- *Corresponding Author:
- Tannaz J. Birdi
The Foundation for Medical Research, 84A, RG Thadani Marg, Worli, Mumbai-400018, India
E-mail: fmr@fmrindia.org
| Date of Submission | 17 September 2013 |
| Date of Decision | 23 March 2014 |
| Date of Acceptance | 31 March 2014 |
| Indian J Pharm Sci 2014;76(3): 229-235 |
Abstract
Diarrhoeal diseases due to enterotoxigenic Escherichia coli continue to be a cause of global concern. Medicinal plants have been gaining popularity as promising antidiarrhoeal agents. In the present study, four antidiarrhoeal plants, viz. Aegle marmelos, Cyperus rotundus, Psidium guajava and Zingiber officinale were screened against a heat-stable toxin-producing enterotoxigenic E. coli strain. Decoctions of these plants were studied for their effect on intracellular killing of the bacterial strain using murine monocytic cell line, J774. [ 3 H] thymidine release assay was used to evaluate the apoptotic/necrotic effect. All plants at concentrations <1% enhanced intracellular killing of the bacteria by J774 cells. However, at higher concentrations, the decoctions induced apoptosis in J774 cells. The study demonstrates that these plants could control diarrhoea caused by heat-stable toxin-producing enterotoxigenic E. coli through their immunomodulatory effect.
Keywords
Enterotoxigenic Escherichia coli, heat-stable toxin, medicinal plants, intracellular killing, J774 cells
Diarrhoeal diseases are a major health concern in developing countries with an estimated 1.8 million deaths per annum [1], with almost 18% of the deaths worldwide being in children under 5 years being attributable to diarrhoeal disease [2]. Despite improvements in public health and economic wealth, it remains an important clinical problem in developed countries as well [3]. It is estimated that infectious diarrhoea will remain a cause of global health concern in the next two to three decades [4]. Although a variety of enteric pathogens are responsible for infectious diarrhoea, Escherichia coli is recognized to be a common cause of gastroenteritis. Among the different strains of E. coli that cause human diarrhoea, enterotoxigenic E. coli (ETEC) alone is responsible for 280 million to 400 million episodes of diarrhoea, leading not only to malnutrition but also about 380,000 deaths annually [5]. ETECs are a cause of concern due to high levels of antibiotic resistance being reported from different regions [6,7]. ETEC strains elaborate two defined groups of enterotoxins: Heatstable (ST) and heat-labile (LT) toxins. The three toxin profiles LT/ST, ST-only and LT-only are found worldwide with varying frequencies.
In recent years, medicinal plants have gained popularity as prospective antidiarrhoeal agents, with a large numbers of studies being published in the past decade across the globe. Most of the studies have focused on the effect of the plants on intestinal motility in experimental models [8]. Although there are reports on the antimicrobial profile of plants against diarrhoeal pathogens, few studies have reported other possible mechanism(s) of action in controlling infectious diarrhoea. With respect to ETECs, the antisecretory activity of medicinal plants against intestinal secretion induced by E. coli enterotoxins has been demonstrated. However, most of the studies have focused on the effect of medicinal plants on E. coli LT toxin only [9-11]. To the best of our knowledge, there is no information available on the effect of medicinal plants against pathogenicity of ST-producing ETEC. Hence, the focus of the present study was to investigate the possible mechanism(s) of action of selected plants against ST-producing ETECs.
Four plants, viz. Aegle marmelos L. Correa (unripe fruit), Cyperus rotundus L. (tubers), Psidium guajava L. (leaves) and Zingiber officinale Rosc. (rhizome) were selected for the present study as they have been reported in the literature to have anti-diarrhoeal activity. The unripe fruit of A. marmelos is known to be one of the ancient and effective anti-diarrhoeal plants, and has also been included in the British Pharmacopoeia [12]. C. rotundus is a popular folk medicine for various stomach disorders [13]. The leaves of P. guajava are widely used globally for diarrhoeal diseases [14] and Z. officinale is a common spice/flavouring agent and also used as a digestive aid [15]. The Chinese and the Americans have included Z. officinale in their official pharmacopoeias [15]. Moreover, these plants had been cited in an ethnobotanical survey of antidiarrhoeal plants [16] and we had also reported their activity against diarrhoeal pathogenesis including their effect on ST-induced diarrhoea [17-20]. However, these studies showed that none of the four plants showed any effect on either the viability of the STproducing ETEC strain TX1 or on the production/ action of ST. As these plants are also known to have immunomodulatory properties [13,14,21,22], we undertook further studies to understand their effect on the ETEC-producing ST using an alternative bioassay. In this study, we demonstrate that one of the possible ways through which medicinal plants can control pathogenicity of ST-producing ETEC could be through enhancement of the intracellular killing of these bacteria by macrophages.
The bacteriological media (Brain Heart Infusion broth, BHI) was purchased from HiMedia Laboratory, Mumbai, India. Dulbecco’s modified Eagle medium (DMEM) and foetal calf serum (FCS) were procured from GibcoBRL, UK. [3H]thymidine was purchased from Bhabha Atomic Research Centre, Mumbai, India and the glass fibre filter paper was purchased from Whatman International Limited, Maidstone, England. All chemicals were from S. D. Fine-Chem Ltd., Mumbai, India. The 24-well and the 96-well flat-bottomed tissue culture plates were purchased from Nunclon, Roskilde, Denmark, and the 55 mm diameter tissue culture plates were obtained from Tarsons, Kolkata, India. The liquid scintillation analyzer used was from PerkinElmer Life and Analytical Sciences, Downers Grove, IL, USA.
The ETEC strain TX1, ST-producing, serotype 078:H12 was obtained from Centre for Disease Control, Atlanta, USA and stored at -80° in BHI containing 20% glycerol. For each assay, a frozen stock of the bacterial strain was revived and passaged in BHI to obtain a logarithmic phase culture. The murine monocytic cell line, J774, was a generous gift from the Department of Biological Sciences, Tata Institute of Fundamental Research, Mumbai, India. The cell line was maintained by passage every 2-3 days in DMEM supplemented with 10% FCS and gentamycin at 37° in 5% CO2 atmosphere.
The unripe fruits of A. marmelos, tubers of C. rotundus, leaves of P. guajava and rhizome of Z. officinale were collected from the Parinche valley, about 53 km south east of Pune city in the state of Maharashtra, India. The plant materials were authenticated at the Naoroji Godrej Centre for Plant Research (NGCPR), Shirwal, Maharashtra, India. Voucher specimen of A. marmelos, C. rotundus and P. guajava were deposited at the Botanical Survey of India (BSI), Western circle, Pune, Maharashtra, India, and that of Z. officinale at the herbarium at the NGCPR (Table 1). The plant materials were shade dried, powdered and stored at 4° until use.
| Botanical name | Common name | Family name | Herbarium number | Part used |
|---|---|---|---|---|
| AeglemarmelosL. | Bengal | Rutaceae | BSI‑124675 | Unripe |
| Correa | Quince, | fruit pulp | ||
| bilva | ||||
| CyperusrotundusL. Nutgrass | Cyperaceae | BSI‑124666 | Tubers | |
| PsidiumguajavaL. | Guava | Myrtaceae | BSI‑124672 | Leaves |
| Zingiberofficinale | Ginger | Zingiberaceae NGCPR‑642 | Rhizomes | |
| Rosc. | ||||
Table 1: Details Of The Plant Material Used In The Study
A crude aqueous extract (decoction) was prepared as described in Ayurvedic text [23], one gram of the powdered plant material was boiled in 16 ml distilled water till the volume reduced to 4 ml. It was centrifuged and filtered through a 0.22 μm membrane before use. For each assay, a fresh decoction was used and diluted to 0.05%, 0.1%, 1%, 5% and 10% as required in DMEM.
To study the effect of the decoctions on the intracellular killing of ST-producing ETEC by J774 cells, the method followed was a modification of the protocol described by Matsunaga et al. [24] Based on our studies reporting the antidiarrhoeal activity of the four plants [17-20], initially, the doses tested were 0.1%, 1%, 5% and 10%. However, it was observed microscopically that all the extracts affected the morphology of J774 cells at the higher concentrations (5% and 10%). Thus, the lower doses of 0.05%, 0.1% and 1% were used for the intracellular assays. E. coli TX1 (1×106/ mL) were added to J774 cells (4×104 cells/well) grown overnight in a 24-well tissue culture plate and incubated for 2 h. The noninternalized bacteria were washed off. The cells were then incubated in DMEM containing 100 μg/mL gentamycin for 3 h in the absence (control) and presence of different concentrations of the decoctions (test). This 3-h interval allowed gentamycin to kill the extracellular bacteria. Thereafter, the decoctions were washed off and the cells were further incubated in fresh DMEM containing gentamycin. After 18-20 h of incubation, the J774 cells were washed and lysed, and the viable intracellular bacteria enumerated by the spread plate technique.
As stated above, the decoctions of all four plants affected the morphology of J774 cells at the higher concentrations tested. This could be either due to apoptosis or necrosis [25,26]. Based on fluorescence microscopy using Hoechst and propidium iodine (data not shown), it was seen that the effect was due to apoptosis. Thus, the quantitation of apoptosis was undertaken using a modification of the [3H] thymidine release assay [27]. Briefly, the cell line J774 (1×104 cells/well) were pulsed with [3H] thymidine (1 μCi/well) in 96-well plates for 48 h. After washing, the cells were incubated for 3 h in DMEM without (control) and with different concentrations of the decoctions as discussed earlier (0.1%, 1%, 5% and 10%, test). The decoctions were then washed off and the macrophages were incubated in fresh DMEM. After 48 h of incubation, the radioactivity trapped onto glass fibre filter paper (GFPP) was measured in a liquid scintillation counter. Because the fragmented DNA of the apoptotic cells would pass through the GFFP, the radioactivity retained on the GFFP corresponded to non-fragmented DNA from the live cells and/ or necrotic cells. Butyrate (10 mM) was used as a positive control for apoptosis. The results have been expressed as the mean± standard error of the percentage values of the test groups relative to control from three independent experiments. The percentage intracellular killing of E. coli TX1 by J774 cells was calculated using the formula [(C or T)/C]×100, whereas the percentage death of J774 cells was calculated using the formula [{C-(C or T)}/C]×100, where C is the mean value of the triplicate readings of the control group and T is the mean value of the triplicate readings of the test groups. Data were analyzed by analysis of variance (ANOVA) and Dunnett’s post-test. P≤0.05 was considered to be statistically significant. The EC50 and IC50 values, wherever applicable, were calculated by non-linear regression analysis using the equation for a sigmoid concentration response curve. All statistical analyses were performed using the software Prism 4.0 (GraphPad Inc.).
The dry weight for each decoction was estimated and was found to be 51.1±0.5 mg/mL (yield 20.4±2.11% w/w) for A. marmelos, 52.2±2.8 mg/mL (yield 20.9±1.12% w/w) for C. rotundus, 27.0±1.25 mg/ mL (yield 10.8±0.5% w/w) for P. guajava and 22.1±0.03 mg/mL (yield 8.84±0.01% w/w) for Z. officinale. Phytoconstituents such as carbohydrates, flavonoids and tannins were reported to be present in the decoctions of all four plants [17-20].
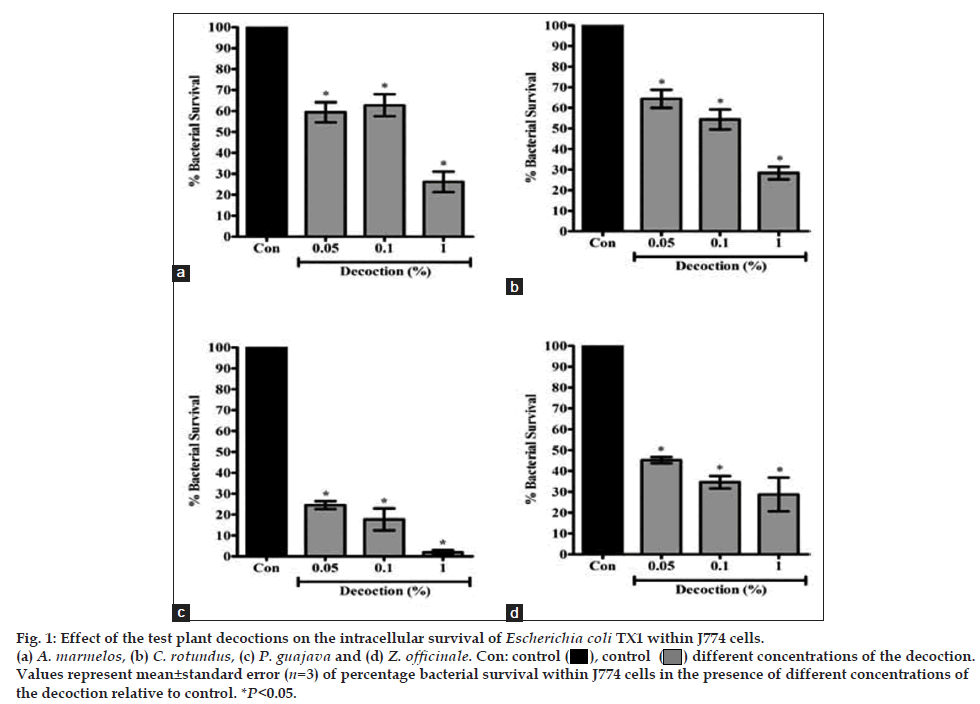
The effect of the decoctions on intracellular killing of E. coli TX1 by J774 cells has been depicted in fig. 1. Because the decoctions of all four plants resulted in cell death at >1% concentration, the data on effect of the decoctions at ≤1% concentration only has been presented. The results show that the decoctions of all four plants significantly enhanced the intracellular killing of E. coli TX1 by J774 cells at <1% concentrations. P. guajava was the most effective, with almost-complete killing of the E. coli at 1% concentration (EC50 value 0.013±0.001%), followed by Z. officinale (EC50 value 0.014±0.008%), A. marmelos (EC50 value 0.051±0.020%) and C. rotundus (EC50 value 0.062±0.046%).
Fig. 1: Effect of the test plant decoctions on the intracellular survival of Escherichia coli TX1 within J774 cells.
(a) A. marmelos, (b) C. rotundus, (c) P. guajava and (d) Z. officinale. Con: control ( ), control (
), control ( ) different concentrations of the decoction.
Values represent mean±standard error (n=3) of percentage bacterial survival within J774 cells in the presence of different concentrations of
the decoction relative to control. *P<0.05.
) different concentrations of the decoction.
Values represent mean±standard error (n=3) of percentage bacterial survival within J774 cells in the presence of different concentrations of
the decoction relative to control. *P<0.05.
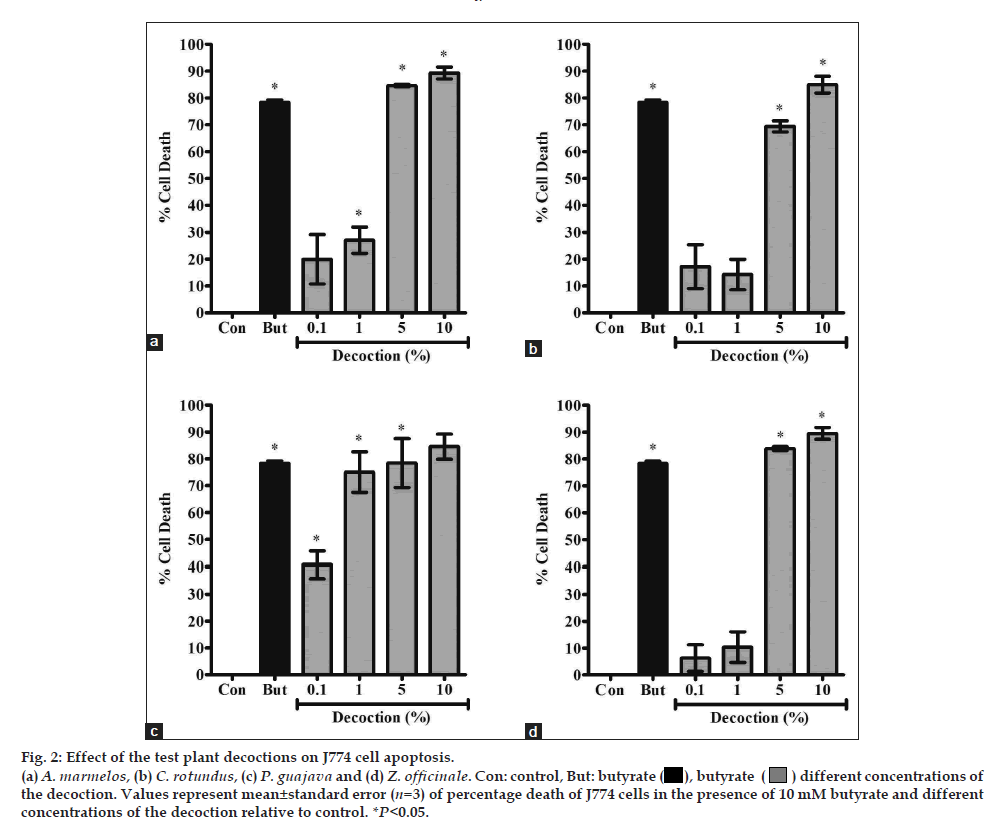
In order to assess the level of macrophage cell death at higher concentrations, the J774 cells were subjected to [3H]thymidine release assay in the presence of the decoctions of all four plants. The results of this assay showed that the decoctions of all four plants resulted in DNA fragmentation in >70% of the J774 cells at 5% and 10% (fig. 2). Among the four plants, P. guajava was found to be the most cytotoxic (IC50 value 0.363±0.337%), followed by A. marmelos (IC50 value 1.593±0.439%). C. rotundus (IC50 value 2.732±0.797%) and Z. officinale (IC50 value 2.246±0.484%) were comparatively less cytotoxic. Under fluorescence microscopy, only 10% of the non-fragmented DNA corresponded to necrotic cells (data not shown).
Fig. 2: Effect of the test plant decoctions on J774 cells apoptosis.
(a) A. marmelos, (b) C. rotundus, (c) P. guajava and (d) Z. officinale. Con: butyrate ( ), butyrate (
), butyrate ( ) different concentrations of the decoction.
Values represent mean±standard error (n=3) of percentage death of J774 cells in the presence of 10mM butyrate and different concentrations of
the decoction relative to control. *P<0.05.
) different concentrations of the decoction.
Values represent mean±standard error (n=3) of percentage death of J774 cells in the presence of 10mM butyrate and different concentrations of
the decoction relative to control. *P<0.05.
Diarrhoeal diseases continue to be a cause of global concern. Because of the limitations of modern medicine in the treatment of diarrhoea and increasing reports of drug resistance, medicinal plants have gained popularity as prospective anti-diarrhoeal agents. In the present study, four anti-diarrhoeal medicinal plants used in traditional medicine, viz. A. marmelos, C. rotundus, P. guajava and Z. officinale were assayed for their ability to stimulate macrophages to kill ST-producing ETEC strain TX1. All four plants have been previously shown to be ineffective in killing strain TX1 or inhibiting the production and action of ST [17-20].
The results of the study show that the decoctions of all four plants significantly enhance intracellular killing of ETEC TX1 by J774 cells at the lower concentrations of 0.05-1%. Although the decoctions of all four plants enhanced intracellular killing of ETEC TX1 at lower concentrations, they induced apoptosis in J774 cells at ≥5% concentrations. The pro-apoptotic activity of the plant decoctions only at higher concentrations could be due to a number of factors. This includes the presence of minimal quantities of some phytoconstituent(s) in the decoction, which therefore exhibit their activity only at higher concentrations. Escribano et al. (1999) had also reported apoptosis of macrophages due to a proteoglycan from Crocus sativus L. even at non cytotoxic concentrations [28]. Apoptosis of macrophages following exposure to the plant decoctions could also be a possible consequence of macrophage activation as it happens following activation with bacterial lipoproteins and lipopolysaccharides [29,30]. Activation of NF-κB induced by different stimuli is an important factor that provides protection against apoptotic killing of macrophages [31]. Constituents such as tannins and flavonoids are known to inhibit the activation of NF-κB [32]. As these phytoconstituents are present in the decoction of the four plants, it is possible that the pro-apoptotic activity observed in the study may have been through the inhibition of activation of NF-κB. It is known that the survival of monocytes following activation depends on the cytokine milieu encountered during differentiation [33,34]. In the present study, the macrophages activated by the plant decoctions could be undergoing apoptosis as they lacked the cytokine environment required for their survival in the in vitro system. Hence, it may be hypothesized that unlike the in vitro system, the plant decoctions may demonstrate a non-apoptotic, immunostimulating activity of macrophages in vivo due to the cytokine environment that would support macrophage survival even at higher concentrations (≥5%) of the plant decoctions. Thus, the J774 cell death induced by the plant decoctions in the present study could be an artefact of the in vitro system, especially because previous reports state that these plants are generally safe [14,22,35,36].
In conclusion, the results of the present study demonstrate that the decoctions of A. marmelos, C. rotundus, P. guajava and Z. officinale could be effective in controlling diarrhoea caused by STproducing ETEC, with P. guajava being the most effective. As these plants were previously shown to be ineffective in controlling ST, the study demonstrates the need for choosing appropriate and, where necessary, multiple bioassays for understanding the various possible mechanisms of action of medicinal plants.
Acknowledgements
This work has been supported by the Indian Council of Medical Research (Grant No. 59/10/2005/BMS/TRM). The authors acknowledge Dr. P. Tetali, NGCPR, for authenticating and providing the plant material. They also thank Dr. Roop Malik for gifting the J774 cell line used for the study.
References
- World Health Organization. World Health Report. Geneva: WHO; 2004.
- World Health Organization, World Health Report. Geneva: WHO; 2005.
- Casburn-Jones AC, Farthing MJ. Management of infectious diarrhoea.Gut 2004;53:296-305.
- Meyrowitsch DW, Bygbjerg IC. Global burden of disease - a race against time. Dan Med Bull 2007;54:32-4.
- World Health Organization. Future directions for research on enterotoxigenic Escherichia coli vaccines for developing countries. WklyEpidemiol Rec 2006;81:97-104.
- Pazhani GP, Chakraborty S, Fujihara K, Yamasaki S, Ghosh A, Nair GB, et al. QRDR mutations, efflux system and antimicrobial resistance genes in enterotoxigenic Escherichia coli isolated from an outbreak of diarrhoea in Ahmedabad, India. Indian J Med Res 2011;134:214-23.
- Usein CR, Tatu-Chitoiu D, Ciontea S, Condei M, Damian M. Escherichia coli pathotypes associated with diarrhea in Romanian children younger than 5 years of age. Jpn J Infect Dis 2009;62:289-93.
- Gutierrez SP, Sanchez MA, Gonzalez CP, Garcia LA. Antidiarrhoeal activity of different plants used in traditional medicine.Afr J Biotech 2007;6:2988-94.
- Chen JC, Huang LJ, Wu SL, Kuo SC, Ho TY, Hsiang CY. Ginger and its bioactive component inhibit enterotoxigenic Escherichia coli heat-labile enterotoxin induced diarrhea in mice. J Agric Food Chem 2007;55:8390-7.
- Chen JC, Ho TY, Chang YS, Wu SL, Li CC, Hsiang CY.Identification of Escherichia coli enterotoxin inhibitors from traditional medicinal herbs by in silico, in vitro, and in vivo analyses. J Ethnopharmacol2009;121:372-8.
- Tai YH, Feser JF, Marnane WG, Desjeux JF. Antisecretory effects of berberine in rat ileum. Am J Physiol 1981;241:G253-8.
- Chopra R. Indigenous drugs of India. Calcutta: Academic Publishers; 1982.
- Singh N, Kulshrestha VK, Gupta MB Bhargava KP. A pharmacological study of Cyperusrotundus. Indian J Med Res 1970;58:103-9.
- Gutierrez RM, Mitchell S, Solis RV. Psidiumguajava: A review of its traditional uses, phytochemistry and pharmacology. J Ethnopharmacol 2008;117:1-27.
- Ernst E, Pittler MH. Efficacy of ginger for nausea and vomiting: A systematic review of randomized clinical trials. Br J Anaesth 2000;84:367-71.
- Tetali P, Waghchaure C, Daswani PG, Antia NH, Birdi TJ. Ethnobotanical survey of antidiarrhoeal plants of Parinche valley, Pune district, Maharashtra, India. J Ethnopharmacol 2009;123:229-36.
- Brijesh S, Daswani P, Tetali P, Antia N, Birdi T. Studies on the antidiarrhoeal activity of Aeglemarmelos unripe fruit: Validating its traditional usage. BMC Complement Altern Med 2009;9:47.
- Birdi T, Daswani P, Brijesh S, Tetali P, Natu A, Antia N. Newer insights into the mechanism of action of Psidiumguajava L. leaves in infectious diarrhoea. BMC Complement Altern Med 2010;10:33.
- Daswani PG, Brijesh S, Tetali P, Birdi TJ. Studies on the activity of Cyperusrotundus Linn.tubers against infectious diarrhea. Indian J Pharmacol 2011;43:340-4.
- Daswani PG, Brijesh S, Tetali P, Antia NH, Birdi TJ. Antidiarrhoeal activity of Zingiberofficinale (Rosc.).CurrSci 2010;98:222-9.
- Pattanayak P, Mohapatra P. Phytopharmacology of Aeglemarmelos (L.) Correa.Pharmacog Rev 2008;2:50-6.
- Ali BH, Blunden G, Tanira MO, Nemmar A. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiberofficinale Roscoe): A review of recent research. Food ChemToxicol 2008;46:409-20.
- Thakkur CG. The Art and Science of pharmacy. In: Introduction to Ayurveda: Basic Indian Medicine. 2nd ed. Jamnagar: GulakunverbaAyurvedic Society; 1976.
- Matsunaga K, Klein TW, Friedman H, Yamamoto Y. Alveolar macrophage cell line MH-S is valuable as an in vitro model for Legionella pneumophilia infection. Am J Respir Cell MolBiol 2001;24:326-31.
- Taraphdar AK, Roy M, Bhattacharya RK. Natural products as inducers of apoptosis: Implication for cancer therapy and prevention. CurrSci 2001;80:1387-96.
- Badisa RB, Chaudhuri SK, Pilarinou E, Rutkoski NJ, Hare J, Levenson CW. Licaniamichauxii Prance root extract induces hsp 70 mRNA and necrotic cell death in cultured human hepatoma and colon carcinoma cell lines. Cancer Lett 2000;149:61-8.
- Duke RC. Apoptosis Methods and Protocols.Vol. 282. New Jersey: Humana Press; 2004.
- Escribano J, Diaz-Guerra MJ, Riese HH, Ontanon J, Garcia-Olmo D, Garcia-Olmo DC, et al. In vitro activation of macrophages by a novel proteoglycan isolated from corns of Crocus sativus L. Cancer Lett 1999;144:107-14.
- Albina JE, Cui S, Mateo RB, Reichner JS. Nitric oxide-mediated apoptosis in murine peritoneal macrophages. J Immunol1993;150:5080-5.
- Davicino R, Mattar A, Casali Y, Porporatto C, Correa S, Micalizzi B. Activation and apoptosis of mouse peritoneal macrophages by extracts of Larreadivaricata Cav. (jarilla). IntImmunopharmacol2006;6:2047-56.
- Xiong A, Clarke-Katzenberg RH, Valenzuela G, Izumi KM, Millan MT. Epstein-Barr virus latent membrane protein 1 activates nuclear factor-κB in human endothelial cells and inhibits apoptosis. Transplantation 2004;78:41-9.
- Nair MP, Mahajan S, Reynolds JL, Aalinkeel R, Nair H, Schwartz SA, et al. The flavonoid quercetin inhibits proinflammatory cytokine (tumor necrosis factor alpha) gene expression in normal peripheral blood mononuclear cells via modulation of the NF-κβ system. Clin Vaccine Immunol 2006;13:319-28.
- Munn DH, Beall AC, Song D, Wrenn RW, Throckmorton DC. Activation induced apoptosis in human macrophages: Developmental regulation of a novel cell death pathway by macrophage colony-stimulating factor and interferon-gamma. J Exp Med 1995;181:127-36.
- Xaus J, Comalada M, Valledor AF, Cardo M, Herrero C, Soler C, et al. Molecular mechanisms involved in macrophage survival, proliferation, activation or apoptosis. Immunobiol 2001;204:543-50.
- Dhankhar S, Ruhil S, Balhara M, Dhankhar S, Chillar AK. Aeglemarmelos (Linn.) Correa: A potential source of phytomedicine. J Med Plants Res 2011;5:1497-507.
- CHEMEXCIL. Selected medicinal plants of India. Bombay: BhartiyaVidyaBhavan’s Swami Prakashanand Ayurveda Research Centre; 1992.