- Corresponding Author:
- R. R. Poonuru
St. Peter’s Institute of Pharmaceutical Sciences, No. 2-4-1211, Vidyanagar, Hanamkonda-506 001
E-mail: yuppieraj@gmail.com
| Date of Submission | 17 May 2014 |
| Date of Revision | 28 August 2014 |
| Date of Acceptance | 02 September 2014 |
| Indian J Pharm Sci 2014;76(6):476-482 |
Abstract
Gastroretentive bimodal drug delivery systems of lamotrigine were developed using immediate release and extended release segments incorporated in a hydroxypropyl methylcellulose capsule and in vitro and in vivo evaluations were conducted. In vivo radiographic studies were carried out for the optimized formulation in healthy human volunteers with replacement of drug polymer complex by barium sulphate and the floating time was noted. Here the immediate release segment worked as loading dose and extended release segment as maintenance dose. The results of release studies of formulations with hydrophillic matrix to formulations with dual matrix hydroxypropyl methylcellulose acetate succinate shown that as the percentage of polymer increased, the release decreased. Selected formulation F2 having F-Melt has successfully released the drug within one hour and hydrophillic matrix composing polyethylene oxide with 5% hydroxypropyl methylcellulose acetate succinate showed a lag time of one hour and then extended its release up to 12th hour with 99.59% drug release following zero order kinetics with R 2 value of 0.989. The Korsmeyer-Peppas equation showed the R 2 value to be 0.941 and n value was 1.606 following non-Fickian diffusion pattern with supercase II relaxation mechanism. Here from extended release tablet the drug released slowly from the matrix while floating.
Keywords
Bimodal drug delivery, lamotrigine, gastroretention, buoyancy, hydroxypropyl methylcellulose acetate succinate, polyox, hydrophilic matrix, lipophillic matrix, dual matrix
Epilepsy is a common chronic neurological disorder with seizures and can occur to any person [1-3]. Epileptic seizures occur due to elevated neuronal activity in the brain [4]. Epileptic patients show seizures though treated with antiepileptics [5,6]. More than 5 crores of people suffer with epilepsy of which only some 1.5 crores people avail proper therapy. Antiepileptics are given alone or as adjunctive therapy and certain drugs are even given for treatment of bipolar disorder as well. Lamotrigine is a good antiepileptic that is administered to control seizures and bipolar disorder with a trade name Lamictal [7]. Lamotrigine is a sodium channel-blocking antiepileptic, which restrains voltage-dependent sodium channels and reduces the release of the glutamate and aspartate to bring brain back to orderliness [8]. It is in use for more than a decade in Europe and more than five years in United States and is showing better efficacy with sufficient tolerability [9]. However, Stevens-Johnson syndrome, drug rash, eosinophilia and systemic symptoms (DRESS) syndrome and toxic epidermal necrolysis might be caused by unregulated plasma concentrations of lamotrigine even leading to mortality [10]. Nevertheless this could be controlled by formulating lamotrigine as a controlled drug delivery system [11]. Moreover the drug displays felicitous absorption profile in gastric environment and the ascending part of the colon. This property additionally with solubility with pH dependency makes it suitable to design as a gastroretentive floating drug delivery system [12]. The drug concentrations can be controlled by formulating bimodal drug delivery systems with combination of immediate release tablet and the floating extended release tablet placed in a hydroxypropyl methylcellulose capsule to have spatiotemporal delivery of the active [13,14]. This study was conducted with an aim to develop buoyant gastroretentive drug delivery system incorporating 100 mg of lamotrigine eudragit complex into two segments sharing equal amounts in immediate and extended segments containing drug equivalent to 25 mg each. The immediate release segment and floating extended hydrophilic polymeric matrix, which would release the drug spatially at stomach in a temporal pattern [15,16]. The immediate release segment releases the drug spontaneously once the capsule shell dissolves in the dissolution medium. The capsule shell dissolved after 3 min [17,18]. The hydroxypropyl methylcellulose acetate succinate provides the one-hour lag time for the extended release segment to release the drug preventing the dose dumping [19].
Materials and Methods
Lamotrigine (Dr. Reddy’s Laboratories, Hyderabad, India) was the active pharmaceutical ingredient. Eudragit E 100, (Evonik Degussa, Mumbai, India), was used to form drug polymer complex, ethanol (S. D. Fine-Chem Ltd., Mumbai, India) was used for the drug polymer complexing solvent for kneading. F-Melt (Fuji Chemical Industry Co., Ltd Toyama-Pref, Japan), was used as a coprocessed excipient for formulation of immediate release segment. Polyplasdone XL-10 (ISP pharmaceuticals, Covington, KY, USA), Primellose (DFE Pharma Goch, Germany), were used as superdisintegrants, Avicel pH 102 (DFE Pharma Goch, Germany) was used as diluent, Aerosil R972 P (Evonik Degussa GMBH, Germany) was used as glidant, Lubripharm (SPI Pharma, Bengaluru, India) was used as lubricant, Effersoda (SPI Pharma, Bengaluru, India), was used as gas generator, hydroxypropyl methylcellulose acetate succinate (HPMC AS, Shinetsu Chemical Co. Ltd. Japan) was used as release retardant, polyethylene oxide WSR 1105 (Colorcon, Verna, Goa, India) was used as hydrophilic matrix forming polymer and Precirol ATO 5 (Glycerol distearate, Gattefosse, Mumbai, India) was used as lipid matrix forming agent. HPMC capsules (ACG capsules, Hyderabad, India) of size 00 were used for incorporating immediate and extended release tablets.
Solubility studies of lamotrigine
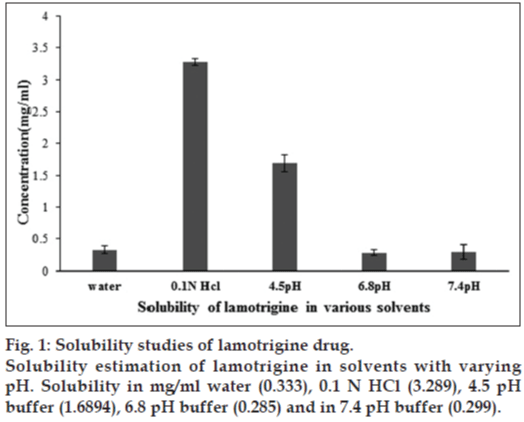
To check the solubility of lamotrigine excess of drug is added to water, 0.1 N HCl, 4.5 pH buffer, 6.8 pH buffer and 7.4 pH buffer and was mixed on a rotary shaker for 48 h and the aliquots are taken, filtered and then estimated spectrophotometrically at 244 nm [20,21].
Preparation of bimodal drug delivery systems of lamotrigine
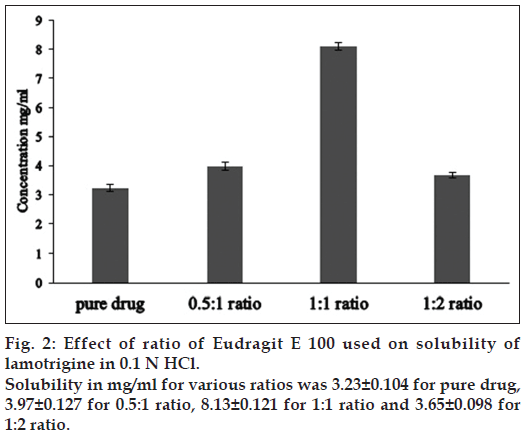
Eudragit E100 and lamotrigine were taken in a mortar at a ratio of 1:1 (eudragit E 100:lamotrigine). To this a small quantity of ethanol was added with trituration to obtain a slurry-like consistency. Then lamotrigine was incorporated slowly into the slurry and trituration was continued further for 15 min to get a soft mass of lamotrigine and eudragit E 100. The soft mass was then transferred to a syringe, extruded out, air dried at room temperature for 24 h, pulverized, passed through sieve no. 60 and was stored in a desiccator over fused calcium chloride [22]. This kneaded material was then made into immediate release tablets and extended release tablets by direct compression method. Different formulations of lamotrigine orodispersible tablets were designed to be prepared by direct compression technique using three super disintegrants, (F-Melt, Primellose, and Polyplasdone XL-10). Super disintegrants at various levels were added keeping all other ingredients constant. Drug-polymer complex, super disintegrants, avicel pH 102, were accurately weighed and passed through a 40-mesh screen to get uniform size particles and mixed in a glass mortar for 15 min. The obtained blend was lubricated with aerosil and glidant (sodium stearyl fumarate) and mixing was continued for further 5 min. The resultant mixture was directly compressed into tablets by using 7 mm round flat faced punch on a rotary tabletting machine. Compression force was kept constant for all formulations. Formulations designed were assigned with formulation codes as shown in Table 1.
| Ingredients | F1 | F2 | F3 | F4 | F5 | F6 |
|---|---|---|---|---|---|---|
| Drug-polymer complex | 50 | 50 | 50 | 50 | 50 | 50 |
| F-Melt type C | 122.5 | 147.5 | 0 | 0 | 0 | 0 |
| Polyplasdone XL-10 | 0 | 0 | 0 | 0 | 7.5 | 12.5 |
| Primellose | 0 | 0 | 7.5 | 12.5 | 0 | 0 |
| Aerosil | 0 | 0 | 5 | 5 | 5 | 5 |
| Lubripharm | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| Avicel PH 102 | 25 | 0 | 135 | 130 | 135 | 130 |
| Total weight of tablet (mg) | 200 | 200 | 200 | 200 | 200 | 200 |
Composition of the immediate release segment of the bimodal drug delivery systems. Drug-polymer complex=Lamotrigine-Eudragit E 100 kneaded complex, Avicel PH 102=Microcrystalline cellulose PH 102
Table 1: Composition of lamotrigine immediate release tablet
Lamotrigine extended release tablets were prepared using the direct compression method. Different formulations of lamotrigine were designed to be prepared by direct compression technique using strategies of hydrophyllic or lipophillic matrices and dual matrix systems. Drug-polymer complex, matrix forming agents, hydroxypropyl methylcellulose acetate succinate, avicel pH 102, were accurately weighed and passed through a 40-mesh screen to get uniform size particles and mixed in a glass mortar for 15 min. The obtained blend was lubricated with aerosil and glidant lubripharm (sodium stearyl fumarate) and mixing continued for an additional 5 min. The resultant mixture was directly compressed into tablets using 7 mm round flat-faced punch on a rotary tabletting machine. Compression force was kept constant for all formulations. Formulations designed were assigned with formulation codes as shown in Table 2.
| Ingredients in mg | FHM | FLM | FDM | FHM (HPMCAS) | FLM (HPMCAS) | FDM (HPMCAS) |
|---|---|---|---|---|---|---|
| Drug-polymer | 50 | 50 | 50 | 50 | 50 | 50 |
| complex | ||||||
| Poly ethylene | 60 | 0 | 60 | 60 | 0 | 60 |
| oxide WSR 1105 | ||||||
| Precirol | 0 | 50 | 50 | 0 | 50 | 50 |
| HPMCAS | 0 | 0 | 0 | 10 | 10 | 10 |
| Avicel PH 102 | 68 | 78 | 18 | 58 | 68 | 8 |
| Effersoda | 20 | 20 | 20 | 20 | 20 | 20 |
| Aerosil R972 P | 1 | 1 | 1 | 1 | 1 | 1 |
| Lubripharm | 1 | 1 | 1 | 1 | 1 | 1 |
| Total weight of | 200 | 200 | 200 | 200 | 200 | 200 |
| tablet |
Composition of various formulas for preparation of matrix tablets of lamotrigine. FHM: formulation with hydrophillic matrix, FLM: formulation with lipophillic matrix, FDM: formulation with dual matrix, FHM (HPMCAS): formulation with hydrophillic matrix with hydroxypropyl methylcellulose acetate succinate, FLM (HPMCAS): formulation with lipophillic matrix with hydroxypropyl methylcellulose acetate succinate, FDM (HPMCAS): formulation with dual matrix with hydroxypropyl methylcellulose acetate succinate
Table 2: Composition of lamotrigine extended release tablet
The prepared immediate and extended release tablets were then incorporated in hydroxypropyl methylcellulose capsule shells. One immediate release tablet and one extended release tablet were placed in the body of the HPMC capsule and then the cap was placed and is then utilized for further studies.
Swelling study of extended release tablets
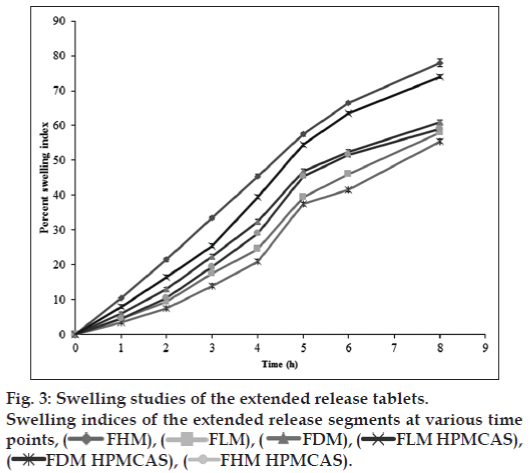
The swelling nature of tablets can be studied by weight gain or water uptake. The water uptake study of the dosage form was conducted by using USP dissolution apparatus-II in a 900 ml of 0.1 N HCl, which was maintained at 37±0.5°, rotated at 50 rpm. At predetermined intervals the tablets were withdrawn and weighed. Percent swelling of the tablet was determined using the formula %WU=((Wt-Wo)/Wo)×100, where, Wt is the weight of the swollen tablet, Wo is the initial weight of the tablet [23].
In vitro drug release study of immediate release tablets
Drug release from the immediate release tablets was studied using USP dissolution apparatus (Electrolab TDT-081, India), type II (Paddle method). Tablets were immersed in a dissolution vessel (n=6) containing 900 ml of 0.1 N HCl (pH 1.2), used as dissolution media at 37±0.5° and stirred at a speed of 50 rpm. The amount of drug released after 5, 10, 15, 30, 45, and 60 min was determined using UV/Vis spectrophotometer (Shimadzu Corporation, 1601, Japan) at 244 nm.
In vitro drug release study of extended release tablets
Drug release from the extended release tablets was studied using USP dissolution apparatus (Electrolab TDT-081, India), type II (Paddle method). Tablets were immersed in a dissolution vessel (n=6) containing 900 ml of 0.1 N HCl (pH 1.2), used as dissolution media at 37±0.5° and stirred at a speed of 50 rpm. The amount of drug released after 0.5, 1, 1.5, 2, 3, 5, 7, 9 and 11 h, was determined using UV/ Vis spectrophotometer (Shimadzu Corporation, 1601, Japan) at 244 nm.
In vitro drug release study of bimodal drug delivery system (capsule)
Drug release from the capsules was studied using USP dissolution apparatus (Electrolab TDT-081, India), type II (Paddle method). Capsules were immersed in a dissolution vessel (n=6) containing 900 ml of 0.1 N HCl, (pH 1.2), used as dissolution media at 37±0.5° and stirred at a speed of 50 rpm. The amount of drug released after 0.25, 0.5, 0.75, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 10, and 12 h, was determined using UV/Vis spectrophotometer (Shimadzu Corporation, 1601, Japan) at 244 nm.
Drug release kinetics
In vitro release data were subjected to model fitting analysis to know the order of drug release by treating the data according to zero order, first order and Higuchi’s release kinetic equations and Korsmeyer-Peppas Eqn [24-28].
In vitro buoyancy study
The floating lag time and the total floating time were determined in the USP dissolution apparatus containing 0.1 N HCl, (pH 1.2), maintained at 37±0.5°. The time between the dropping of the tablet and time to rise and float at the surface of the medium was considered as floating lag time. The duration of time the dosage form remained constantly on the surface of medium was considered as the total floating time [29-32].
In vivo evaluation of floating
Six healthy male human volunteers in the age group of 18-30 and 55-70 kg body weight were selected for the study. The Floating tablets excluding drug (lamotrigine) and replaced with barium sulphate was administered orally. During the study they were served standard breakfast and water was allowed to be taken. The first X-ray was taken after 15 min of the tablet administration and further images were taken after at 1, 4 and 8 h.
Results and Discussion
The solubility of lamotrigine was found to increase with decrease in pH with high solubility at acidic pH and low solubility in alkaline pH (fig. 1). The complexation with Eudragit improvised the solubility of lamotrigine (fig. 2). All formulations showed a minimum of 50% rise in weight after water uptake for a period of 8 h with hydrophillic matrix systems FHM and FHM (HPMC AS) showing high swelling index of more than 70% due to presence of poly ethylene oxide WSR 1105 (Polyox, fig. 3). Most of the formulations were found to have good buoyancy properties as shown in Table 3. The capsules floated immediately and then the shells got dissolved within 3 min and further the immediate and extended release segments dropped down into the dissolution medium. Later on the immediate release tablets disintegrated rapidly with extended release tablets floating over the surface within a minute due to the effervescence because of the reaction between effersoda and 0.1 N HCl. (fig. 4).
| Formulation | Floating lag time in s | Total floating time in h |
|---|---|---|
| FHM | 20 | 16 |
| FLM | 74 | 7 |
| FDM | 48 | 9 |
| FHM (HPMCAS) | 17 | 18 |
| FLM (HPMCAS) | 67 | 8 |
| FDM (HPMCAS) | 38 | 10 |
Floating characteristics of different extended release buoyant tablets of lamotrigine. FHM: formulation with hydrophillic matrix, FLM: formulation with lipophillic matrix, FDM: formulation with dual matrix, FHM (HPMCAS): formulation with hydrophillic matrix with hydroxypropyl methylcellulose acetate succinate, FLM (HPMCAS): formulation with lipophillic matrix with hydroxypropyl methylcellulose acetate succinate, FDM (HPMCAS): formulation with dual matrix with hydroxypropyl methylcellulose acetate succinate
Table 3: Properties of buoyant tablets
Figure 4: In vitro floating pictures.
Fate of bimodal drug delivery system in vitro represented in numbered pictures. 1: dropping of bimodal drug delivery system, 2: capsule
floating on surface initially, 3: capsule shell dissolving in medium within 3 minutes, 4: separation of immediate release and extended release
tablets, 5: extended release tablet floating 27 seconds after shell dissolution, 6: initiation of disintegration of immediate release tablet,
7: complete disintegration of immediate release tablet, 8: floating after 4 h, 9: floating after 8h, 10: floating after 12 h.
In vivo studies were conducted on healthy volunteers for confirming the floating of gastroretentive tablets in the stomach and to observe the gastric floating time using X–ray radiography. Buoyancy studies were done with the optimized formulation and the location of the tablet in the stomach was noted (fig. 5). The tablets floated over a period of 8 h in the gastric environment. The release profile was studied segmentally for immediate release tablets and extended release tablets separately and also after combining them by placing them in a HPMC capsule. It was observed that the immediate release tablets have released the drug for one hour time followed by the extended release segment releasing the drug for the next 11 h to contribute the total release for 12 h. The immediate release tablets release profile (fig. 6) and the extended release tablets release profile (fig. 7) were noted. The combination showed effective drug release pattern (fig. 8) with 99.59% drug release for 12 h. The use of HPMC AS provided required lag time for the extended release to release the drug during the period when immediate release was releasing to prevent the dose dumping. The selection of the best formulation was done based on the drug release profile of bimodal drug delivery system. The floating lag time and the total floating time of the extended release segment were as shown in Table 3 and the R2 values were as shown in Table 4.
| Formulation | Zero order | First order | Higuchi | Hixson-Crowell | Korsmeyer Peppas | n value |
|---|---|---|---|---|---|---|
| FHM | 0.900 | 0.970 | 0.945 | 0.997 | 0.970 | 0.795 |
| FLM | 0.920 | 0.960 | 0.941 | 0.991 | 0.980 | 0.793 |
| FDM | 0.913 | 0.970 | 0.927 | 0.954 | 0.957 | 0.890 |
| FHM (HPMCAS) | 0.989 | 0.722 | 0.786 | 0.897 | 0.941 | 1.606 |
| FLM (HPMCAS) | 0.979 | 0.864 | 0.755 | 0.931 | 0.948 | 1.685 |
| FDM (HPMCAS) | 0.984 | 0.892 | 0.720 | 0.946 | 0.944 | 1.601 |
Drug release kinetics of dosage form by fitting the release into Zero order, First order, Higuchi, Hixson–Crowell and Korsmeyer Peppas models. FHM: formulation with hydrophillic matrix, FLM: formulation with lipophillic matrix, FDM: formulation with dual matrix, FHM (HPMCAS): formulation with hydrophillic matrix with hydroxypropyl methylcellulose acetate succinate, FLM (HPMCAS): formulation with lipophillic matrix with hydroxypropyl methylcellulose acetate succinate, FDM (HPMCAS): formulation with dual matrix with hydroxypropyl methylcellulose acetate succinate
Table 4: Release kinetics model fitting and R2 values
Selected formulation F2 (having F-Melt) has successfully released the drug within one hour and FHM (HPMC acetate succinate, hydrophillic matrix composing polyethylene oxide with 5% HPMC acetate succinate) showed a lag time of one hour and then extended its release up to 12th h with 99.59% drug release following zero order kinetics with R2 value of 0.989. The Korsmeyer-Peppas Eqn. showed the R2 value to be 0.941 and n value was 1.606 following non-Fickian diffusion patterns with supercase II relaxation mechanism. Here extended release tablet floated in 17 seconds and did float for a period of 18 h in vitro and for more than 8 h in vivo and released slowly from the matrix while floating. Drug release from bimodal drug delivery system when compared with marketed formulation (fig. 8) showed a similarity factor, F2 value of 51.7 indicating that the release profile of the bimodal drug delivery system is similar to that of the marketed formulation.
Bimodal gastroretentive drug delivery systems of lamotrigine were successfully developed. The optimized formulation, unlike conventional formulations seemed to be promising for improving the release as well as patient acceptance. The control of release could help in overcoming the toxic effects of unregulated levels of lamotrigine.
References
- Commission on Epidemiology and Prognosis, International League against Epilepsy, Guidelines for epidemiologic studies on epilepsy. Commission on Epidemiology and Prognosis, International League Against Epilepsy. Epilepsia 1993;34:592-6.
- Blume WT, Luders HO, Mizrahi E, Tassinari C, van Emde Boas W,semiology: Report of the ILAE task force on classification and terminology. Epilepsia 2001;42:1212-8.
- Sander JW. The epidemiology of epilepsy revisited. CurrOpinNeuro 2003;16:165-70.
- Fisher RS, van Emde Boas W, Blume W, Elger C, Genton P, Lee P, et al. Epileptic seizures and epilepsy: Definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia 2005;46:470-2.
- Cascino GD. Epilepsy: Contemporary perspectives on evaluation and treatment. Mayo ClinProc 1994;69:1199-211.
- Engel J Jr. Surgery for seizures. N Engl J Med 334 1996;10:647-52.
- McNamara JO. Pharmacotherapy of the Epilepsies. In: Brunton LL, Chabner BA, Knollman BC, editors. Goodman and Gilman’s the Pharmacological Basis of Therapeutics. 12th ed. New York: McGraw-Hill; 2011.p. 583-108.
- Coulter DA. Antiepileptic drug cellular mechanisms of action: where does lamotrigine fit in? J Child Neurol 1997;12(Suppl 1):S2-9.
- Bialer M, Johannessen SI, Kupferberg HJ, Levy RH, Loiseau P, Perucca E. Progress report on new antiepileptic drugs: A summary of the Sixth Eilat Conference (EILAT VI). Epilepsy Res 2002;51:31-71.
- Anderson GD, Saneto RP. Current oral and non-oral routes of antiepileptic drug delivery.Adv Drug Deliv Rev 2012;64:911-8.
- Rheims S, Ryvlin P. Once-daily lamotrigine extended release for epilepsy management. Expert Rev Neurother 2009;9:167-73.
- Raja Sekhar Reddy P, ChandrasekharaRao G. Spatio temporal release of lamotrigine by buoyant gastroretentive drug delivery: Development and evaluation. Int J Pharm PharmSci 2014;6:604-10.
- Li YH, Zhu JB. Modulation of combined-release behaviors from a novel tablets-in-capsule system. J Control Release 2004;95:381-9.
- Makoto I, Kenichi A, Minoru H, Hashezime, Kawamura M. A novel approach to sustained pseudoephedrine release: Differentially coated mini-tablets in HPMC capsules. Int J Pharm 2008;359:46-52.
- Timmermans J, Moes AJ. How well floating dosage do forms float? Int J Pharm 1990;62:207-16.
- Hwang SJ, Park H, Park K. Gastric retentive drugdelivery systems.Crit Rev Ther Drug Carrier Syst 1998;15:243-84.
- Bechgaard H, Nielsen GH. Controlled-release multiple-units and single-unit doses.A literature review. Drug DevInd Pharm 1978;4:53-67.
- Chiwele I, Jones BE, Podczeck F. The shell dissolution of various empty hard capsules.Chem Pharm Bull 2000;48:951-6.
- Nagai T, Sekigawa F, Hoshi N. Applications of HPMC and HPMCAS aqueous film coating of pharmaceutical dosage forms. In: McGinity JM. editors. Aqueous polymeric coatings for pharmaceutical dosage forms. New York: Marcel Dekker; 1989. p. 81-152.
- Martins MT, Clesio SP, Martin S. Development of a dissolution test for lamotrigine in tablet form using an ultraviolet method. Braz J Pharm Sci 2010;46:179-86.
- Jouyban A, Soltanpour S, William EA. Improved prediction of drug solubilities in Ethanol+water mixtures at various temperatures. Biomed Inter 2010;1:19-24.
- Shinde VR, Shelake MR, Shetty SS. Enhanced solubility and dissolution rate of lamotrigine by inclusion complexation and solid dispersion technique. J Pharm Pharmacol 2008;60:1121-9.
- Arora G, Malik K, Arora S. Formulation and evaluation of controlled release matrix mucoadhesive tablets of domperidone using Salvia plebeian gum. J Adv Pharm Technol Res 2011;2:163-9.
- Dash S, Murthy PN, Nath L, Chowdhury P. Kinetic Modeling on Drug Release from Controlled Drug Delivery Systems. Acta Pol Pharm 2010;67:217-23.
- Higuchi T. Mechanism of sustained action medication theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J Pharm Sci 1963;52:1145-9.
- Korsmeyer RW, Gurny R, Peppas NA. Mechanism of solute release from porous hydrophilic polymers.Int J Pharm 1983;15:25-35.
- Peppas NA. Analysis of Fickian and non-Fickian drug release from polymers. Pharm ActaHelv 1985;60:110-1.
- Phaechamuda T, Yodkhum K, Ritthidej G. Drug release, water sorption and erosion behavior of three-layered matrix tablets consisting of chitosan and xanthan gum. Asian J Pharm 2008;3:260-8.
- Prajapati ST, Patel LD, Patel DM. Studies on formulation and in vitro evaluation of floating matrix tablets of domperidone. Indian J Pharm Sci 2009;71:19-23.
- Putta RK, Hiremath D, Raje S. Design and evaluation studies on novel floating tablets for peptic ulcer treatment. J Adv Pharm Edu Res 2011;2:159-76.
- Gande S, Rao Y. Sustained-release effervescent floating matrix tablets of baclofen: Development, optimization and in vitro-in vivo evaluation in healthy human volunteers. Daru 2011;19:202-9.
- Raja Sekhar Reddy P, ChandrasekharaRao G. Development of buoyant controlled release drug delivery systems of atazanavirsulphate: Effect of various diluents and lubricants on drug release. PharmanestInt J Adv Pharm Sci 2014;5:1947-57.
 FHM), (
FHM), ( FLM), (
FLM), ( FDM), (
FDM), ( FLM HPMCAS),
(
FLM HPMCAS),
(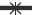 FDM HPMCAS), (
FDM HPMCAS), ( FHM HPMCAS).
FHM HPMCAS).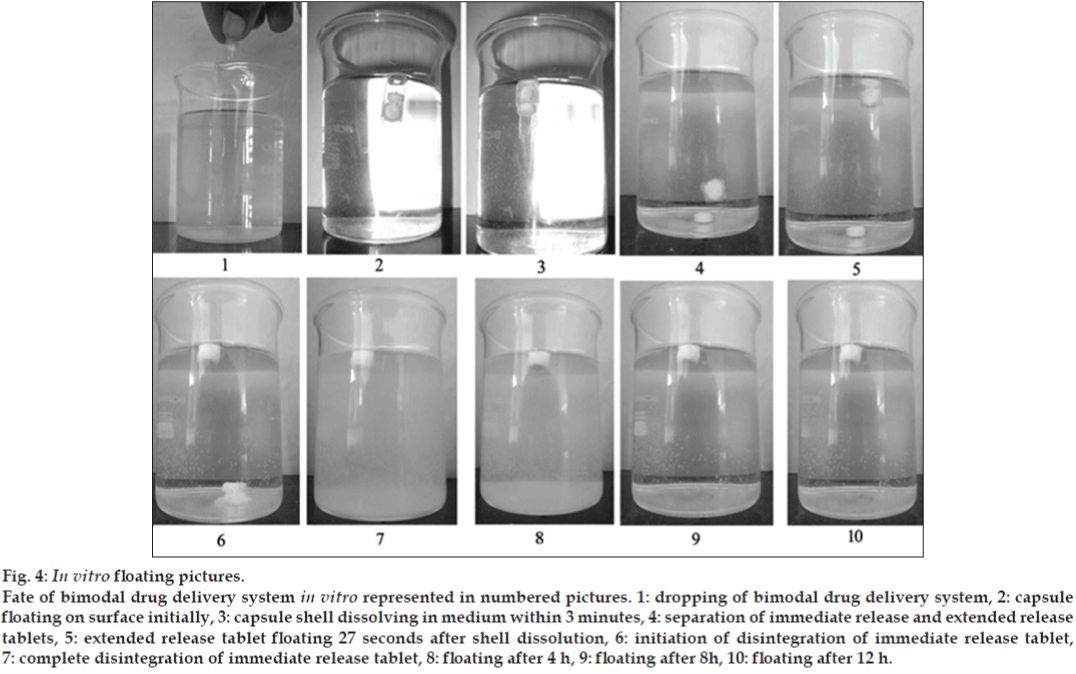
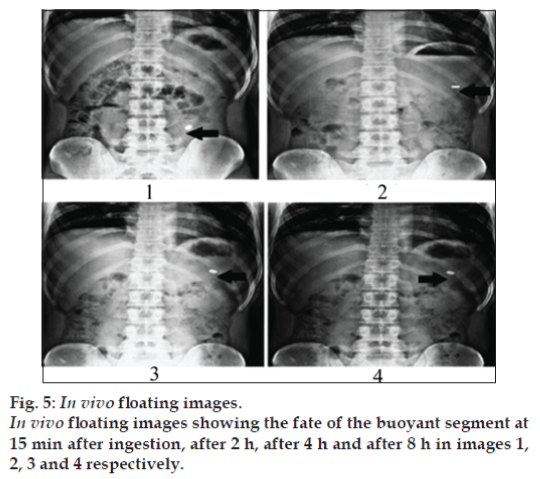
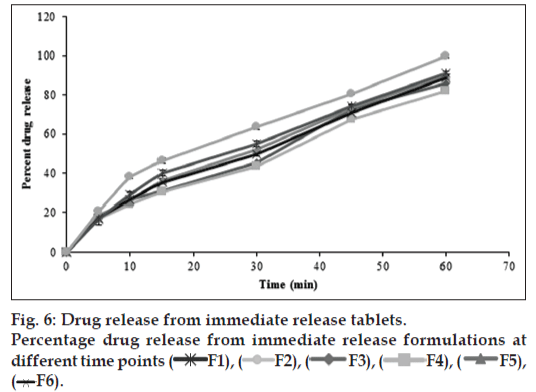
 F1), (
F1), ( F2), (
F2), ( F3), (
F3), ( F4), (
F4), ( F5),
(
F5),
( F6).
F6).
 ) FHM, (
) FHM, ( ) FLM, (
) FLM, ( ) FDM, (
) FDM, ( ) FHM
HPMCAS, (
) FHM
HPMCAS, ( ) FLM HPMCAS and (
) FLM HPMCAS and ( ) FDM HPMCAS.
) FDM HPMCAS.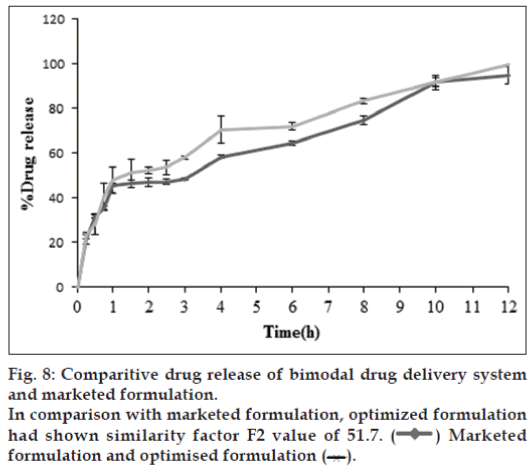
 ) Marketed
formulation and optimised formulation (
) Marketed
formulation and optimised formulation ( ).
).



