- Corresponding Author:
- N. G. Gobade
Nitte Gulabi Shetty Memorial Institute of Pharmaceutical Sciences, Paneer, Derelakatte, Mangalore-574 160, India
E-mail: nagnathgobade@gmail.com
| Date of Submission | 19 July 2010 |
| Date of Revision | 09 December 2011 |
| Date of Acceptance | 11 December 2011 |
| Indian J Pharm Sci, 2012, 74 (1): 69-72 |
Abstract
The aim of the present study was to design an asymmetric membrane capsule, an osmotic pump-based drug delivery system of ethyl cellulose for controlled release of terbutaline sulphate. asymmetric membrane capsules contains pore-forming water soluble additive, sorbitol in different concentrations in the capsule shell membrane, which after coming in contact with water, dissolves, resulting in an in situ formation of a microporous structure. The terbutaline sulphate is a β-adrenoreceptor agonist widely used in the treatment of asthma. The oral dosage regimen of terbutaline sulphate is 5 mg twice or thrice daily, the plasma half-life is approximate 3-4 h and it produces GI irritation with extensive first pass metabolism. Hence, terbutaline sulphate was chosen as a model drug with an aim to develop controlled release system. Different formulations of ethyl cellulose were prepared by phase inversion technique using different concentrations of sorbitol as pore forming agent. It was found that the thickness of the prepared asymmetric membrane capsules was increased with increase in concentration of ethyl cellulose and pore forming agent, i.e. sorbitol. The dye release study in water and 10% sodium chloride solution indicates that, the asymmetric membrane capsules follow osmotic principle to release content. The pores formed due to sorbitol were confirmed by microscopic observation of transverse section of capsule membrane. Data of in vitro release study of terbutaline sulphate from asymmetric membrane capsules indicated that, the capsules prepared with 10% and 12.5% of ethyl cellulose and 25% of sorbitol released as much as 97.44% and 76.27% in 12 h, respectively with zero order release rate. Hence asymmetric membrane capsule of 10% ethyl cellulose and 25% of sorbitol is considered as optimum for controlled oral delivery of terbutaline sulphate.
Keywords
Asymmetric membrane capsule, ethyl cellulose, sorbitol, terbutaline sulphate
Asymmetric membrane capsule (AMC) is a non-disintegrating, single core osmotic system, consisting of drug filled in shells which are made up of water insoluble polymers by phase inversion technique. The membrane or shell of these capsules contain pore forming water soluble additive, which after coming in contact with water, dissolve, resulting in an in situ formation of a microporous structure. The advantages of AMCs are; higher rate of water influx, controlling the release of freely soluble drugs, pH independent release and minimize the exposure of drug to the gastrointestine (so that, reduced gastric irritation and degradation of drugs).
The mechanism of drug release from an AMC consists of imbibitions of water through the membrane into the core, dissolution of soluble components (including drug) in the core, and pumping of the solution out of pores in the membrane. The imbibition of water through the membrane is driven by its thermodynamic activity gradient between the external medium, e.g., receptor solution or gastric/intestinal fluids and the osmotic agent(s) in the core. Dissolution of the soluble components within the core produces the activity gradient and establishes the osmotic pressure difference between the core and external environment. The approximately constant dosage form volume means that the volume of drug solution delivered will be roughly equal to the volume of water imbibed within a given time interval. As water diffuses into the core, the volume of the imbibed water creates a hydrostatic pressure difference across the membrane, which forces the solution out through the pores in the coating. The rate of flow (dv/dt) of water into the device can be represented as: dv/dt = Ak/h (DP-DR) where k = Membrane permeability, A = Area of the membrane, Dp = Osmotic pressure difference, DR = Hydrostatic pressure difference, h = Thickness of the membrane.
The in vitro release rate of a drug from an asymmetric membrane capsule was dependent on the capsule shell composition as well as the fill (core) formulation. For a given shell composition, the release was dependent on osmotic pressure (solubility) of the core ingredients and for a given core composition, the release is depends on the capsule shell permeability. Sustained zero-order drug release can be achieved using AMC devices while the concentration of dissolved drug within the fluid portion of the core remains constant. When the drug concentration in core fluid falls below the saturation, then the release rate declines [1,2].
The terbutaline sulphate (TBS) is a β-adrenoreceptor agonist widely used in the treatment of asthma. The oral dosage regimen of TBS is 5 mg twice or thrice daily, the plasma half-life is approximate 3-4 h. The drug produces GI irritation with extensive first pass metabolism and hence has a poor oral bioavailability of only 14.8%. Therefore, TBS was chosen as a model drug with the objective of improving bioavailability and therefore therapeutic efficacy with lower doses and hence better patient compliance.
The aim of the present study is to optimize the formula for AMCs of ethyl cellulose (EC) to deliver water soluble drug TBS in controlled manner and to study the effect of sorbitol as pore forming agent on drug release.
TBS was obtained from Astra IDL, Bangalore, India, as a complimentary gift sample and other excipients like ethyl cellulose (EC), acetone, methanol was purchased from Merck Specialities Pvt. Ltd. of analytical grade. Sorbitol was purchased from Hebei Shengxue Glucose Co. Ltd., China.
AMCs were prepared by phase inversion process [2], in which the membrane was precipitated on glass mould pins having a diameter of 6, 7 and 8 mm, so as to form two sizes of capsules. The glass mould pins ware dipped into polymer solutions containing EC dissolved in acetone, ethanol and varying concentration of sorbitol as shown in Table 1. Quenching was done by 10% w/v glycerin for 10 min which resulted in the formation of an asymmetric membrane. The quenched pins were withdrawn and air dried. The capsules were stripped off the pins, trimmed to size and stored in desiccators [3,4].
| Ingredients | Formulation code | Sealing solution | |||||
|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | ||
| EC (% w/v) | 10 | 10 | 12.5 | 12.5 | 15 | 15 | 15 |
| Sorbitol (% w/v) | 15 | 25 | 15 | 25 | 15 | 25 | 00 |
| Acetone (% v/v) | 50 | 50 | 50 | 50 | 50 | 50 | 50 |
| Ethanol (% v/v) | 25 | 15 | 22.5 | 12.5 | 20 | 10 | 35 |
Table 1: Formulae for the preparation of the AMCs
The thickness of the asymmetric membrane of prepared capsules was measured using a screw gauge. From each formulation, thickness was measured for three different capsules and its mean was considered. The prepared AMCs were filled with water soluble dye, amaranth, sealed by sealing solution and placed in 50 ml distilled water. The pattern of dye diffusing through the asymmetric membrane wall of AMCs was observed for two hours and afterwards these was transferred to 50 ml 10% NaCl solution to observe the effect of osmotic pressure on dye diffusion [5-6]. Transverse section of prepared capsules was prepared and observed under the microscope, to confirm the formation of the pores.
Each prepared capsule was filled with 200 mg mixture of water soluble drug TBS (20 mg) and lactose (180 mg) and sealed with sealing solution as given in Table 1. The sealing solution was prepared using the composition given in Table 1 with the exception of sorbitol.
The filled capsules were subjected to release study using USP dissolution apparatus II (100 rpm, 37°) in 500 ml of distilled water as dissolution medium. The samples were withdrawn at specific time intervals and analyzed by UV spectrophotometer at λmax 277nm.
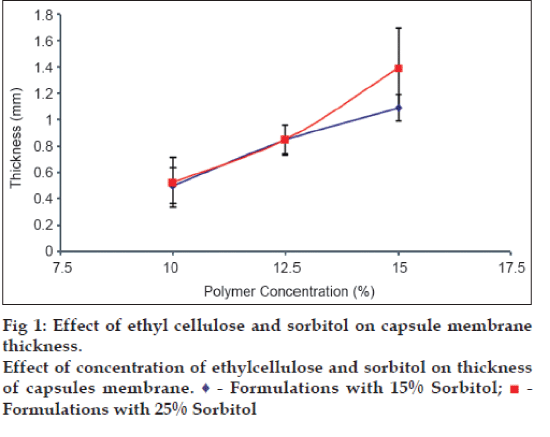
Six AMCs of EC were prepared by phase inversion technique, using sorbitol as pore forming agent. All AMCs appeared to be white and opaque with no visible physical defect. Membrane thickness was found to be increased with increase in concentration of EC. The thickness of the capsule wall also increased with increase in concentration of pore forming agent (fig. 1).
The release of amaranth dye from dye encapsulated AMCs was observed in distilled water after an average time period of 28 min from capsules A, B, C and D,which was continuing for another 2.5 h. But no release was observed from E and F capsules, even after 2 h, due to their higher thickness. So these capsules were rejected for in vitro drug release study. However, no release was observed when the same capsules were transferred into 10% sodium chloride solution. In such a case, the osmotic effect gets nullified, suggesting that the prepared AMCs follow osmotic principle for the release of encapsulated material.
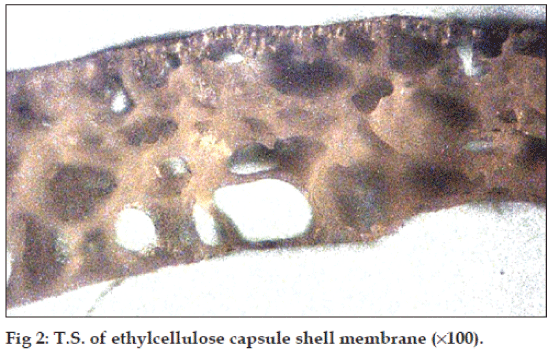
The pores formed were confirmed by observing a transverse section of capsule shell under microscope (100X) and found that the porous nature and size of pores increase with increase in concentration of sorbitol (fig. 2).
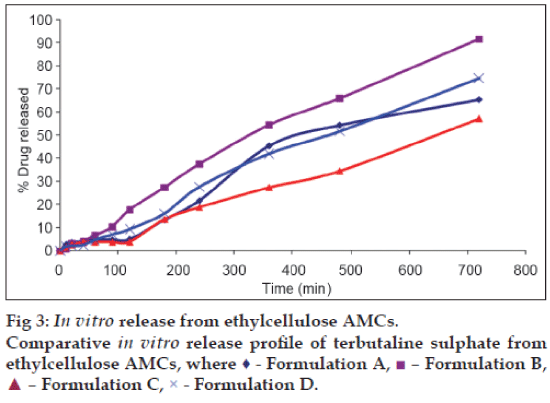
Among the formulations, capsule E and F were already rejected in dye release study since the greater thickness of membrane, hindered release of dye up to 4 h from the capsules. And from the remaining four capsules (A, B, C and D), capsule B (10% EC, 25% sorbitol), capsule C (12.5%EC, 15% sorbitol) and capsule D (12.5% EC, 25% sorbitol) follow the zero order release model and capsule A (10% EC, 15% sorbitol) followed the Hixson-Crowell order release model (Tables 2 and 3, fig. 3).
| Formulation code | Kinetic Parameters | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zero order | First order | Matrix-Higuchi | Peppas | Hixson Crowell | ||||||||
| k | R2 | k | R2 | k | R2 | k | R2 | k | R2 | |||
| A | 0.0991 | 0.9764 | 0.0015 | 0.977 | 1.945 | 0.8835 | 0.228 | 0.9203 | 0.0004 | 0.9786 | ||
| B | 0.1353 | 0.9943 | 0.0028 | 0.958 | 2.704 | 0.9260 | 0.077 | 0.9921 | 0.0007 | 0.9872 | ||
| C | 0.0761 | 0.9927 | 0.0010 | 0.976 | 1.480 | 0.8848 | 0.158 | 0.9437 | 0.0003 | 0.9841 | ||
| D | 0.1059 | 0.9956 | 0.0017 | 0.978 | 2.078 | 0.9001 | 0.115 | 0.9753 | 0.0005 | 0.9892 | ||
Table 2: Kinetic analysis of terbutaline sulphate from formulations
| Dissolution | Formulation Code | |||
|---|---|---|---|---|
| parameters | A | B | C | D |
| Q30 (%) | 3.77 | 4.06 | 2.28 | 3.17 |
| Q60 (%) | 7.45 | 8.12 | 4.56 | 6.35 |
| Q180 (%) | 21.23 | 24.36 | 13.69 | 19.06 |
| Q360 (%) | 39.23 | 48.72 | 27.38 | 38.13 |
| Q720 (%) | 66.57 | 97.44 | 54.77 | 76.27 |
| T10(min) | 81.2 | 73.8 | 131.4 | 94.3 |
| T30(min) | 263.7 | 221.6 | 394.3 | 283.1 |
| T50(min) | 485.4 | 369.4 | 657.2 | 471.9 |
| T80(min) | 976.9 | 591.0 | 1051.5 | 755.1 |
| T90(min) | 1260.7 | 664.9 | 1182.9 | 849.5 |
| Best fitting model | Hix. Crowell | Zero order | Zero order | Zero order |
Qt ? Quantity in % of drug released at time t. TQ ? Time in min required to release quantity Q of drug.
Table 3: Comparison of in vitro release parameters of terbutaline sulphate from ethylcellulose AMCs
The TBS release from capsule C (54.77%) was lesser as compared to capsule B (97.44%) and capsule D (76.27%) after 12 h. So capsule B was considered as an optimum AMC formulation for sustained release of TBS.
AMCs prepared with EC as polymer and sorbitol as pore former released the contents by osmotic principle. As the concentration of sorbitol was increased with same concentration of EC, the pore formation and drug release rate from capsules was also increased. But pore forming effect decreased with increase in concentration of EC. Capsule B (10% EC, 25% sorbitol) has better release profile for terbutaline sulphate and can be considered as promising control release formulation.
Acknowledgements
The authors would like thank the Nitte Education Trust, Mangalore for providing the necessary facilities for carrying out this investigation. Also thanks to Astra IDL, Bangalore for complimentary gift sample of terbutaline sulphate.
References
- Thombre AG, Cardinal JR, DeNoto AR, Gibbes DC. Asymmetricmembrane capsule for osmotic drug delivery II. In vitro and in vivodrug release performance. J Control Release 1999;57:65-73.
- Methiowitz E. Encyclopedia of controlled drug delivery system. Vol. 2.New York: John Wiley and Sons. Inc; 1999. p. 731.
- Thombre AG, Cardinal JR, DeNoto AR, Herbig SM, Smith KL.Asymmetric membrane capsule for osmotic drug delivery I.
- Development of a manufacturing processes. J Control Release1999;57:55-64.
- Philip AK, Pathak K. Osmotic flow through asymmetric membrane:A mean for controlled delivery of drug with varying solubility. AAPSPharmaSciTech 2006;7:56.
- Chauhan CS, Chaudhary PK. Controlled porosity osmotic pump for thedelivery of flurbiprofen. Control Drug Deliv 2006;3:193-8.
- Chauhan CS, Ranawat MS, Chaudhary PK. Fabrication and evaluationof an asymmetric membrane osmotic pump. Indian J Pharm Sci2007;69:748-52.