- *Corresponding Author:
- G. Xiao
Department of Neurology, Dongtai People's Hospital, Dongtai, Jiangsu 224200, China
E-mail: yarrowshaw@hotmail.com
| Date of Received | 29 November 2021 |
| Date of Revision | 26 May 2022 |
| Date of Acceptance | 07 November 2022 |
| Indian J Pharm Sci 2022;84(5):1323-1327 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
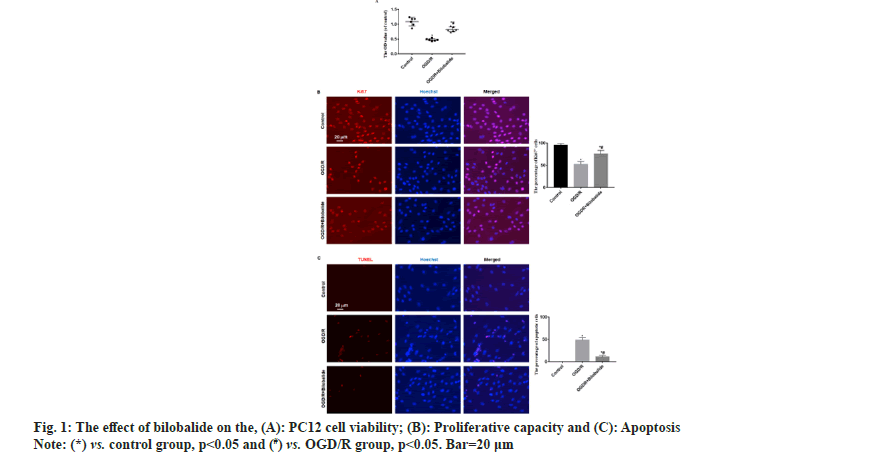
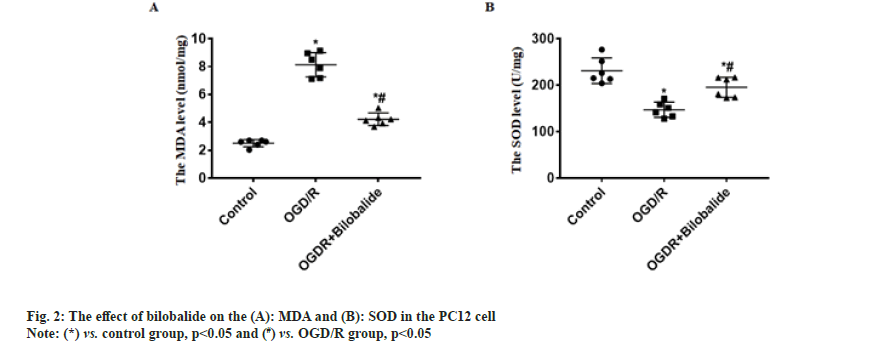
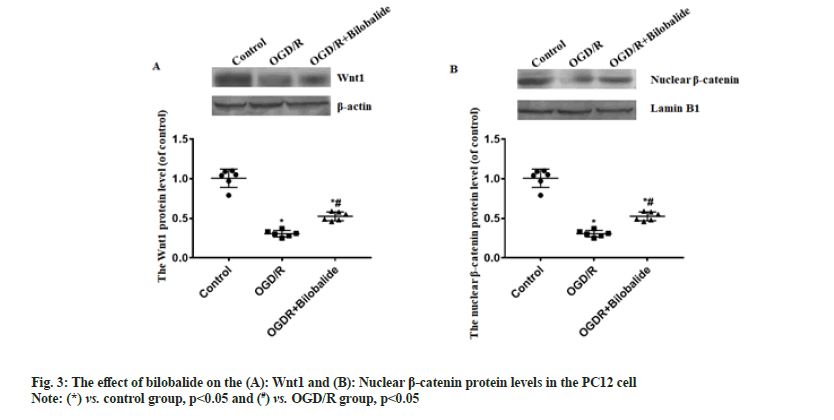
To explore the protective effect of bilobalide on the pheochromocytoma cell injury induced by oxygen-glucose deprivation/reperfusion in vitro. To simulate ischemia-reperfusion condition, the pheochromocytoma cell injury was induced by oxygen-glucose deprivation/reperfusion in vitro. The cells were divided into control, oxygen-glucose deprivation/reperfusion and oxygen-glucose deprivation/ reperfusion+bilobalide groups. The cell viability, proliferative capacity, malondialdehyde level, superoxide dismutase level, apoptosis, Wnt1 protein level and nuclear beta-catenin protein level were assayed. Compared with control group, the cell viability, proliferative capacity, superoxide dismutase level, Wnt1 protein level and nuclear beta-catenin protein level decreased in the oxygen-glucose deprivation/ reperfusion group, malondialdehyde level and the percentage of apoptotic cells increased in the oxygenglucose deprivation/reperfusion group, while the cells were treated with bilobalide in the oxygen-glucose deprivation/reperfusion+bilobalide group, all the above-mentioned observation indicators recovered to a certain extent, but still did not reach the level of the control group. Bilobalide protects pheochromocytoma cell from oxygen-glucose deprivation/reperfusion induced injury in vitro through inhibiting oxidative stress via activating Wnt1/beta-catenin pathway.
Keywords
Bilobalide, ischemia, cell damage, apoptosis, neuroprotective agents
Cerebrovascular disease has become an important cause that threatens the health and quality of life of the middle-aged and elderly people. Cerebral ischemia-induced cerebral infarction is the most common and it is also a key factor causing disability and death in the elderly worldwide[1]. Cerebral ischemia can cause obstacles to the transport of substances such as sugar and oxygen, and induce nerve cell damage. Even if blood circulation in the brain tissue is restored after therapy, ischemia-reperfusion injury will occur[2]. Therefore, it is particularly important to find effective protective drugs. Neuroprotection is an important link in the treatment strategy of ischemic stroke, which refers to disrupting the neuronal death cascade by blocking ischemic signaling pathways. With the in depth research on the pathophysiological mechanism of ischemic stroke, the development of neuroprotective agents has become a hot spot in the research and development of ischemic stroke treatment drugs. Bilobalide is an extract of Ginkgo biloba, the sesquiterpene lactone compound[3]. At present, the data results of multiple studies have shown that bilobalide has various biological functions, including anti-oxidative stress, anti-inflammatory, scavenging oxygen free radicals, improving energy metabolism, anti-excitatory amino acid toxicity, protecting mitochondria, inhibiting nerve cell apoptosis[4-6]. It has a wide range of neuroprotective effects. However, the role and mechanism of bilobalide in Cerebral Ischemia-Reperfusion Injury (CIRI) remain unclear. In this study, we used the Pheochromocytoma (PC12) cell to make the Oxygen-Glucose Deprivation/Reperfusion (OGD/R) model in vitro and the cell model was treated with bilobalide, then the cell viability, proliferative capacity, Malondialdehyde (MDA) level, Superoxide Dismutase (SOD) level, apoptosis, Wnt1 protein level and nuclear β-catenin protein level were assayed. This study aims to clarify the protective effect of bilobalide on ischemia-reperfusion injury in vitro and the possible mechanism. The PC12 cell was purchased from Tongpal Biotechnology Co., Ltd (Shanghai, China) and cultured in the Dulbecco's Modified Eagle Medium (DMEM) (Gibco, United States of America (USA)). The cells were divided into control, OGD/R and OGD/R+bilobalide groups. The cell model was prepared based on a previous study[7]. The OGD/R cell model was prepared in glucose-free DMEM under 37°, 0.2 % Oxygen (O2), 5 % Carbon dioxide (CO2), 95 % Nitrogen (N2) and cultured for 3 h. Then, the cells were cultured in glucose-containing DMEM with 10 % Fetal Bovine Serum (FBS) under 37°, 5 % CO2 for 24 h. Cells in the control group were cultured normally. The bilobalide (MUST, China) solution was prepared with normal sodium containing 8 % ethanol. During the above cell culture, the medium of OGD/R+bilobalide group contained 25 mg/l bilobalide and the medium of control, OGD/R groups contained the equal concentration of ethanol. The dose selection of bilobalide was based on a previous study[8]. The PC12 cells (3×103/ml) were cultured into 96-well plates according to the above grouping and culture methods. After the culture, 10 μl of CCK-8 solution was added to the cells, incubated at 37° for 2 h and then the Optical Density (OD) value at 450 nm was measured with a microplate reader (BioTek microplate reader, USA). The cells of each group were blocked after the culture medium was removed, then the cells were incubated with primary antibody:rabbit anti-Ki67 (1:800, Abcam, UK) for 4 h in a humidified chamber at 37°, then the cells were incubated with second antibody:Alexa Fluor® 568-labeled goat anti-rabbit Immunoglobulin G (IgG) (1:1000, Abcam, UK) for 2 h in a humidified chamber at 37°. The Hoechst 33342 (1:2000, Beyotime, China) was used to stain the cells nuclei for 15 min at 37°. The Ki67+ cells were observed by a fluorescence microscope (Leica, Germany). The cells of each group were taken and the MDA and SOD levels in the cells were measured by MDA detection kit (thiobarbituric acid method) and SOD detection kit (xanthine oxidation method) respectively according to the instructions. The TUNEL kit (Beyotime, China) was used to assess apoptosis according to the instruction. The Hoechst 33342 (1:2000, Beyotime, China) was used to stain the cells nuclei for 15 min at 37°. The apoptotic cells were observed with a fluorescence microscope (Leica, Germany). The Wnt1 and nuclear β-catenin protein levels process were performed according to a previous study[9]. Briefly, the total protein and the nuclear protein were extracted by Radioimmunoprecipitation Assay (RIPA) buffer with 100 mM of phenylmethylsulphonyl fluoride (Beyotime, China) and nuclear protein extraction kit (Active Motif, USA) respectively, then separated proteins were transferred onto Polyvinylidene Fluoride (PVDF) membranes, then the membranes were incubated with primary antibodies and secondary antibodies in turn. The primary antibodies were; rabbit anti-β-catenin (1:1000, Abcam), rabbit anti-Wnt1 (1:1000, Abcam), rabbit anti-β-catenin (1:1000, Abcam), mouse anti-Lamin B1 (1:2000, Abcam). The second antibodies were; goat-anti-mouse or goat-anti-rabbit Horseradish Peroxidase (HRP)-conjugated IgG (1:1500, Abcam). ImageMaster™ 2D Platinum (Amersham Biosciences, USA) was used to calculate optical densities. The statistical software Statistical Package for the Social Sciences (SPSS) 21.0 was used to analyze the data in this study. The data was expressed by Mean±Standard Deviation (x̄ ±SD) . The One-way Analysis of Variance (ANOVA) was used to analyze the difference among groups and the difference compared using the Bonferroni method. p<0.05 was considered statistically significant. The results of CCK-8 showed the OD value of OGD/R group was the lowest, the OD value of OGD/R+bilobalide group was more than that in the OGD/R group but was lower than that in the control group (p<0.05) as shown in fig. 1A. The results of Ki67 immunofluorescence showed Ki67 positive cells percentage of OGD/R group was the lowest, the Ki67 positive cells percentage of OGD/R+bilobalide group was more than that in the OGD/R group but was lower than that in the control group (p<0.05) as shown in fig. 1B. The results of TUNEL assay showed almost no apoptotic cells were seen in the control group, more apoptotic cells were seen in OGD/R group and fewer apoptotic cells were in OGD/R+bilobalide group (p<0.05) as shown in fig. 1C. Compared with control group, the MDA level increased and SOD level decreased in the OGD/R group (p<0.05), while the cells were treated with bilobalide in the OGD/ R+bilobalide group, the MDA and SOD levels recovered to a certain extent, but still did not reach the level of the control group (p<0.05) as shown in fig. 2. Compared with control group, the Wnt1 and nuclear β-catenin protein levels significantly decreased in the OGD/R group (p<0.05), while the cells were treated with bilobalide in the OGD/R+bilobalide group, the Wnt1 and nuclear β-catenin protein levels recovered to a certain extent, but still did not reach the level of the control group (p<0.05) as shown in fig. 3. CIRI refers to a phenomenon in which the blood supply to the brain is restored after a period of ischemia and the brain function is not improved, but a serious disorder occurs, the key pathological process of the disease development[10]. The occurrence of CIRI in the brain tissue further aggravates the brain injury, which is a pathological process involving multiple factors and multiple mechanisms. Current research believes that it mainly involves various mechanisms such as oxidative stress, inflammatory response, excitatory amino acid toxicity, intracellular calcium overload, energy metabolism disorders and free radical damage, which ultimately lead to nerve cell death[10-14]. In this study, the PC12 cell injury induced by OGD/R in vitro, and the results showed the cell viability, proliferative capacity significantly decreased and the percentage of apoptotic cells increased in the OGD/R group, while the cells were treated with bilobalide in the OGD/R+bilobalide group, all the above-mentioned observation indicators recovered to a certain extent, but still did not reach the level of the control group. The results indicated that bilobalide can protect the PC12 cell from injury induced with OGD/R in vitro. The process of oxidative stress is an important role that cannot be ignored. Reducing oxidative stress injury may play a key role in reducing CIRI and downstream pathophysiological responses[15]. After oxidative stress occurs, a large number of reactive oxygen species are generated. The final product of peroxidation reaction is MDA, and MDA can affect the activity of mitochondrial respiratory chain complexes and key enzymes in mitochondria, so MDA can reflect the degree of lipid peroxidation in the body and the degree of cellular oxidative damage[16,17]. SOD is the main antioxidant enzyme and its level represents the antioxidant capacity in the body[18]. In this study, the MDA level increased and SOD level decreased in the OGD/R group, while the cells were treated with bilobalide in the OGD/R+bilobalide group, the MDA and SOD levels recovered to a certain extent. The results indicated that bilobalide can reduce the oxidative stress level in the PC12 cell OGD/R injury in vitro. Wnt/β-catenin signaling pathway is one of the important regulatory signaling pathways in the body, which can affect many cell life activities in the body, such as regulating cell cycle, promoting cell division, affecting cell growth and apoptosis, etc. This signaling pathway is also involved in the pathophysiological process of various diseases such as tumors, cardiovascular and cerebrovascular diseases and aging[19,20]. One of the hallmarks of the activation of the Wnt/β-catenin signaling pathway is the accumulation of β-catenin into the nucleus, which activates the downstream gene expression changes of this signal, thereby exerting biological effects. In this study, the Wnt1 and nuclear β-catenin levels decreased in the OGD/R group, while the cells were treated with bilobalide in the OGD/R+bilobalide group, Wnt1 and nuclear β-catenin levels recovered to a certain extent. The results indicated bilobalide can activate the Wnt1/ β-catenin signaling pathway in the PC12 cell OGD/R injury in vitro. In summary, bilobalide protects PC12 cell from OGD/R induced injury in vitro through inhibiting oxidative stress via activating Wnt1/β-catenin pathway.
Ethical approval:
This study was approved by the Ethics Committee of Dongtai People's Hospital (2021-dtry-L-004).
Authors’ contributions:
Xiaofeng Yu and Jie Hou have contributed equally to this work.
Acknowledgements:
This work was supported by Nantong Science and Technology Project (JC2019079, YYZ17097).
Conflict of interests:
The authors declared no conflict of interests.
References
- Jiang M, Liu X, Zhang D, Wang Y, Hu X, Xu F, et al. Celastrol treatment protects against acute ischemic stroke-induced brain injury by promoting an IL-33/ST2 axis-mediated microglia/macrophage M2 polarization. J Neuroinflammation 2018;15(1):78.
[Crossref] [Google Scholar] [PubMed]
- Gu Y, Zhou C, Piao Z, Yuan H, Jiang H, Wei H, et al. Cerebral edema after ischemic stroke: Pathophysiology and underlying mechanisms. Front Neurosci 2022;16:988283.
[Crossref] [Google Scholar] [PubMed]
- Feng Z, Sun Q, Chen W, Bai Y, Hu D, Xie X. The neuroprotective mechanisms of ginkgolides and bilobalide in cerebral ischemic injury: A literature review. Mol Med 2019;25(1):57.
[Crossref] [Google Scholar] [PubMed]
- Song LJ, Sui RX, Wang J, Miao Q, He Y, Yin JJ, et al. Targeting the differentiation of astrocytes by bilobalide in the treatment of Parkinson’s disease model. Int J Neurosci 2022:1-8.
[Crossref] [Google Scholar] [PubMed]
- Su Q, Dong J, Zhang D, Yang L, Roy R. Protective effects of the bilobalide on retinal oxidative stress and inflammation in streptozotocin-induced diabetic rats. Appl Biochem Biotechnol 2022.
[Crossref] [Google Scholar] [PubMed]
- Lu J, Xie L, Liu K, Zhang X, Wang X, Dai X, et al. Bilobalide: A review of its pharmacology, pharmacokinetics, toxicity and safety. Phytother Res 2021;35(11):6114-30.
[Crossref] [Google Scholar] [PubMed]
- Zheng T, Shi Y, Zhang J, Peng J, Zhang X, Chen K, et al. MiR-130a exerts neuroprotective effects against ischemic stroke through PTEN/PI3K/AKT pathway. Biomed Pharmacother 2019;117:109117.
[Crossref] [Google Scholar] [PubMed]
- Liu Q, Jin Z, Xu Z, Yang H, Li L, Li G, et al. Antioxidant effects of ginkgolides and bilobalide against cerebral ischemia injury by activating the Akt/Nrf2 pathway in vitro and in vivo. Cell Stress Chaperones 2019;24(2):441-52.
[Crossref] [Google Scholar] [PubMed]
- Lu X, Chen X, Xing J, Lian M, Huang D, Lu Y, et al. miR-140-5p regulates the odontoblastic differentiation of dental pulp stem cells via the Wnt1/β-catenin signaling pathway. Stem Cell Res Ther 2019;10(1):226.
[Crossref] [Google Scholar] [PubMed]
- Zeng M, Zhang R, Yang Q, Guo L, Zhang X, Yu B, et al. Pharmacological therapy to cerebral ischemia-reperfusion injury: Focus on saponins. Biomed Pharmacother 2022;155:113696.
[Crossref] [Google Scholar] [PubMed]
- Yu L, Wang Y, Tang J, Shu Z, Han X. Astragaloside IV ameliorates cerebral ischemia-reperfusion injury via up regulation of PKA and Cx36. Neuroreport 2022;33(15):656-62.
[Crossref] [Google Scholar] [PubMed]
- Zhu F, Xiong J, Yi F, Luo E, Huang C, Li R. Albiflorin relieves cerebral ischemia-reperfusion injury by activating Nrf2/HO-1 pathway. Histol Histopathol 2022:18518.
[Crossref] [Google Scholar] [PubMed]
- Zhang H, Wang X, Chen W, Yang Y, Wang Y, Wan H, et al. Danhong injection alleviates cerebral ischemia-reperfusion injury by inhibiting autophagy through miRNA-132-3p/ATG12 signal axis. J Ethnopharmacol 2022;300:115724.
[Crossref] [Google Scholar] [PubMed]
- Liu Y, Qu X, Yan M, Li D, Zou R. Tricin attenuates cerebral ischemia/reperfusion injury through inhibiting nerve cell autophagy, apoptosis and inflammation by regulating the PI3K/Akt pathway. Human Exp Toxicol 2022;41:9603271221125928.
[Crossref] [Google Scholar] [PubMed]
- Ten VS, Starkov A. Hypoxic-ischemic injury in the developing brain: The role of reactive oxygen species originating in mitochondria. Neurol Res Int 2012;2012:542976.
[Crossref] [Google Scholar] [PubMed]
- Pau MC, Zinellu E, Fois SS, Piras B, Pintus G, Carru C, et al. Circulating malondialdehyde concentrations in obstructive sleep apnea (OSA): A systematic review and meta-analysis with meta-regression. Antioxidants 2021;10(7):1053.
[Crossref] [Google Scholar] [PubMed]
- Tsikas D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal Biochem 2017;524:13-30.
[Crossref] [Google Scholar] [PubMed]
- Pong K. Oxidative stress in neurodegenerative diseases: Therapeutic implications for superoxide dismutase mimetics. Expert Opin Biol Ther 2003;3(1):127-39.
[Crossref] [Google Scholar] [PubMed]
- Ring A, Kim YM, Kahn M. Wnt/catenin signaling in adult stem cell physiology and disease. Stem Cell Rev Rep 2014;10(4):512-25.
[Crossref] [Google Scholar] [PubMed]
- Mo Z, Zeng Z, Liu Y, Zeng L, Fang J, Ma Y. Activation of Wnt/Beta-catenin signaling pathway as a promising therapeutic candidate for cerebral ischemia/reperfusion injury. Front Pharmacol 2022;13:914537.
[Crossref] [Google Scholar] [PubMed]