- *Corresponding Author:
- Yaping Wei
Department of Oncology,
Fu Xing Hospital,
Capital Medical University,
Xicheng District,
Beijing 100038,
China
E-mail: yapingwei708@gmail.com
| This article was originally published in a special issue, “New Advancements in Biomedical and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2022:84(2) Spl Issue “200-206” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Ovarian cancer is a lethal malignancy with poor prognosis and low overall survival rate due to late diagnosis and asymptotic behaviour at early stages. The aim of this investigation was to evaluate the synergistic effect of β-Caryophyllene and dexmedetomidine on cell proliferation, apoptosis and tumor growth inhibition in ovarian cancer.
Human ovarian epithelial cells were cultured with dexmedetomidine alone, β-Caryophyllene alone or β-Caryophyllene together with dexmedetomidine for 24 h and analyzed for cell proliferation with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay. Enzyme-linked immunoassay based kit was used to determine apoptotic deoxyribonucleic acid fragmentation. Western blotting was used to determine expression levels of target proteins. The induction of experimental human ovarian epithelial cells tumor in rat model was achieved through the injection of human ovarian epithelial tumor cells subcutaneously into the middle left side of the mice after anesthetization with pentobarbital (35 mg/kg) at 2.8×106 cells in 400 μl of phosphate buffered saline. We found that β-Caryophyllene and dexmedetomidine combination significantly enhances the anti-proliferative effects on human ovarian epithelial cells compared to individual treatments. Furthermore, β-Caryophyllene and dexmedetomidine combination significantly enhances apoptotic cell death compared to individual treatments. Furthermore, β-Caryophyllene and dexmedetomidine combination significantly causes up-regulation of peroxisome proliferator-activated receptor-gamma coactivator-1 alpha and mitochondrial transcription factor A compared to individual treatments. Finally, we observed that β-Caryophyllene and dexmedetomidine combination significantly suppressed tumor growth in mice without changing body weight compared to individual treatments. β-Caryophyllene in combination with dexmedetomidine synergistically enhances anti-cancer effects in ovarian cancer through adenosine monophosphate-activated protein kinase activation and peroxisome proliferator-activated receptor-gamma coactivator-1α/mitochondrial transcription factor A over expression. The results point towards the potential combinational use of β-Caryophyllene and dexmedetomidine in ovarian cancer treatment.
Keywords
β-Caryophyllene, dexmedetomidine, ovarian cancer, apoptosis, tumor growth
Ovarian Cancer (OC) is a lethal malignancy, affecting large numbers of women across the globe [1]. Due to its asymptotic behaviour, the maximum number of patients is diagnosed at later disease stages. Only 2.5 % of women are diagnosed of OC worldwide but the death rate is 5 %. Poor prognosis results show decreased 5 y overall survival rate which stands at 25 %-30 % [2]. In 2018 alone, around 22 000 new patients and 14 000 deaths due to OC were expected in United States. OC is divided into three main subtypes including epithelial OC, germ cell OC and sex cord-stromal OC [1]. Various factors contribute to the etiology of this disease including biological, physical, chemical, genetic factors, immune factors, carcinogenic factors and poor lifestyle choices. Intraperitoneal chemotherapy has revealed significant improvements in later stages of epithelial OC but in 2012, only less than 50 % of the patients were subjected to treatment with intraperitoneal chemotherapy due to higher number of side effects [3]. OC has a very high relapse rate as well as develop high chemotherapy resistance, resulting in high mortality and poor prognosis [4]. An increased overall survival rate was reported after secondary surgery in case of relapsing, thus it may be considered for the patients who have a disease-free time interval of half a year. Lethality of OC gets amplified mainly of poor prognosis, diagnosis at later stages, drug resistance and side effects of chemotherapy. Therefore there is a pressing need to overcome the shortcomings of conventional chemotherapeutic agents and move to novel and effective ones.
Dexmedetomidine (Dex) is a commonly used sedative agent during perioperative period. It has been reported that Dex induce anti-apoptotic effects and inhibits tumor growth in ovarian cancer [5-7]. It is worth to note that the molecular mechanism responsible for the Dex associated cancer induction is still unknown. Isolation of biologically active compounds from nature and their use in cancer therapy has emerged vastly in past decade [8]. Such active compounds are aimed to impact different signaling pathways in cancer cells so to induce apoptosis and death of target cells [9]. Use of such naturally obtained compounds is presenting a great scope in the field of cancer research and therapy [10]. β-Caryophyllene (BCP), a naturally obtained product from has been reported to induce apoptosis and cell death in various cancer cell lines [11].
Mitochondria are known as the “powerhouse” of the cell because they create the vital energy required for cellular functions. Peroxisome proliferatoractivated receptor gamma co-activator 1 (PGC-1?) has emerged as an important factor for biogenesis of mitochondria [12,13]. PGC-1? has been found to change the transcriptional activity of numerous critical mitochondrial genes, leading to an increase in mitochondrial Deoxyribonucleic Acid (mtDNA) [14].
Despite the fact that numerous chemotherapy procedures are utilised to treat human sarcomas, however, for high-grade sarcomas such therapies have proven ineffective [15]. Several treatment options against high-grade sarcomas have previously been reported. Reduced mitochondrial numbers have been associated to neoplastic transformation and/or tumor progression, including apoptosis resistance, in a number of investigations [16]. PGC-1? controls the activities of several nuclear receptors and transcriptional factors responsible for mitochondrial biogenesis [17]. A lower number of mitochondria have been reported in osteosarcoma. Forced increase of mitochondria number in human sarcoma cell lines has been reported to induce mitochondrial apoptosis and therefore cell death [18]. Changes in copy number of mtDNA have been well reported in human cancers [19,20]. Furthermore, mitochondrial dysfunction has been associated with cancer progression via Mitochondrial Transcription Factor A (TFAM) silencing [21]. The present study aims to investigate the possible synergistic role of BCP and Dex on proliferation, apoptosis and tumor growth in ovarian cancer.
Materials and Methods
Cell culture and treatment:
Human ovarian epithelial cells (SV-40) were acquired from American Type Culture Collection (ATCC). Cells were grown in culture for 24 h in Dulbecco’s modified eagle medium mixed with 10 % fetal bovine serum and supplemented with antibiotic solution (100 U/ml). Cells were either left untreated or treated with Dex (1 nm) alone or together with BCP (4 μm) in 12-well plates.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay:
This assay was performed to assess the cell survival after tangeretin treatment. In brief, SV-40 cancer cell line was harvested at logarithmic phase of growth and exposed to either Dex (1 nm) alone, BCP (4 μm) alone or BCP together with Dex for 24 h with each well plate holding 8×103 cells. At the end of treatment time, MTT stock solution of 5 mg/ml concentration and volume 100 μl was supplemented to cells with 4 h of incubation. The formazan crystals then produced are dissolved with the use of dimethyl sulfoxide and thereafter absorbance was measured at 540 nm using a microplate reader. Percent cell value was taken from the results of triplicate reactions.
Apoptosis assay:
To carry out the apoptotic assay, SV-40 cells were cultured in 96-well plates for 24 h. Cells were exposed to either Dex (1 nm) alone, BCP (4 μm) alone or BCP together with Dex for 24 h. Cell death assay kit (Enzyme- Linked Immunoassay (ELISA) based) was used to determine apoptosis (manufacturer’s instructions were followed).
Wound healing assay:
For wound healing assay purposes, SV-40 cells were cultured in 12-well plate overnight. With the help of 10 μl tip, a scratch was created on the monolayer cells. After scratch, cells were washed once with culture media to remove floating cells. Cells were kept untreated or exposed to either Dex (1 nm) alone, BCP (4 μm) alone or BCP together with Dex for 24 h. Images of fresh scratch were captured immediately with the help of a digital camera. After treatment completion, cells were washed thrice with culture media and followed by capturing of pictures of the scratch. The scratch area was calculated using Image-Pro Plus software (Media Cybernetics, USA). Cell migration was determined by calculating the scratch closure.
Protein extraction:
Preparation of SV-40 cell lysate was achieved using lysis buffer (NP-40). To prevent proteolysis of cell lysate, halt protease inhibitor cocktail (Thermo Scientific) was used. Centrifugation (3000 rpm for 10 min) of cell lysate was performed to obtain supernatant. Bradfords assay was used to determine protein concentration.
Western blotting:
Protein samples were prepared as described by Waza et al. [22]. Detection of target proteins were determined using specific antibodies: anti-p-Adenosine monophosphateactivated protein kinase (AMPK)α (Thr172), anti- AMPKα, anti-PGC-1α, anti-TFAM, anti-caspase-3/9, anti-PARP and α-GAPDH. Horseradish peroxidase conjugated anti rabbit was used as secondary antibody and incubated at room 37° for 2 h. Enhanced Chemiluminescent (ECL) kits was used to visualize the protein bands in the membrane and analysed using FR- 200 system (Shanghai FURI Technology).
Quantitative Polymerase Chain Reaction (qPCR):
SV-40 cells were exposed to either Dex (1 nm) alone, BCP (4 μm) alone or BCP together with Dex for 24 h. It was followed by the quantification of mtDNA relative to nuclear DNA. Extraction of genomic DNA was carried out using DNA extraction kit (Sigma-Aldrich). It was followed by Polymerase Chain Reaction (PCR) amplification of mtDNA using specific primers.
Animal model of ovarian cancer:
Bagg and Albino nude mice (30 in number) were used for the present study. The experimental ovarian tumor model was created in the mice as described earlier [23]. In the present study, animals were followed up for 40 d with 5 groups of rats as follows, Group 1 as control with vehicle (Sham); Group 2 rats with liver tumor induced (model), Group 3 rats administered with Dex (0.5 mg/kg per d) (Dex) for 2 w prior to ovarian tumor induction; Group 4 rats administered with BCP (20 mg/ kg per d) for 2 w prior to ovarian tumor induction and Group 5 rats administered with Dex (0.5 mg/kg per d) and BCP (20 mg/kg per d) (Dex+BCP) for 2 w prior to ovarian tumor induction and continued even after ovarian tumor induction till the end of the experimental periods.
For the induction of ovarian tumor, SV-40 tumor cells were injected subcutaneously into the middle left side of the mice after anesthetization with pentobarbital (35 mg/kg) at 2.8×106 cells in 400 μl of phosphate-buffered saline. Normal saline doses were given to sham and model mice groups. A regular body weight check up was performed for every day till the experiment was finished [24].
Statistical Analysis:
Statistical Package for the Social Sciences (SPSS) software was used to carry out statistical analysis. Experimental values were given as mean and standard error of mean. Statistical significance was measured with Analysis Of Variance (ANOVA) and for multiple comparisons purposes post-hoc test was used (p<0.05 as statistically significant).
Results and Discussion
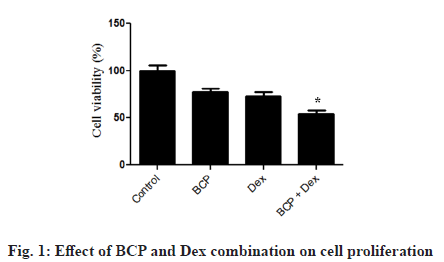
SV-40 cell viability was determined using MTT assay against Dex treatment alone or together with β-Caryophyllene. Dex decreased cell viability of SV-40 cells; however in combination with BCP significantly restrict cell proliferation (fig. 1).
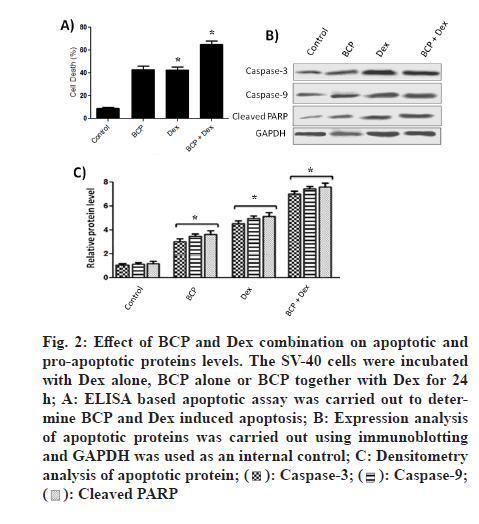
To look for β-Caryophyllene-induced death in SV-40 cells, apoptotic assay was carried out by using Cell Death Detection ELISA method. During apoptosis cells exclude fragmented DNA and histone from nucleus to the cytoplasm. ELISA based kit is used to measure fragmented DNA and histones in cytoplasm. We found that Dex treatment decreases cell apoptosis compared to control (fig. 2A). Similarly, BCP treatment decreases cell apoptosis compared to control (fig. 2A). However, BCP in combination with Dex significantly counters cell apoptosis.
Figure 2: Effect of BCP and Dex combination on apoptotic and pro-apoptotic proteins levels. The SV-40 cells were incubated with Dex alone, BCP alone or BCP together with Dex for 24 h; A: ELISA based apoptotic assay was carried out to determine BCP and Dex induced apoptosis; B: Expression analysis of apoptotic proteins was carried out using immunoblotting and GAPDH was used as an internal control; C: Densitometry analysis of apoptotic protein; 

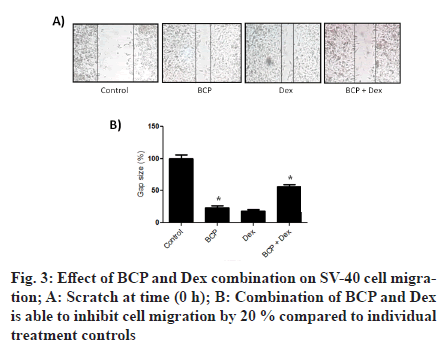
To look for the underlying mechanism of BCP and Dex-induced death in SV-40, western blotting was carried out. As shown in fig. 2B caspase-3/9 levels and cleaved PARP gets increased after BCP treatment. Similarly, caspase-3/9 levels and cleaved Poly(ADPRibose) Polymerase (PARP-1) PARP gets increased after Dex treatment. However, BCP in combination with Dex significantly increases caspase-3/9 levels and cleaved PARP. Densitometry analysis (fig. 2C) showed that BCP treatment increases expression levels of caspase-3, caspase-9 and cleaved PARP by 2.8, 2 and 4 fold respectively, compared to control. Similarly, Dex treatment increases expression levels of caspase-3, caspase-9 and cleaved PARP by 2, 2.5 and 3 fold respectively, compared to control. However, BCP in combination with Dex significantly increased expression levels of caspase-3, caspase-9 and cleaved PARP by 1.5, 2 and 2 fold respectively, compared to individual controls. The effects of BCP treatment on SV-40 cell migration were assessed with the woundhealing assay (fig. 3). We found that the migration of SV-40 cells is inhibited by 40 % and 35 % with BCP and Dex treatments compared to control. However, BCP in combination with Dex significantly inhibited migration of cells compared to individual controls.
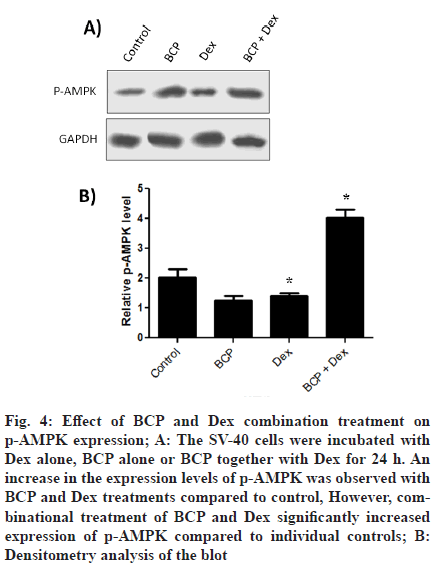
To look for expression levels of p-AMPK, cells were either treated with Dex or BCP together with Dex for 24 h. It was observed that individual treatments of BCP and Dex treatment increased expression of p-AMPK cells (fig. 4A). However, combinational treatment of BCP and Dex significantly increased expression of p-AMPK. Densitometry analysis (fig. 4B) showed that BCP and Dex treatments increases expression levels of p-AMPK by 3 and 1.5 fold respectively compared to control). However, combinational treatment of BCP and Dex significantly increased expression of p-AMPK by 2 folds compared to individual controls.
Figure 4: Effect of BCP and Dex combination treatment on p-AMPK expression; A: The SV-40 cells were incubated with Dex alone, BCP alone or BCP together with Dex for 24 h. An increase in the expression levels of p-AMPK was observed with BCP and Dex treatments compared to control, However, combinational treatment of BCP and Dex significantly increased expression of p-AMPK compared to individual controls; B: Densitometry analysis of the blot
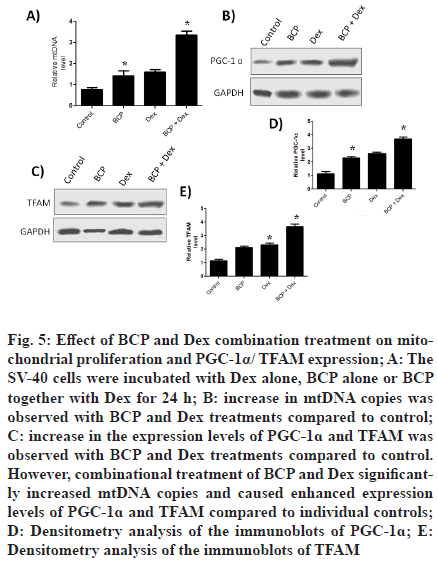
To look for impact of treatments on the mtDNA copies, cells were incubated with Dex alone, BCP alone or BCP together with Dex for 24 h. It was found that BCP and Dex treatments increased mtDNA copies compared to control (fig. 5A). Expression levels of PGC-1? and TFAM were determined after treating cells with were incubated with Dex alone, BCP alone or BCP together with Dex for 24 h. It was observed that BCP and Dex treatments increased expression of PGC-1? (fig. 5B) and TFAM (fig. 5C) compared to control. However, combinational treatment of BCP and Dex significantly increased expression of PGC-1? and TFAM compared to individual controls. Densitometry analysis showed that BCP and Dex treatments increases expression levels of PGC-1? and TFAM by 3.5 and 2.5 fold respectively compared to control (fig. 5D and fig. 5E). However, combinational treatment of BCP and Dex increased expression of PGC-1? and TFAM by 1.5 and 2 fold respectively compared to individual controls.
Figure 5: Effect of BCP and Dex combination treatment on mitochondrial proliferation and PGC-1α/ TFAM expression; A: The SV-40 cells were incubated with Dex alone, BCP alone or BCP together with Dex for 24 h; B: increase in mtDNA copies was observed with BCP and Dex treatments compared to control; C: increase in the expression levels of PGC-1? and TFAM was observed with BCP and Dex treatments compared to control. However, combinational treatment of BCP and Dex significantly increased mtDNA copies and caused enhanced expression levels of PGC-1? and TFAM compared to individual controls; D: Densitometry analysis of the immunoblots of PGC-1?; E: Densitometry analysis of the immunoblots of TFAM
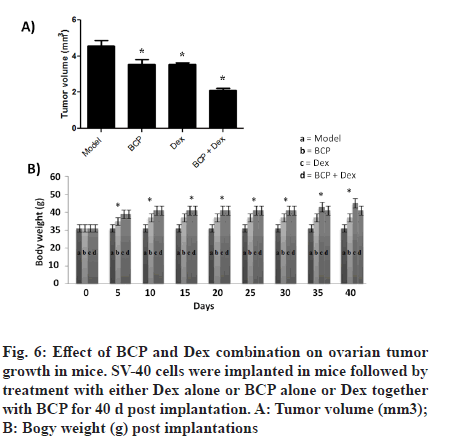
In vivo antitumor ability of BCP was determined in mice model of SV-40 cell xenograft (fig. 6A). It was observed that BCP and Dex treatments of SV-40 cell implanted models inhibit tumor growth compared to control groups. However, combinational treatment of BCP and Dex of SV-40 cell implanted models significantly inhibits tumor growth compared to individual groups (fig. 6A). However, it was observed that treatments did not affect body weight of mice during study period (fig. 6B).
Despite tremendous advances in the medical field, the clinical use of most of the anti-ovarian tumor chemotherapeutics are limited due to toxicity towards normal cells, lack of sensitivity and selectivity to tumor cells, poor pharmacokinetics, Multidrug Resistance (MDR) etc [25,26]. Therefore, there is an urgent need to look for new anti-tumor targets for treating ovarian tumor successfully [27]. The present study was carried out to look for the possible synergistic anti-cancer role of BCP and Dex in ovarian cancer. First of all, we investigated the impact of BCP and Dex on proliferation of ovarian cancer cell line [28,6]. As shown in fig. 1, combination of BCP and Dex treatments significantly inhibited proliferation of SV-40 cells compared to individual controls (fig. 1). Furthermore, BCP and Dex treatments significantly enhanced apoptosis induced cell death compared to individual treatment controls (fig. 2A).
Cell survival and death are mainly decided by the expression dynamics of pro- and anti-apoptotic proteins [22]. Expression levels of pro-apoptotic proteins like caspases get up-regulated during cell death [29]. We observe significant up-regulation of caspase 3 and 9 proteins after combinational treatments of BCP and Dex to individual treatment controls (fig. 2B). Cleavage of PARP proteins (113 kDa into two smaller fragments 89 and 24 kDa is observed during apoptosis [30]. In the present study, combinational treatments of BCP and Dex induced significant PARP cleavage compared to individual treatment controls. Furthermore, BCP and Dex has been reported to inhibit migration of ovarian cancer cells [28,6]. We reported that the ovarian cancer cell migration is significantly inhibited by the combinational treatments of BCP and Dex compared to individual treatment controls (fig. 3).
Various metabolic stresses induce AMP-activated protein kinase activation through its phosphorylation of threonine 172 [31,32]. AMPK activation through inhibition of the mammalian Target Of Rapamycin (mTOR) pathway leads to reduced proliferation of cells [33,34]. We observe significant increase in the expression of p-AMPK after combinational treatments of BCP and Dex compared to individual treatment controls (fig. 3).
A lower number of mitochondria have been reported in number of cancers [35,36]. Forced increase of mitochondria number in human cancer cell lines has been reported to induce mitochondrial apoptosis and therefore cell death [18]. Changes in copy number of mtDNA have been well reported in human cancers [19,20]. In the current study, we observe significant increase in the mtDNA copy number after combinational treatments of BCP and Dex compared to individual treatment controls (fig. 5A). PGC-1? has been found to control mitochondrial biogenesis. It has been reported that mitochondrial dysfunction is associated with cancer progression via TFAM silencing [21]. An increase in the expression levels of PGC-1a and TFAM was observed after combinational treatments of BCP and Dex compared to individual treatment controls (fig. 5B and fig. 5C). We further reported that combinational treatments of BCP and Dex of SV-40 cell implanted in mice model significantly reduced tumor growth compared to individual treatment controls without affecting body weight (fig. 6).
We conclude that combination of BCP and Dex suppressed ovarian cancer cell proliferation via apoptosis through p-AMPK activation. Furthermore, mitochondrial biogenesis and expression levels of PGC-1α and TFAM got up-regulated by combinational treatment of BCP and Dex. Finally, combination of BCP and Dex treatment significantly reduced ovarian tumor growth in mice model. In the current study, combination therapy of BCP and Dex has emerged as an effective therapeutic strategy against ovarian cancer.
References
- Reid BM, Permuth JB, Sellers TA. Epidemiology of ovarian cancer: A review. Cancer Biol Med 2017;14(1):9-32.
[Crossref] [Google Scholar] [PubMed]
- Paoletti X, Lewsley LA, Daniele G, Cook A, Yanaihara N, Tinker A, et al.Assessment of progression-free survival as a surrogate end point of overall survival in first-line treatment of ovarian cancer: A systematic review and meta-analysis. JAMA Netw Open 2020;3(1):e1918939.
[Crossref] [Google Scholar] [PubMed]
- Sun CC, Bodurka DC, Weaver CB, Rasu R, Wolf JK, Bevers MW, et al.Rankings and symptom assessments of side effects from chemotherapy: Insights from experienced patients with ovarian cancer. Support Care Cancer 2005;13(4):219-27.
[Crossref] [Google Scholar] [PubMed]
- Arnold M, Rutherford MJ, Bardot A, Ferlay J, Andersson TM, Myklebust TÅ, et al.Progress in cancer survival, mortality, and incidence in seven high-income countries 1995–2014 (ICBP SURVMARK-2): A population-based study. Lancet Oncol 2019;20(11):1493-505.
[Crossref] [Google Scholar] [PubMed]
- Cai QH, Tang Y, Fan SH, Zhang ZF, Li H, Huang SQ, et al.In vivo effects of dexmedetomidine on immune function and tumor growth in rats with ovarian cancer through inhibiting the p38MAPK/NF-κB signaling pathway. Biomed Pharmacother 2017;95:1830-7.
[Crossref] [Google Scholar] [PubMed]
- Tian H, Hou L, Xiong Y, Cheng Q. Dexmedetomidine upregulates microRNA-185 to suppress ovarian cancer growth via inhibiting the SOX9/Wnt/β-catenin signaling pathway. Cell Cycle 2021;20(8):765-80.
[Crossref] [Google Scholar] [PubMed]
- Zheng L, Jia R, Zhao J. Dexmedetomidine regulates proliferation, apoptosis, migration, and invasion in ovarian cancer cells via MiR-155-HIF-1α axis. Med Sci Monit 2019;25:10164.
[Crossref] [Google Scholar] [PubMed]
- Graham JG, Quinn ML, Fabricant DS, Farnsworth NR. Plants used against cancer: An extension of the work of Jonathan Hartwell. J Ethnopharmacol 2000;73(3):347-77.
[Crossref] [Google Scholar] [PubMed]
- Hsu S, Singh B, Schuster G. Induction of apoptosis in oral cancer cells: Agents and mechanisms for potential therapy and prevention. Oral Oncol 2004;40(5):461-73.
[Crossref] [Google Scholar] [PubMed]
- Cragg GM, Pezzuto JM. Natural products as a vital source for the discovery of cancer chemotherapeutic and chemopreventive agents. Med Princ Pract 2016;25(Suppl.2):41-59.
[Crossref] [Google Scholar] [PubMed]
- Amiel E, Ofir R, Dudai N, Soloway E, Rabinsky T, Rachmilevitch S. β-Caryophyllene, a compound isolated from the biblical balm of gilead (Commiphora gileadensis), is a selective apoptosis inducer for tumor cell lines. Evid Based Complement Alternat Med 2012;2012:872394.
[Crossref] [Google Scholar] [PubMed]
- Scarpulla RC. Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochim Biophys Acta 2011;1813(7):1269-78.
[Crossref] [Google Scholar] [PubMed]
- LeBleu VS, O’Connell JT, Gonzalez Herrera KN, Wikman H, Pantel K, Haigis MC, et al.PGC-1α mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat Cell Biol 2014;16(10):992-1003.
[Crossref] [Google Scholar] [PubMed]
- Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 1998;92(6):829-39.
[Crossref] [Google Scholar] [PubMed]
- Bajpai J, Susan D. Adjuvant chemotherapy in soft tissue sarcomas… Conflicts, consensus, and controversies. South Asian J Cancer 2016;5(1):15-9.
[Crossref] [Google Scholar] [PubMed]
- Masuike Y, Tanaka K, Makino T, Yamasaki M, Miyazaki Y, Takahashi T, et al.Esophageal squamous cell carcinoma with low mitochondrial copy number has mesenchymal and stem-like characteristics, and contributes to poor prognosis. PLoS One 2018;13(2):e0193159.
[Crossref] [Google Scholar] [PubMed]
- Cantó C, Auwerx J. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr Opin Lipidol 2009;20(2):98.
[Crossref] [Google Scholar] [PubMed]
- Xie H, Lev D, Gong Y, Wang S, Pollock RE, Wu X, et al.Reduced mitochondrial DNA copy number in peripheral blood leukocytes increases the risk of soft tissue sarcoma. Carcinogenesis 2013;34(5):1039-43.
[Crossref] [Google Scholar] [PubMed]
- O'Hara R, Tedone E, Ludlow A, Huang E, Arosio B, Mari D, et al.Quantitative mitochondrial DNA copy number determination using droplet digital PCR with single-cell resolution. Genome Res 2019;29(11):1878-88.
[Crossref] [Google Scholar] [PubMed]
- Reznik E, Miller ML, ?enbabao?lu Y, Riaz N, Sarungbam J, Tickoo SK, et al.Mitochondrial DNA copy number variation across human cancers. Elife 2016;5:e10769.
[Crossref] [Google Scholar] [PubMed]
- Araujo LF, Siena AD, Plaça JR, Brotto DB, Barros II, Muys BR, et al.Mitochondrial transcription factor A (TFAM) shapes metabolic and invasion gene signatures in melanoma. Sci Rep 2018;8(1):1-4.
- Waza AA, Andrabi K, Hussain MU. Adenosine-triphosphate-sensitive K+ channel (Kir6. 1): A novel phosphospecific interaction partner of connexin 43 (Cx43). Exp Cell Res 2012;318(20):2559-66.
[Crossref] [Google Scholar] [PubMed]
- Kobayashi Y, Kashima H, Wu RC, Jung JG, Kuan JC, Gu J, et al.Mevalonate pathway antagonist suppresses formation of serous tubal intraepithelial carcinoma and ovarian carcinoma in mouse models. Clin Cancer Res 2015;21(20):4652-62.
[Crossref] [Google Scholar] [PubMed]
- Fu B, Yin G, Song K, Mu X, Xu B, Zhang X. Indirubin-3′-oxime (IDR3O) inhibits proliferation of osteosarcoma cells in vitro and tumor growth in vivo through AMPK-activation and PGC-1α/TFAM up-regulation. Dokl Biochem Biophys 2020;495(1):354-60.
[Crossref] [Google Scholar] [PubMed]
- Wang L, Xue GB. Catalpol suppresses osteosarcoma cell proliferation through blocking epithelial-mesenchymal transition (EMT) and inducing apoptosis. Biochem Biophys Res Commun 2018;495(1):27-34.
[Crossref] [Google Scholar] [PubMed]
- Xie L, Ji T, Guo W. Anti-angiogenesis target therapy for advanced osteosarcoma. Oncol Rep 2017;38(2):625-36.
[Crossref] [Google Scholar] [PubMed]
- Liu R, Fu C, Sun J, Wang X, Geng S, Wang X, et al.A new perspective for osteosarcoma therapy: Proteasome inhibition by MLN9708/2238 successfully induces apoptosis and cell cycle arrest and attenuates the invasion ability of osteosarcoma cells in vitro. Cell Physiol Biochem 2017;41(2):451-65.
[Crossref] [Google Scholar] [PubMed]
- Arul S, Rajagopalan H, Ravi J, Dayalan H. Beta-caryophyllene suppresses ovarian cancer proliferation by inducing cell cycle arrest and apoptosis. Anticancer Agents Med Chem 2020;20(13):1530-7.
[Crossref] [Google Scholar] [PubMed]
- Bartke T, Siegmund D, Peters N, Reichwein M, Henkler F, Scheurich P, et al.p53 upregulates cFLIP, inhibits transcription of NF-κB-regulated genes and induces caspase-8-independent cell death in DLD-1 cells. Oncogene 2001;20(5):571-80.
[Crossref] [Google Scholar] [PubMed]
- Chaitanya GV, Alexander JS, Babu PP. PARP-1 cleavage fragments: Signatures of cell-death proteases in neurodegeneration. Cell Commun Signal 2010;8(1):1-1.
[Crossref] [Google Scholar] [PubMed]
- Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol 2011;13(9):1016-23.
[Crossref] [Google Scholar] [PubMed]
- He G, Zhang YW, Lee JH, Zeng SX, Wang YV, Luo Z, et al.AMP-activated protein kinase induces p53 by phosphorylating MDMX and inhibiting its activity. Mol Cell Biol 2014;34(2):148-57.
[Crossref] [Google Scholar] [PubMed]
- Dowling RJ, Zakikhani M, Fantus IG, Pollak M, Sonenberg N. Metformin inhibits mammalian target of rapamycin dependent translation initiation in breast cancer cells. Cancer Res 2007;67(22):10804-12.
[Crossref] [Google Scholar] [PubMed]
- Kimura N, Tokunaga C, Dalal S, Richardson C, Yoshino KI, Hara K, et al.A possible linkage between AMP?activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR) signalling pathway. Genes Cells 2003;8(1):65-79.
[Crossref] [Google Scholar] [PubMed]
- Wallace DC. Mitochondria and cancer. Nat Rev Cancer 2012;12(10):685-98.
[Crossref] [Google Scholar] [PubMed]
- Chatterjee A, Dasgupta S, Sidransky D. Mitochondrial subversion in cancer. Cancer prevention research. 2011 May 1;4(5):638-54.
[Crossref] [Google Scholar] [PubMed]