patw. & C. R. Kulk. Nat Prod J 2018;10(1):87-93.
- Bharadwaj VT. New record of mangrove lichens on Andhra Pradesh and Orissa states of India. Studies Fungi 2019;4(1):90-3.
- Haritha P, Patnaik SK, Tatipamula VB. Chemical and pharmacological evaluation of manglicolous lichen Graphis ajarekarii Patw. & C. R. Kulk. Vietnam J Sci Technol 2019;57(3):300-8.
- Tatipamula VB, Vedula GS. In vitro anti inflammatory and cytotoxicity studies of two mangrove associated lichens, Dirinaria consimilis and Ramalina leiodea extracts. Hygeia J D Med 2018;10(1):16-26.
- Bharadwaj VT, Sastry GV, Murthy KS. A note on the occurrence of lichens on Vainateya Godavari mangroves in East Godavari district of Andhra Pradesh India. Studies Fungi 2018;3(1):302-8.
- Tatipamula VB, Vedula GS. Antarvediside A-B from manglicolous lichen Dirinaria consimilis (Stirton) D.D. Awasthi and their pharmacological profile. In: Program Book of abstracts, Youmares 9, Oldenburg, Germany; 2018. p.135.
- Günter S. Review Mangroves and Mountains: Silviculture at Ecological Margins. In: Silviculture in the Tropics. Berlin, Heidelberg: Springer; 2011. p. 299-323.
- Hong PN, San HT. Mangroves of Vietnam. Iucn 1993.
- Huneck S, Yoshimura I. Identification of lichen substances. In: Identification of lichen substances. Berlin, Heidelberg: Springer;1996. p. 11-123.
- Tatipamula VB, Vedula GS. Antimicrobial and anti-tubercular activities of metabolites and semi-synthetic derivatives from lichen Ramalina leiodea (Nyl.) Nyl. J Serb Chem Soc 2019;84(6):555-62.
- Kashiwadani H, Kalb K. The genus Ramalina in Brazil. The Lichenologist 1993;25(1):1-31.
- Stark JB, Walter ED, Owens HS. Method of isolation of usnic acid from Ramalina reticulata. J Am Chem Soc 1950;72(4):1819-20.
- Gulluce M, Aslan A, Sokmen M, Sahin F, Adiguzel A, Agar G, et al. Screening the antioxidant and antimicrobial properties of the lichens Parmelia saxatilis, Platismatia glauca, Ramalina pollinaria, Ramalina polymorpha and Umbilicaria nylanderiana. Phytomedicine 2006;13(7):515-21.
- Marshak A, Barry GT, Craig LC. Antibiotic Compound isolated from the Lichen Ramalina veticulata. Science (Washington) 1947:394-5.
- Paudel B, Bhattarai HD, Lee HK, Oh H, Shin HW, Yim JH. Antibacterial activities of Ramalin, usnic acid and its three derivatives isolated from the Antarctic lichen Ramalina terebrata. Zeitschrift für Naturforschung C 2010;65(1-2):34-8.
- Paudel B, Bhattarai HD, Koh HY, Lee SG, Han SJ, Lee HK, et al. Ramalin, a novel nontoxic antioxidant compound from the Antarctic lichen Ramalina terebrata. Phytomedicine 2011;18(14):1285-90.
- González AG, Barrera JB, Pérez EM, Padrón CE. Chemical constituents of the lichen Ramalina hierrensis. Planta Medica 1992;58(02):214-8.
- Gasulla F, Guéra A, Barreno E. A simple and rapid method for isolating lichen photobionts. Symbiosis 2010;51(2):175-9.
- Esimone CO, Adikwu MU. Antimicrobial activity and cytotoxicity of Ramalina farinacea. Fitoterapia 1999;70(4):428-31.
- Le Pogam P, Herbette G, Boustie J. Analysis of lichen metabolites, a variety of approaches. In: Recent Advances in Lichenology. New Delhi: Springer; 2015. p. 229-61.
- Talluri MR, Ketha A, Battu GR, Tadi RS, Tatipamula VB. Protective nature of Aurelia aurita against free radicals and Streptozotocin-induced diabetes. Bangladesh J Pharmacol 2018;13:287-95.
- Tatipamula VB, Kolli MK, Lagu SB, Paidi KR, Reddy RP, Yejella RP. Novel indolizine derivatives lowers blood glucose levels in streptozotocin-induced diabetic rats: A histopathological approach. Pharmacol Rep 2018;71:233-42.
- Tatipamula VB, Vedula GS, Sastry AVS. Antarvediside A-B from manglicolous lichen Dirinaria consimilis (Stirton) D.D. Awasthi and their pharmacological profile. Asian J Chem 2019;31(4):805-12.
- Tatipamula VB, Killari KN, Ketha A, Vedula GS. Taxithelium napalense has aptitude to act against free radicals and diabetes. Bangladesh J Pharmacol 2017;12:197-203.
- Sastry AVS, Vedula GS, Tatipamula VB. In-vitro biological profile of mangrove associated lichen, Roccella montagnei extracts. Inventi Impact: Ethnopharmacology 2018;2018(3):153-8.
- Huneck S, Yoshimura I. Data of Lichen Substances. In: Identification of Lichen Substances. Berlin, Heidelberg: Springer; 1996. p. 125-446.
- Tatipamula VB, Vedula GS, Paidi KR, Annam SSP. Nutraceutical value of lichens, Graphis ajarekarii and Parmotrema tinctorum and their implications in diabetes. Inventi Impact: Nutraceuticals 2018;2018(3):189-94.
- Tatipamula VB, Haritha P, Rao GSNK, Ketha A, Yejella PR. Isolation and Characterization of metabolites from Clathria procera Ridley extract and Evaluation of its antidiabetic effects in Streptozotocin-induced diabetic rats. J Exp Appl Anim Sci 2019;3(1):35-56.
 ). Mean±SD (n=3). One-way ANOVA followed by t-test with p<0.05 was statistically significant
). Mean±SD (n=3). One-way ANOVA followed by t-test with p<0.05 was statistically significant