- Corresponding Author:
- P. Chattopadhyay
Cellular Biology Laboratory, College of Pharmacy, IFTM, Lodhipur Rajput, Moradabad - 244 001
E-mail: chatto-pronobesh@rediffmail.com
| Date of Submission | 25 May 2009 |
| Date of Revision | 13 May 2010 |
| Date of Acceptance | 24 July 2010 |
| Indian J Pharm Sci, 2010, 72 (4): 437-441 |
Abstract
Ischemic and reperfusion injury leads to necrosis and apoptosis. Mitochondrial enzymes and antiapoptotic gene plays an important role in necrosis and apoptosis. The aim of this study was to investigate the role of Bcl-2 expression in alternations in mitochondrial energy regulation during hepatic ischemia and reperfusion and role in necrosis and apoptosis. Total 12 Wistar rats were divided into sham-operated control group (I) and ischemia and reperfusion group (II). Mitochondrial tri carboxylic acid cycles marker enzymes, respiratory marker enzymes, apoptotic cells, necrotic cells and Bcl-2 expression was measured. Number of necrotic and apoptotic cells were increased in ischemic and reperfusion group with reducing tri carboxylic acid cycles marker enzymes, respiratory marker enzymes and decreasing of Bcl-2 expression. On the basis of our findings it may be concluded that suppression of Bcl-2 gene, inhibition of tri carboxylic acid cycles and respiration rate, adenosine tri phosphate production in mitochondria is a pathophysiological consequences which provides a clue for necrosis and apoptosis in hepatic ischemic and reperfusion injury.
Keywords
Bcl-2 gene, liver ischemia, mitochondrial enzymes, reperfusion injury
Bcl-2 is an antiapoptotic gene that was originally found in over expression of B-cell lymphoma [1]. Recent studies suggested that Bcl-2 has an important role in regulating mitochondrial metabolism and function [2,3], and that its protective effect may not be limited to an apoptotic role only. Ischemia and reperfusion (I/R) injury is a phenomenon whereby cellular damage is caused by hypoxic condition and further damaged caused by resortoration of oxygen delivery in the liver. Deprivations of oxygen, nutrients, or growth factors are important to cause ischemic and reperfusion injury which leads to necrosis and apoptosis during hepatic I/R injury [4].
Oxidant stress-induced cytotoxicity is mediated through oxidation of pyridine nucleotides, mitochondrial calcium overload, generation of superoxide radicals, formation of membrane permeability transition pores leading to breakdown of the mitochondrial membrane potential [5]. It has been suggested that antiapoptotic family members such as Bcl-2 oppose release of cytochrome c release and blocking or opening of a cytochrome c release pathway. Mitochondrial generation of ATP occurs via the F1Fo-ATPase, which generated from tri carboxyl pathway (TCA) pathway. Cell energetic depends on the appropriate transport of ADP into mitochondria and transport of ATP to the cytosol.
During ischemia, when lack of oxygen inhibits mitochondrial electron transport and mitochondrial generation of ATP, the F1Fo-ATP can in reverse and consume TCA generated ATP [6,7]. Thus Bcl-2 mediated alternation in adenine nucleotide transport has important implications of cell energetic and ATP synthesis.
There are no reports on the relationship between the mitochondrial TCA pathway and apoptosis in ischemia followed by reperfusion injury; in addition, there is a lack of information to correlate Bcl-2 gene expression with TCA pathway during ischemia followed by reperfusion injury. In this study, our objective was to obtain insight into between the relations with ischemia -reperfusion induced necrosis and apoptosis specifi c overexpression of Bcl-2 suppresses cell death as well as well as potential mechanisms by which Bcl-2 might modulate cellular metabolism.
Materials and Methods
Dithiothreitol was purchased from Sisco Laboratories, Mumbai, India. CGT-CAT-AAC-TAA-AGA-CACCCC and TTC-ATC-TCC-AGT-ATC-CGA-CTC sequence primer were custom-synthesized from Integrated DNA Technologies, Inc. Milpitas, CA. Ampli Taq Polymerase and 1 kbp DNA ladder were purchased from Bangalore Geni, Bangalore, India. AMV reverse transcriptase and RNase were procured from Boehringer, Mannheim, Mannheim, Germany. Protease inhibitor procured from Amersham Pharmacia Biotech, Uppsala, Sweden, Mouse Bcl-2 antibody and glyceraldehydes-3-phosphate dehydrogenase (G-3- PDH) from Bio source International, NY, USA and Propidium iodides, Annexin V-FITC, 4,6-diamidino- 2-phenylindole Hoechst 33258 were procured from Sigma Chemical Co., St. Louis, MO, USA. If not mentioned other chemicals were procured from Hi- Media, Mumbai.
Animal grouping
Twelve Wistar rats were divided into sham-operated group (I, n=6) and ischemia and reperfusion group (II, n=6). All animals received human care in compliance with the Guide for the Care and Use of Laboratory Animals. Experimental protocols were reviewed and approved by Institutional Animals Ethical Committee. The hepatic I/R protocol were performed as per procedure of Chattopadhyay et al. [8]
Tissue procurement
After sacrifice, portions of the ischemic and nonischemic liver lobe were snap frozen in liquid nitrogen and stored at -70° for reverse- transcription polymerase chain reaction (RT-PCR), western blot, mitochondria isolation, flow-cytometry and fl uorescence microscopy.
Isolation of mitochondrial enzyme and assay of TCA, respiratory marker enzymes
The mitochondria of liver were isolated by the method of Jonshon and Lardy [9]. The activities of TCA marker mitochondrial enzymes malate dehydrogenase (MDH) [10], succinate dehydrogenase (SDH) [11], α-ketoglutarate dehydrogenase (α-KGDH) [12], NADH dehydrogenase [13] and cytochrome -C- oxidases [14] were determined.
Measurement of intracellular ATP
Hepatocytes were prepared by the collagenase perfusion method [15] and isolated hepatocytes (4×102 cells/ml) were supplemented with 3 ml of ice cold 6% (w/v) perchloric acid, sonicated and left at 0o for 20 min. The extracts were centrifuged to remove protein and neutralized with ice cold 6 M NaOH/0.5 M morpholine sulphonic acid. ATP content was determined in neutralized cellular extracts by the luciferase-luciferin assay [16].
Reverse transcriptase polymerase chain reaction assay
Total cytoplasmic RNA of each group was isolated from liver tissue by the Trizol method (according to GIBCO specifi cation). Polymerase chain reaction (PCR) primer was against rat Bcl-2 sequences obtained from gene bank (Fastaf). The sense primer used was a 21–mer with a sequences of CGT-CATAAC- TAA-AGA-CAC-CCC and the reverse primer was also a 21 mer with a sequences of TTC-ATCTCC- AGT-ATC-CGA-CTC. The product length was 234 base pairs Tm of 52.3o. The cDNA was amplifi ed by PCR amplification with Ampli Taq Polymerase. Amplified product was resolved by electrophoresis on 1.5% agarose gels (Sigma, St Louis, USA), stained with ethidium bromide and visualized under ultraviolet light. A 1 kbp DNA ladder molecular weight marker was run on every gel to confirm expected molecular weight of the amplification product. Bands were quantitatively measured by densitometry analysis system (Molecular Analyst/ PC, Windows software for Bio-Rad’s (Hercules CA). Image Analysis System Version 1.5, and the data are expressed in relative optical density (OD) units.
Western blot analysis
Cell lysates were prepared from liver and lysed in a buffer contains 1% triton X-100 10 mM Tris (PH 7.4), 150 mM NaCl, 2 mg/ml aprotinin and 10 mlmol and phenylmethyl sulfonyl fluoride (PMSF). Protein samples (50 μg) were analyzed by sodium dodecyl sulphate (SDS) poly acrylamide gel electrophoresis (PAGE) under reducing condition transferred overnight to nylon membrane. Both were incubated with rabbit anti- mouse/rat Bcl-2 primary antibody, followed by peroxides-labeled goat anti-rabbit secondary antibody (Bio source international, USA) and bound antibody were detected by enhanced chemiluminescences. Bands were quantitatively measured by densitometry analysis system.
Flow cytometry analysis
Hepatocytes (1×109/l) of each group were washed with phosphate buffer saline (PBS) at 4º and exposed to pre-chilled ethanol at 4º for 30 min. They were then washed again with PBS, exposed to PI 50 mg/l, 0.1% Triton X-100, 0.01 mmol/l EDTA (Na)2 and RNase 50 mg/l at normal temperature in darkness for 12-24 h. Specimen were then subjected to the FACS-420 Flow Cytometry Analyzer (New York, Becton Dickinson and Company, USA) to evaluate apoptosis levels.
Fluorescence microscopy
Cells were washed with PBS and fixed with PBSbuffered (pH 7), 4% formaldehyde, 1.5% methanol solution (CDH, Mumbai) at 4° for 15 min, then washed three times with PBS (Life Technologies, Inc.). Twenty five microlitre cell suspension was taken in a PCR Tubes and 5 μl ethidium bromide and acridine orange (100 mg/ml) added. Next, the cover slips were put on slides coated with buffered mounting medium consisting of 90% glycerol, 10% PBS with 0.1% NaN3 and 3% DABCO (triethylenediamine, Sigma Chemical Co., St. Louis, MO), to prevent fading. Examination was done with inverted Olympus microscope (Japan).
Statistical analysis
Data are expressed as mean (SD). The signifi cance of difference was analyzed by one-way ANOVA followed by Tukey’s post-hoc test. P<0.05 was considered signifi cant.
Results and Discussion
Data in Table 1 represents the activities of TCA cycle marker enzymes (MDH, SDH, α- KGDH) and respiratory marker enzymes of mitochondria (NADH dehydrogenase and cytochrome –C-oxidases) in the liver of sham operated control and I/R group of rats. The activities of mitochondrial TCA marker enzymes and respiratory marker enzymes showed signifi cantly (P<0.05) decreased in group II rats as compared group I.
| Groups | MDH# | SDH† | α-KGDH‡ | NADH* | cyt–C*† |
|---|---|---|---|---|---|
| Sham-operated (Group I) | 319.11 ± 14.0 | 247.44 ± 24.8 | 74.20 ± 5.8 | 121.47 ± 9.7 | 0.34 ± 0.02 |
| I/R Injury (Group II) | 198.11 ± 24.2a | 107.02 ± 15.1 | 24.51 ± 9.0a | 60.44 ± 7.5a | 0.15 ± 0.04a |
Results are expressed as mean±SD (n=6). P<0.05a Comparison are made between sham operated control (group I) with I/R injury (group II) #expressed as nmol of NADH oxidized per min per mg protein for MDH. †expressed as nmol of succinate oxidized per min per mg protein for SDH. ‡expressed as nmol of ferrocyanide formed per hour per mg protein for αKGDH.*expressed as nmol of NADH oxidized per min per mg protein for NADH dehydrogenase.*†expressed as nmol per min per mg of protein for cytochrome-C-oxidases.
Table 1: Mitochondrial tca and respiratory marker enzymes in liver after ischemia reperfusion.
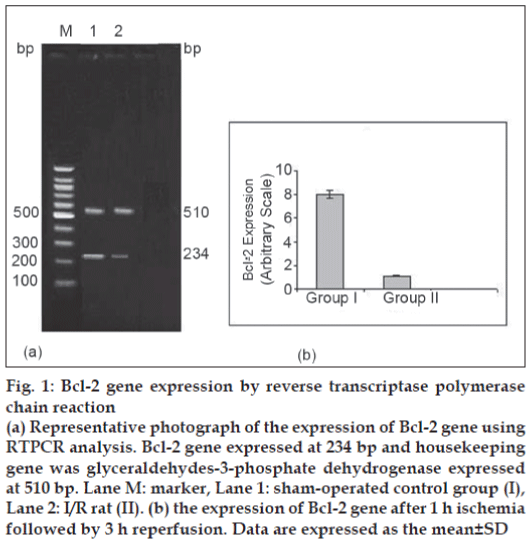
In the present study, 20 μl Bcl-2 and GPDH PCR products were separated by electrophoresis and stained with EB. PCR products of Bcl-2, GPDH were expressed at 234 bp and 510 bp, respectively (fig. 1). Expression of Bcl-2 gene in group II was lower (P<0.05) in comparison with sham-operated group after 1 h ischemia followed by 3 h reperfusion.
Figure 1: Bcl-2 gene expression by reverse transcriptase polymerase chain reaction
(a) Representative photograph of the expression of Bcl-2 gene using RTPCR analysis. Bcl-2 gene expressed at 234 bp and housekeeping gene was glyceraldehydes-3-phosphate dehydrogenase expressed at 510 bp. Lane M: marker, Lane 1: sham-operated control group (I), Lane 2: I/R rat (II). (b) the expression of Bcl-2 gene after 1 h ischemia followed by 3 h reperfusion. Data are expressed as the mean ± SD
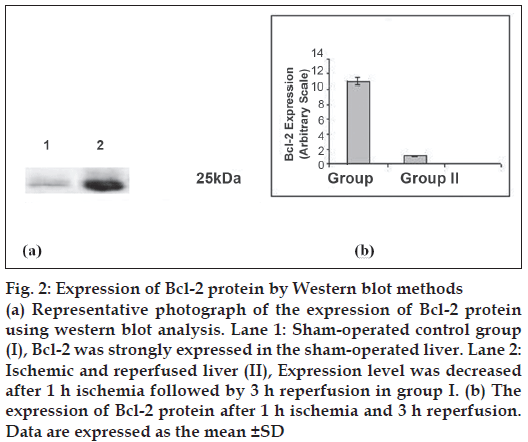
Bcl-2 protein was expressed at 25 kDa (fig. 2) and decreased lower (P<0.05) level in group II as compared to sham-operated group after 1 h ischemia followed by 3 h reperfusion. Percentage of necrosis, apoptosis of hepatocytes and percentage ATP production are shown in Table 2. Percentage of necrosis and apoptosis signifi cantly (P<0.05) increased and percentage ATP content significantly (P<0.05) decreased in I/R group with compared to sham operated rat.
Figure 2: Expression of Bcl-2 protein by Western blot methods
(a) Representative photograph of the expression of Bcl-2 protein using western blot analysis. Lane 1: Sham-operated control group (I), Bcl-2 was strongly expressed in the sham-operated liver. Lane 2: Ischemic and reperfused liver (II), Expression level was decreased after 1 h ischemia followed by 3 h reperfusion in group I. (b) The expression of Bcl-2 protein after 1 h ischemia and 3 h reperfusion. Data are expressed as the mean ± SD
| Group | % of Necrotic cells | % of Apoptotic cells | % of ATP production |
|---|---|---|---|
| Sham-operated control Group (I) | 1.06±0.2 | 1.30±0.5 | 100 (Base value) |
| Ischemia and reperfusion Group (II) | 28.44±4.7a | 14.44±6.0a | 12.49 |
Mean±SD, n=6. aComparison are made between sham-operated control (group I) with I/R injury (group II).
Table 2: Apoptotic and necrotic cells and atp levels in liver after ischemia reperfusion.
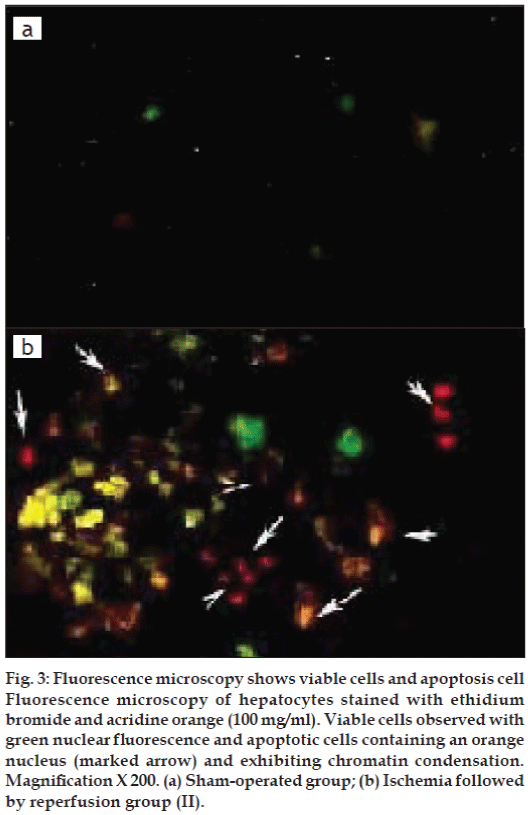
Apoptosis was evaluated based on some distinct morphological features such as cell shrinkage, chromatin condensation, oligonucleosomal DNA fragmentation and finally break down of the cell into smaller units (apoptotic bodies). Sham operated rat showed re no sign of apoptosis (fig. 3a) whereas reperfusion of the ischemic liver caused severe hepatocellular apoptosis after 1 h ischemia followed by 3 h reperfusion in Group II (fig. 3b).
Figure 3: Fluorescence microscopy shows viable cells and apoptosis cell Fluorescence microscopy of hepatocytes stained with ethidium bromide and acridine orange (100 mg/ml). Viable cells observed with green nuclear fl uorescence and apoptotic cells containing an orange nucleus (marked arrow) and exhibiting chromatin condensation. Magnifi cation X 200. (a) Sham-operated group; (b) Ischemia followed by reperfusion group (II).
Mitochondrion is most susceptible organelle for oxidative damage during I/R. Ex vivo models of I/R have demonstrated that decline in activities of mitochondrial electron transport chain components as well as Krebs cycle marker enzymes leads to mitochondrial damage [17]. The magnitude of decline in activity of electron transport chain components is not directly proportional to decline in ADP linked respiration in I/R injury [18]. Moreover, defects in Krebs cycle enzymes are detrimental factors and are rate limiting steps in mitochondrial respiration in I/R injury [19]. Therefore, investigation of Krebs cycle marker enzymes and mitochondrial respiration were most important parameter in our study.
In this study we found that suppression of Bcl-2 showed increased numbers of necrotic and apoptotic cells. These findings are consistent with previous study that overexpression of Bcl-2 reduced ischemia/ reperfusion injury [20]. In addition, this study provides new insight into the mechanism by which Bcl-2 mediates hepato-protective. Depletion of ATP was observed during I/R injury, which provides a evidence that the rate of mitochondrial free radical production is inversely proportional to the rate of electron transport and this observation supports previous work [21].
Mitochondrial degeneration and dysfunction has been reported in I/R injury. The decrease activities of TCA cycle (MDH SDH α-KGDH) enzymes were observed in I/R induced injury rats. The impaired mitochondrial function, which may contribute to the pathogenesis of I/R induced toxicity, might be a reason for observed decrease in the activities of TCA cycle enzymes [22]. Under ischemic conditions (anaerobic phase), ATP produced by TCA using glucose or storage glycogen which can account for 30-50% of total ATP production [22]. The inhibition of mitochondrial respiration by low oxygen supply results in stimulation of glycolytic flux [23]. This phenomenon is known as Pasteur effect and indicates that in hepatocytes TCA and mitochondrial respiration closely functionally connected [24]. The activities of respiratory marker enzymes NADH dehydrogenase and cytochrome –C-oxidases were lowered in I/R induced rats. Decreased activity of mitochondrial and respiratory marker enzymes in ischemic condition has been previously reported [25]. Much less is known about the mitochondrial energy production in the process of necrosis and apoptosis. Studies preformed to understand the relation between apoptosis, necrosis, Bcl-2 overexpression and mitochondrial energy production and respiratory enzymes.
In conclusion, we fi nd that Bcl-2, in addition reducing ischemic followed by reperfusion induced necrosis and apoptosis, decline ATP production by inhibition of TCA cycles as well as inhibition of mitochondrial respiration. Although it is difficult to analyze the pathophysiological mechanism of apoptosis involved in I/R mediated injury, our finding showed that the suppression of Bcl-2 gene and inhibition of TCA cycles may provide a clue.
References
- Cleary ML, Sklar J. Nucleotide sequence of at (14:18) chromosomal breakpoint in follicular lymphoma and demonstration of a breakpoint cluster region ner a transcriptionally active locus on chromosome 18. ProcNatlSci USA 1998;82:7439-43.
- Garland JM, Halestrap A. Energy metabolism during apoptosis: Bcl-2 Promoter’s survival in hepatopoitic cells induced to apoptosis by growth factor withdrawal by stabilizing a form of metabolism arrest. J BiolChem 1997;272:4680-8.
- Manfredi G, Kwong JQ, Oca–Cossio JA, Woischinick M, gajewski CD, Martushova K, et al. Bcl-2 improves oxidative posphorylation and modulates adenine nucleotide translocations in mitochondria of cells harboring mutant mtDNA. J BiolChem 2003;278:5639-46.
- Cory S, Huang DC, Adams JM. The Bcl-2 family: Roles in cell survival oncogenesis. Oncogene 2003;22:8590-607.
- Carden DL, Young JA, Granger DN. Pulmonary microvascular injury after intestinal ischemia-reperfusion: Role of P-selectin. J ApplPhysiol 1993;75:2529-34.
- Vuorinen K, Ylitalo K, Peuhkurienen K, Raatikainen P, Ala–Rami A, Hassienen IE. Mechanisms of ischemic preconditioning in rat myocardium: Roles adenosine, cellular energy state and mitochondrial F1Fo- ATPase. Circulation 1995;9:2810-8.
- Di Lisa F, Blank PS, Colonna R, Gambassi G, Silverman HS, Stren MD, et al. Mitochondrial membrane potentials in single living adult rat cardiac myocytes exposed to anoxia or metabolic inhibition. J Physiol 1995;486:1-13.
- Chattopadhyay P, Sharma AK, Wahi AK. Folic acid protects hepatobiliary function in ischemic reperfusion of rat liver. Indian J Gastroenrtol 2007;26:95-6.
- Jonshon D, Lardy H. Isolation of liver kidney mitochondria. In: Estarbook RW, editor. Methods in Enzymology. Vol. 10. London: Academic Press; 1947. p. 94-6.
- Mehler AH, Kornberg A, Grisolia S, Ochoa S. The enzymatic mechanism of oxidation- reductions between malate or isocitrate or pyruvate. J BiolChem 1948;174:961-77.
- Slater EC, Bonner WD. The effect of fluoride on the succinic oxidases system. Biochem J 1952;52:185-96.
- Reed LJ, Mukherjee RB. α-Ketoglutarate dehydrogenase complex from Escherichia coli. In: Lowenstein JM, editor. Methods in Enzymology. Vol. 13. London: Academic Press; 1969. p. 3-11.
- Minakami S, Ringler RL, Singer TP. Studies on the respiratory chain linked dihydrodiphospho pyridine nucleotide dehydrogenase: Assay of the enzyme in particulate and insoluble preparations. J BiolChem 1962;237:569-76.
- Pearl W, Cancercao J, Zweifach BW. Micro determination of cytochrome oxidase in rat tissues by the oxidation of N-phenyl-phenylenediamine of ascorbic acid. J HistochemCytochem 1963;11:102-4.
- Kreamer Bl, Staecker JL, Sawada N, Sattler GL, Hsia MT, Pitot HC. Use of low- speed, iso-density percoll centrifugation method to increase the viability of isolated rat hepatocytes preparations. In Vitro Cell Dev Biol 1986;22:201-11.
- Havertown PA. Chrono-Long Diagnostic Protocol. Manual for testing with the whole-blood aggregometer model 550. Havertown, PA: Chrono-Long Corp; 1987.
- Lucas DT, Szweda LI. Cardiac reperfusion injury: Aging, lipid peroxidation, and mitochondrial dysfunction. Proc Natl Acad Sci USA 1998;95:510-4.
- Lucas DT, Szweda LI. Declines in mitochondrial respiration during cardiac reperfusion: Age- dependent inactivation of α-ketoglutaratedehydogenase. Proc Natl Acad Sci USA 1999;96:6689-93.
- Weiss RG. Tricarboxylic acid cycle activity in postischemic rat hearts. Ciculation 1993;87:270-82.
- Chen Z, Chua CC, Ho YS, Hamdy RC, Chua BH. Overexpression of Bcl-2 attenuates apoptosis and protects against myocardial I/R injury in transgenic mice. Am J Physiol 2001;280:H2312-20.
- Matsuyama S, Lipoid J, Deveraux Q, Tsien R, Reed J. Changes in intra- mitochondrial and cystolic pH: Early events that modulate caspase activation during apoptosis. Nat Cell Biol 2000;2:318-25.
- Malarkodi KP, Balachander AV, Varalakshmi P. Influence of lipoic acid on adrianycin induced nephotoxicity in rats. Mol Cell Biochem 2003:247;15-22.
- Niu X, Artur P, Abas L, Whisson M, Guppy M. Carbohydrate metabolism in human platelets. BiochemBiophysActa 1996;396:1-10.
- Guppy M, Whisson ME, Sabaratnam R, Witheres P, Brand K. Alternative fuels for platelet storage: A metabolic study. Vox Sang 1990;59:146-52.
- Vijayapadma V, Shyamaladevi CS. Effect of fish oil on mitochondrial respiration in isoproterenol induced myocardial infraction in rats. Indian J Exp Biol 2001;40:268-72.