- *Corresponding Author:
- Xiangling Li
Department of Nephrology, Affiliated Hospital of Weifang Medical University, Weicheng, Weifang 261031, China
E-mail: lixiangling163@163.com
| This article was originally published in a special issue, “Current Trends in Pharmaceutical and Biomedical Sciences” |
| Indian J Pharm Sci 2022:84(5) Spl Issue “99-108” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The current study examined the relationship between renal cell carcinoma-related risk and gene polymorphisms in the interleukin-10, vitamin D receptor and cytochrome P450 1A1 genes. Using the preferred reporting items for systematic reviews and meta-analyses criteria, the link between gene polymorphisms and renal cell carcinoma was studied by utilizing the Web of Science, Embase, PubMed and Wanfang databases. The CC and TT genotypes along with the interleukin-10-819C/T C-allele were found to be substantially associated with renal cell carcinoma risk in the Chinese residents. Furthermore, significant relationships between the vitamin D receptor, FokI FF genotype and FokI F allele, ApaI A allele, ApaI AA and aa genotypes and renal cell carcinoma risk were observed in Asian populations. The cytochrome P450 1A1 MspI (rs4646903) polymorphism was substantially linked to an elevated risk of renal cell carcinoma in the T allele, TT genotype. However, no significant relationships were found between renal cell carcinoma risk and the single nucleotide polymorphisms interleukin-10-1082A/G, vitamin D receptor TaqI and cytochrome P450 1A1 Ile462Val (rs1544410).
Keywords
Renal cell carcinoma, interleukin-10, vitamin D receptor, cytochrome P450 1A1, gene polymorphisms
With more than 270 000 new cases and 100 000 fatalities each year, Renal Cell Carcinoma (RCC) is one of the most prevalent malignancies of the urinary system and the most common kind of kidney cancer. The tolerance of traditional cancer treatments like radiation therapy and chemotherapy, the higher recurrence rate and distant metastases, the prognosis and 5 y survival rates of cancer patients are relatively poor[1]. Globally, the incidence and accompanying death rate are now on the rise. Importantly, men have around double the morbidity and death rates compared to women. Although the origin of RCC is unknown, epidemiological studies have revealed that various threatening factors, including cigarette smoking, obesity, hypertension, a family record of cancer and poor nutrition habits, are strongly associated with the disease. As a result, new diagnostic tools and prognostic biomarkers are critical for predicting RCC advancement.
Interleukin-10 (IL-10), which is an anti-inflammatory cytokine, has an overall anti-inflammatory effect. Therefore, its circulating level can be employed as a biomarker for various illnesses, including RCC. The IL-10 gene is localized to chromosome 1q and the degree of IL-10 production is impacted by three biallelic polymorphisms in the IL-10 promoter region, namely, 592C/A (rs1800872), 1082G/A (rs1800896) and 819C/T (rs1800871). Also, the IL-10 gene of humans has five exons and is located on chromosome 1q32.1[2]. Genetic polymorphisms including Fok1 (rs2228570), TaqI (rs731236), ApaI (rs7975232) and BsmI (rs1544410), restriction fragment length polymorphisms, which is related to a range of illnesses, have an impact on Vitamin D Receptor (VDR), which regulates the vitamin D’s activity[3]. The Cytochrome P450 1A1 (CYP1A1) enzyme is the most efficacious in converting carcinogens of people, specifically aromatic polycyclic hydrocarbons into highly reactive intermediates[4]. In the CYP1A1 gene, recent research has focused mainly on two non-synonymous Single Nucleotide Polymorphisms (SNPs)-Adenine (A) to Guanine (G) transformation at codon 462 in exon 7 (Ile462Val, rs1048943) and Thymine (T) to Cytosine (C) transformation in the noncoding 39-flanking region (MspI, rs4646903)[5]. These alterations may impact gene expression and function, probably changing the balance between toxicant detoxification and metabolic activation consequently, adding to a person’s vulnerability to cancer.
A previous study has found that environmental and genetic factors have a major impact on the occurrence and development of RCC. RCC risk is highly related to CYP1A1, VDR and IL-10 gene variations. As shown by Yang et al. the ApaI mutant AA and AC genes were significantly connected to an elevated risk of RCC in comparison with the CC genotype[6]. Also, Zhou et al. discovered that the VDR’s ApaI A gene, ApaI AA and aa gene, BsmI B gene and Fok1 FF gene have all been linked to RCC susceptibility[7]. Despite this, Lin et al. and her colleagues discovered no significant relationships between RCC risk and VDR BsmI and TaqI gene polymorphisms in Asian and Caucasian populations, which may be paradoxical[3]. Based on case-control studies, a meta-analysis was done to resolve the controversial results reported and the study aims to assess the role of IL-10, VDR and CYP1A1 gene polymorphisms on the RCC risk.
Materials and Methods
Search strategy:
Two independent researchers (Xu and Yang) reviewed the published research work in the Web of Science, Embase, PubMed and Wanfang databases before 12 Oct 2021 using the keywords sets as follows: “Interleukin-10” or “IL-10” or “CYP1A1” or “P4501A1” or “VDR” or “vitamin D receptor” and “renal cancer or “renal cell carcinoma” or RCC”. Besides, the references given in chosen articles are retrieved for the purpose of finding more potential eligible studies. Two authors performed the process without any discussion.
Inclusion criteria: Inclusion criteria were the following-The IL-10, VDR and CYP1A1 gene polymorphisms and RCC were investigated in case- control studies; at least 2 groups for comparison of control group vs. case group and the study satisfies the conditions for the Hardy-Weinberg Equilibrium (HWE).
Exclusion criteria: Exclusion criteria included review articles and editorials, case reports, no reporting of IL-10, VDR or CYP1A1 gene polymorphism or RCC prognosis and no inquiry detailed genotype data of IL-10, VDR and CYP1A1 in RCC.
Data extraction:
From each eligible study, two independent researchers collected the following information. First author’s surname, origin, year of publication, location, number of cases, control basis of the matched group and controls for IL-10, VDR and CYP1A1 genotype. The discussion was used to address the existing disparities between the two sets of data.
The validated Newcastle-Ottawa Scale (NOS) for non-randomized research was employed to evaluate the study quality. Research might earn one star in each of the selection and exposure categories, as well as two stars in the comparability category. In our meta-analysis, only high-quality studies with scores greater than 5 were included.
Statistical investigation:
Based on the five genetic comparison models, the strength of the link between the RCC susceptibility and three gene polymorphisms was assessed using pooled Odds Ratios (ORs) with 95 % Confidence Intervals (CIs). The models include the recessive, allele, homozygous, dominant and heterozygous models. The outcomes of dichotomous data were shown using ORs as well as 95 % CIs were generated. To determine the statistical value of the pooled ORs, a test was used. The heterogeneity of the selected research was evaluated using I2. In this investigation, the fixed-effect model (a Mantel-Haenszel approach) was used, since p values of 0.05 and I2>50 % were regarded to indicate considerable heterogeneity. Otherwise, a DerSimonian and Laird approach was used, which is also known as the random-effect model. To determine the cause of considerable heterogeneity, subgroup analyses were carried out. When there were more than two included publications to assess, we conducted a subgroup analysis. Stratification studies were undertaken to identify possible sources of variability among factors such as ethnicity, age groups, sample size, genotyping technologies and control types. Additionally, a sensitivity analysis was done to examine the impact of every study on the whole findings and avoid the possibility that the existence of separate studies may cause the pooled findings to be reversed. Egger’s linear regression test, funnel plots and Begg’s rank correlation test were employed to identify publication bias in the present studies. A statistically significant value of p<0.05 was included. For all statistical studies, STATA 16.0 was employed.
Results and Discussion
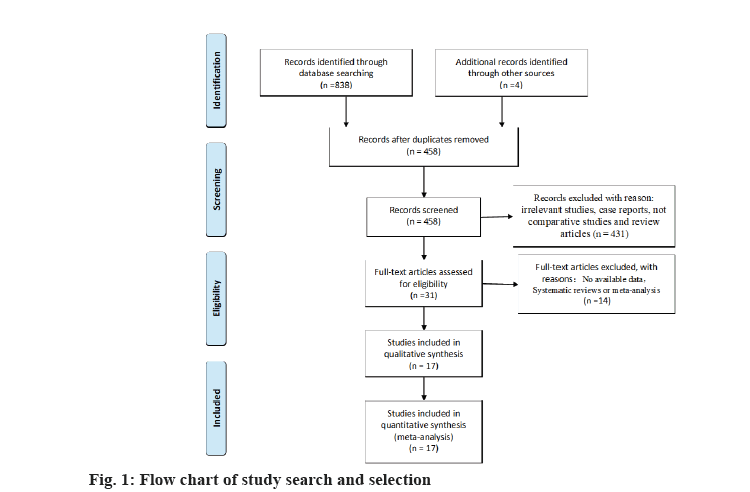
During the search, 838 studies were found in the PubMed (n=163), Embase (n=252), Web of Science (337) and Wanfang (n=86) databases. Following the removal of 380 duplicate articles, further publications were deleted by checking the title and abstract. The total of reviews, conference papers, meta-analyses, letters and abstracts from conferences, editorials, brief surveys and notes were 431. Furthermore, the total of publications involving animals or in vitro studies is 30.
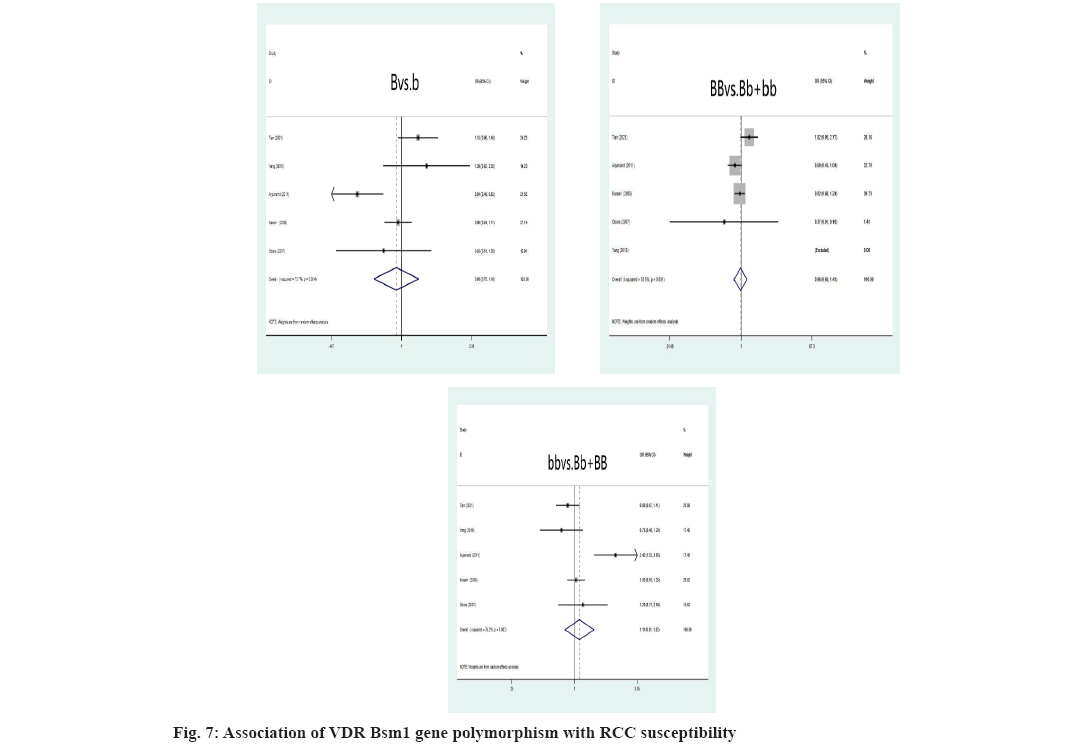
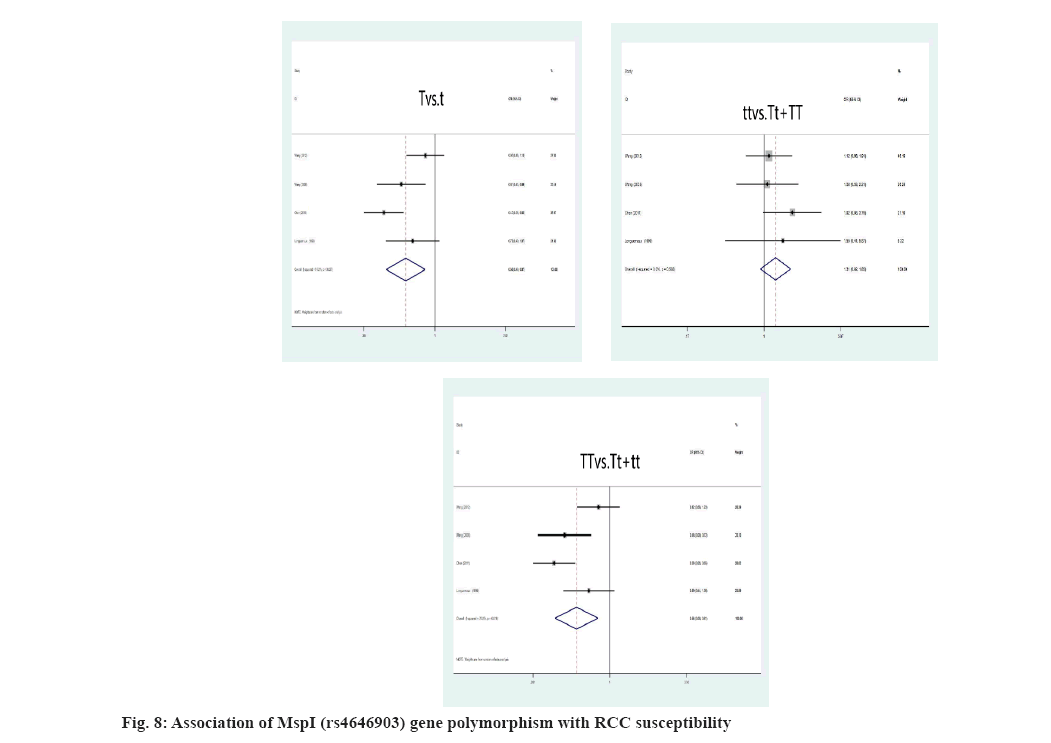
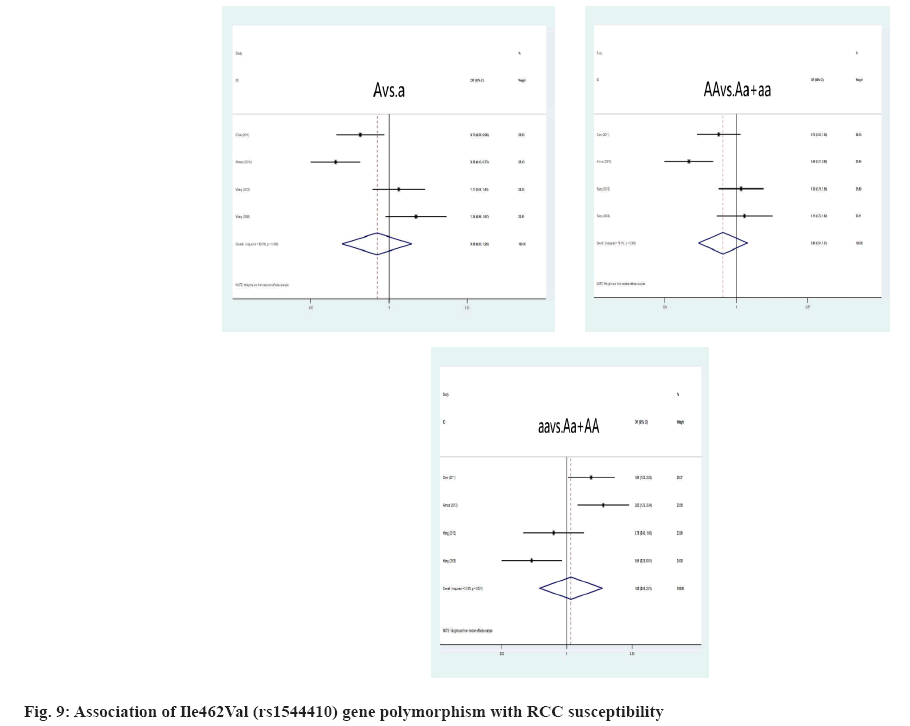
We rejected 14 papers after reading the complete text of these studies for the following reasons, other genes or SNPs of renal illness were researched; the study was not pertinent to the gene; other illnesses were included, but there was no data available for the study. In addition, four studies were discovered through other means. Finally, for this meta-analysis, 17 studies were kept. The flowchart representing the process of selecting and searching the literature is shown in fig. 1. Furthermore, 3 studies have discovered a correlation for both the incidence of RCC and IL-10 gene polymorphism (Table 1). There into, 2 studies assessed IL-10-819C/T gene polymorphism and 3 studies[8-10] analyzed IL- 10-1082A/G gene polymorphism[8,9]. In addition, 7 studies[6,11-16] illustrated the connection between VDR gene polymorphism and the susceptibility to RCC in Table 2. Among these, 5 studies focused on VDR FokI rs2228570 gene polymorphism, 3 studies[6,11-13,16] on VDR ApaI rs7975232, 5 studies[6,11-14] on BsmI rs1544410 and 5 studies[6,11,13-15] on VDR TaqI rs731236. Simultaneously, 5 studies[17-21] retrieved the association between CYP1A1 gene polymorphism with RCC in Table 3. Among these, 4 studies[17-19,21] mainly focused on MspI (rs4646903) gene polymorphism and Ile462Val (rs1544410) gene polymorphism, respectively[17,19-21].
| Gene sites | Author | Year | Ethnicity | Country | Source of control | Case | Control | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AA | AG | GG | Total | AA | AG | GG | Total | ||||||
| IL-10-1082A/G | Chang | 2016 | Asian | China | PB | 71 | 16 | 5 | 92 | 414 | 107 | 29 | 550 |
| Romero | 2009 | Granada | Spain | PB | 42 | 62 | 22 | 126 | 58 | 87 | 30 | 175 | |
| IL-10-819C/T | CC | CT | TT | Total | CC | CT | TT | Total | |||||
| Chang | 2016 | Asian | China | PB | 62 | 26 | 4 | 92 | 310 | 209 | 61 | 580 | |
| Romero | 2009 | Granada | Spain | PB | 81 | 37 | 9 | 127 | 98 | 63 | 14 | 175 | |
Note: PB: Population-Based study
Table 1: Effects of IL-10 Gene Polymorphism on RCC Risk
| Gene sites | Author | Year | Ethnicity | Country | Source of Control | Case | Control | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FF | Ff | ff | Total | FF | Ff | ff | Total | ||||||
| FokI | Tian | 2021 | Asian | China | PB | 191 | 143 | 32 | 366 | 432 | 263 | 40 | 735 |
| Yang | 2016 | Asian | China | PB | 61 | 171 | 70 | 302 | 64 | 159 | 79 | 302 | |
| Southard | 2012 | Caucasian | Finland | PB | 22 | 66 | 64 | 152 | 48 | 144 | 113 | 305 | |
| Arjumand | 2011 | Asian | Indian | PB | 40 | 94 | 62 | 196 | 38 | 98 | 114 | 250 | |
| Karami | 2008 | Caucasian | United States | HB | 149 | 376 | 286 | 811 | 199 | 492 | 338 | 1029 | |
| ApaI | AA | Aa | aa | Total | AA | Aa | aa | Total | |||||
| Tian | 2021 | Asian | China | PB | 182 | 149 | 35 | 366 | 475 | 226 | 34 | 735 | |
| Yang | 2016 | Asian | China | PB | 35 | 153 | 114 | 302 | 18 | 135 | 149 | 302 | |
| Obara | 2007 | Asian | China | PB | 23 | 52 | 60 | 135 | 11 | 71 | 68 | 150 | |
| TaqI | TT | Tt | tt | Total | TT | Tt | tt | Total | |||||
| Tian | 2021 | Asian | China | PB | 200 | 139 | 27 | 366 | 460 | 241 | 34 | 735 | |
| Yang | 2016 | Asian | China | PB | 0 | 41 | 261 | 302 | 0 | 30 | 272 | 302 | |
| Karami | 2008 | Caucasian | United States | HB | 97 | 361 | 320 | 778 | 137 | 438 | 402 | 977 | |
| Obara | 2007 | Asian | China | PB | 0 | 31 | 104 | 135 | 1 | 37 | 112 | 150 | |
| Ikuyama | 2002 | Asian | Japan | HB | 1 | 19 | 82 | 102 | 8 | 70 | 126 | 204 | |
| BsmI | BB | Bb | bb | Total | BB | Bb | bb | Total | |||||
| Tian | 2021 | Asian | China | PB | 211 | 130 | 25 | 366 | 466 | 236 | 33 | 735 | |
| Yang | 2016 | Asian | China | PB | 0 | 47 | 255 | 302 | 0 | 37 | 265 | 302 | |
| Arjumand | 2011 | Asian | Indian | PB | 50 | 88 | 58 | 196 | 83 | 130 | 37 | 250 | |
| Karami | 2008 | Caucasian | United States | HB | 81 | 370 | 324 | 775 | 112 | 474 | 407 | 993 | |
| Obara | 2007 | Asian | Japan | PB | 0 | 33 | 102 | 135 | 1 | 41 | 108 | 150 | |
Note: PB: Population-Based study and HB: Hospital-Based study
Table 2: Summary of the Effects of VDR Gene Polymorphism on RCC Risk
| Restriction sites | Author | Year | Ethnicity | Country | Case | Control | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wt/Wt (TT) | Wt/Vt (CT) | Vt/Vt (CC) | Total | Wt/Wt (TT) | Wt/Vt (CT) | Vt/Vt (CC) | Total | |||||
| MspI (rs4646903) | Wang | 2012 | Asian | China | 89 | 87 | 31 | 207 | 113 | 91 | 32 | 236 |
| Chen | 2011 | Asian | China | 80 | 83 | 18 | 181 | 237 | 94 | 19 | 350 | |
| Wang | 2008 | Asian | China | 62 | 64 | 17 | 143 | 96 | 40 | 17 | 153 | |
| Longuemaux | 1999 | Caucasian | France | 114 | 52 | 5 | 171 | 156 | 50 | 4 | 210 | |
| Ile462Val (rs1544410) | Ile/Ile (AA) | Ile/Val (GA) | Val/Val (GG) | Total | Ile/Ile (AA) | Ile/Val (GA) | Val/Val (GG) | Total | ||||
| Ahmad | 2013 | Asian | India | 53 | 98 | 45 | 196 | 112 | 106 | 32 | 250 | |
| Wang | 2012 | Asian | China | 106 | 80 | 21 | 207 | 116 | 90 | 30 | 236 | |
| Chen | 2011 | Asian | China | 77 | 63 | 41 | 181 | 174 | 122 | 54 | 350 | |
| Wang | 2008 | Asian | China | 69 | 66 | 23 | 158 | 56 | 48 | 35 | 139 | |
Table 3: Summary of the Effects of CYPA1 Gene Polymorphism on RCC Risk
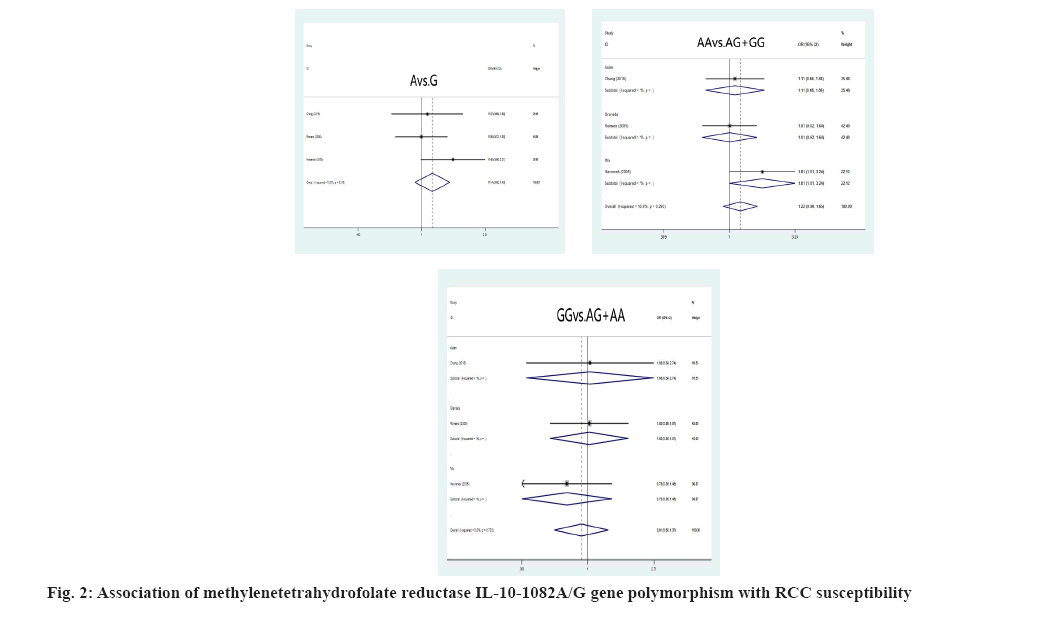
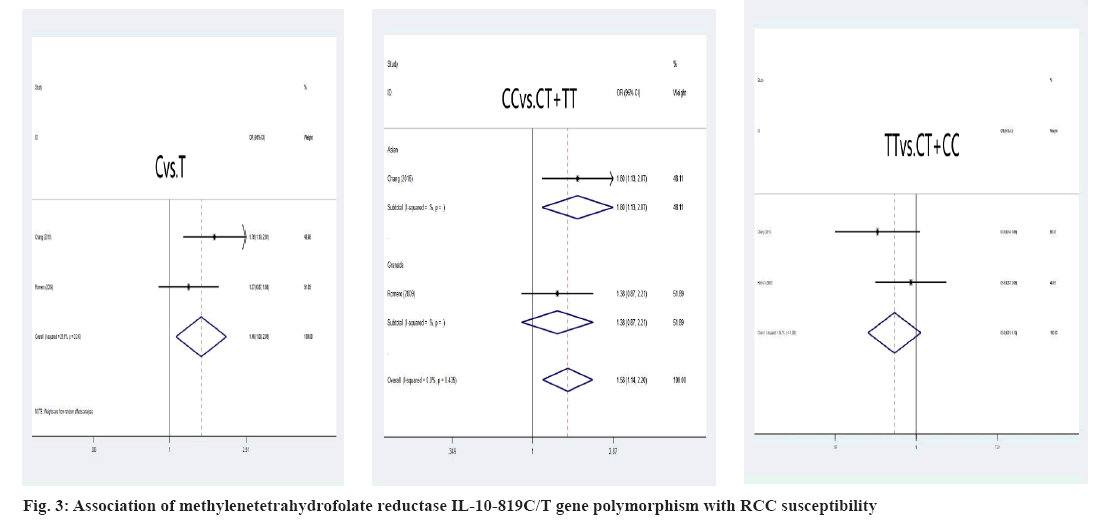
Association between RCC susceptibility and IL-10 gene polymorphism was explained here. The A allele and genotype of IL10-1082A/G were not linked with the incidence of RCC in the Chinese and Grenada populations (A allele: OR=1.14, 95 % CI: (0.92-1.43), p=0.233; AA genotype: OR=1.22, 95 % CI: (0.90-1.65), p=0.196; GG genotype: OR=0.91, 95 % CI: 0.60-1.37, p=0.639) as shown in fig. 2. The IL-10-819C/T C allele and CC genotypes, on the other hand, had shown significant correlation of RCC susceptibility (C allele: OR=1.76, 95 % CI: 1.19; 2.61, p=0.005; CC genotype: OR=1.80, 95 % CI: 1.13; 2.87, p=0.013) in the Chinese residents was shown in fig. 3.
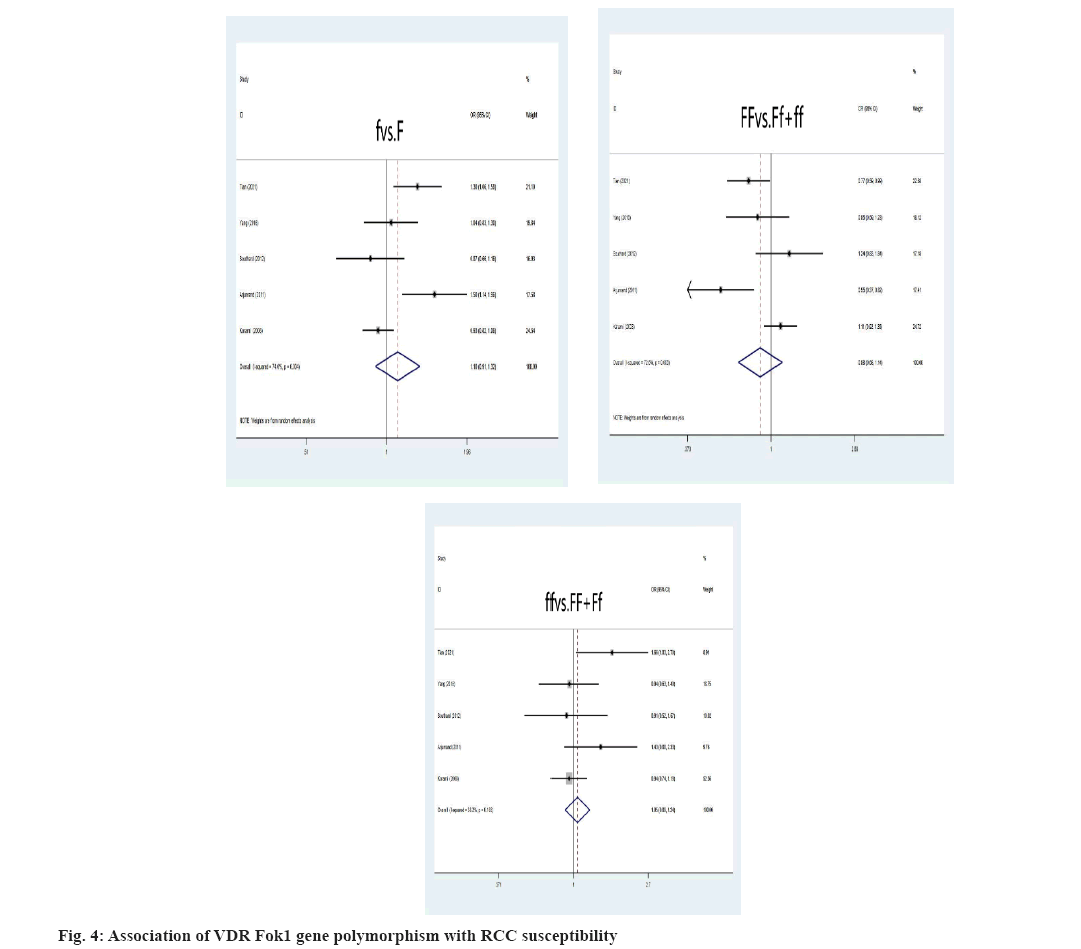
Association between VDR gene polymorphism and RCC susceptibility was explained here. The VDR FokI allele and genotype were not relevant to an elevated risk of RCC (f vs. F: OR=1.10, 95 % CI: (0.91-1.32), p=0.326; ff vs. FF+Ff: OR=1.05, 95 % CI: (0.89-1.24), p=0.575; FF vs. Ff+ff: OR=0.88, 95 % CI: (0.68-1.14), p=0.328) was shown fig. 4. As depicted, the F allele and FF genotype remain linked to a higher risk of developing RCC, instead of ff genotype in the Asian population. Additionally, neither the ff nor FF genotypes nor the VDR FokI f allele was linked to an elevated risk of developing RCC in Caucasians. Asians were more likely to develop RCC if they had the VDR ApaI A allele, AA or aa genotype (A vs. a: OR=2.57, 95 % CI: (1.81-3.65), p=0.000; AA vs. Aa+aa: OR=2.22, 95 % CI: (1.58-3.11), p=0.000; aa vs. AA+Aa: OR=0.65, 95 % CI: (0.49-0.88), p=0.004) (fig. 5). The correlation between RCC susceptibility and the VDR TaqI gene wasn’t statistically significant, T allele: OR=0.97, 95 % CI: (0.71-1.31), p=0.817; tt genotype: OR=1.00, 95 % CI: (0.57-1.77), p=0.996; TT genotype: OR=1.03, 95 % CI: (0.73-1.45), p=0.87 (fig. 6). In general populations, there are no known significant relationships between the VDR BsmI B allele, BB genotype, or bb genotype with RCC risk, B allele: OR=0.95, 95 % CI: (0.75- 1.20), p=0.649; BB genotype: OR=0.96, 95 % CI: (0.65; 1.41), p=0.824; bb genotype: OR=1.12, 95 % CI: (0.82-1.53), p=0.498 (fig. 7). There is no link between the VDR BsmI gene polymorphism and RCC risk in Asians or Caucasians, according to research that was stratified by ethnicity.
Link amongst CYPA1 gene polymorphism and RCC susceptibility was explained here. The high risk of RCC is closely associated with the MspI polymorphism, when the genotype is TT (T genotype: OR=0.56, 95 % CI: (0.38; 0.81), p=0.009; T allele: OR=0.65, 95 % CI: (0.49; 0.87), p=0.003) (fig. 8). For all genetic models, the correlation of the Ile462Val polymorphism and RCC risk was not significant, A allele: OR=0.88, 95 % CI: (0.60; 1.69), p=0.503; AA genotype: OR=0.80, 95 % CI: (0.54; 1.21), p=0.824; aa genotype: OR=1.01, p=0.800 (fig. 9). However, we identified a substantial link between CYPA1 gene polymorphism and a low risk of RCC among Indians with the AA genotype, A allele and aa genotype, A genotype: OR=0.46, 95 % CI: (0.31; 0.68), p=0.000; aa genotype: OR=0.20, 95 % CI: (1.23; 3.34), p=0.005.
IL-10 is an anti-inflammatory cytokine that regulates cellular homeostasis. The IL-10-819C/T C allele, along with the CC and TT genotypes, had shown a significant correlation with the incidence of RCC in the Chinese population.
A phase I enzyme called CYP1A1 can impact the metabolism of environmental contaminants as well as modify cancer susceptibility. It is required for polycyclic aromatic hydrocarbon phase I metabolism and hormone metabolism. CYP1A1 dysfunction can cause Deoxyribonucleic Acid (DNA), lipid and protein damage, which can lead to carcinogenesis[7].
VDR is found on the long arm of chromosome 12q12-q14 and has been associated with cancer risk through a variety of single nucleotide polymorphisms. Each mutation is called after the target sequence that was originally used to characterize it. The VDR BsmI and TaqI polymorphisms, in general, are unrelated to the risk of developing RCC. According to a meta- analysis conducted by Zhou et al. three genotypes (ApaI AA, Fok1 FF and BsmI BB) and the Fok1 f allele are all linked to the risk of RCC in Asians[7]. There was more reliability than Zhou et al. since it contained more research in our analysis. Nunes et al. revealed that the rs228570 VDR gene polymorphism, particularly the protective f allele, showed a significant correlation with arterial hypertension[22]. Furthermore, vitamin D can also influence cell proliferation, differentiation and death in a variety of organs and protects against some forms of cancer. Moreover, the relevance between the haplotype blocks of those genes and RCC must be studied. For starters, some subgroup studies used a tiny sample size, which might not be illustrative of the entire population. Second, a rigorous study in the control group contradicted HWE. In addition, just a few electronic databases were examined. More study is needed to understand the pathways that connect VDR, IL-10 and cell cancer. As a result, our findings should be taken into account and additional case-control studies are needed to corroborate our findings.
In the present study, the gene-gene and gene- environment interactions are not described such as participant’s average food consumption, macronutrient nutritional content and use of vitamin D supplements, especially vitamin D or energy. Even though cancer is a complex interaction between inherited and environmental factors, the authors did not even look into these nutritional elements. Finally, it may not provide any more information on its impact on cell cancer.
Author’s contributions:
Ning Xu made the substantial contributions to the conception and design of the work; Ning Xu, Maoquan Yang searched selected materials and extracted data; Ning Xu wrote this manuscript; Xiangling Li, Jie Liu, Suzen Li and Zihao Zhu revised the paper carefully and also contributed to the statistical analyses. All authors have read and approved the final manuscript.
Funding:
This work was supported by the Clinical Research Project Program of Affiliated Hospital of Weifang Medical University (2021wyfylcyj03) and Shandong Provincial Nature Fund Joint Special Fund Project (ZR2018LH006).
Conflict of interests:
The authors declared no conflict of interest.
References
- Wang J, Liu L, Long Q, Bai Q, Xia Y, Xi W, et al. Decreased expression of JMJD3 predicts poor prognosis of patients with clear cell renal cell carcinoma. Oncol Lett 2017;14(2):1550-60.
[Crossref] [Google scholar] [PubMed]
- Zhang F, Yang Y, Lei H, Qiu J, Wang Y, Hu D, et al. A meta-analysis about the association between 1082G/A and 819C/T polymorphisms of IL-10 gene and risk of type 2 diabetes. Hum Immunol 2013;74(5):618-26.
[Crossref] [Google scholar] [PubMed]
- Lin ZJ, Zhang XL, Yang ZS, She XY, Xie Y, Xie WJ. Relationship between Vitamin D receptor gene polymorphism and renal cell carcinoma susceptibility. J Cancer Res Ther 2018;14(4):820-5.
[Google scholar] [PubMed]
- Meng FD, Ma P, Sui CG, Tian X, Jiang YH. Association between cytochrome P450 1A1 (CYP1A1) gene polymorphisms and the risk of renal cell carcinoma: A meta-analysis. Sci Rep 2015;5(1):1-6.
[Crossref] [Google scholar] [PubMed]
- Salinas-Sánchez AS, Sánchez-Sánchez F, Donate-Moreno MJ, Rubio-del-Campo A, Serrano-Oviedo L, Gimenez-Bachs JM, et al. GSTT1, GSTM1 and CYP1B1 gene polymorphisms and susceptibility to sporadic renal cell cancer. Urol Oncol 2012;30(6):864-70.
[Crossref] [Google scholar] [PubMed]
- Yang C, Li J, Li Y, Wu D, Sui C, Jiang Y, et al. The vitamin D receptor gene ApaI polymorphism is associated with increased risk of renal cell carcinoma in Chinese population. Sci Rep 2016;6(1):1-6.
[Crossref] [Google scholar] [PubMed]
- Zhou T, Li H, Xie WJ, Zhong Z, Zhong H, Lin ZJ. Association of methylenetetrahydrofolate reductase, vitamin D receptor and interleukin-16 gene polymorphisms with renal cell carcinoma risk. Technol Cancer Res Treat 2019 ;18:1533033819859413.
[Crossref] [Google scholar] [PubMed]
- Chang WS, Liao CH, Tsai CW, Hu PS, Wu HC, Hsu SW, et al. The role of IL-10 promoter polymorphisms in renal cell carcinoma. Anticancer Res 2016;36(5):2205-9.
[Google scholar] [PubMed]
- Romero JM, Sáenz-López P, Cózar JM, Carretero R, Canton J, Vazquez F, et al. A polymorphism in the interleukin-10 promoter affects the course of disease in patients with clear-cell renal carcinoma. Hum Immunol 2009;70(1):60-4.
[Crossref] [Google scholar] [PubMed]
- Sáenz López P, Vázquez Alonso F, Romero JM, Carretero R, Tallada Buñuel M, Ruiz Cabello F, et al. Polimorfimos en genes de respuesta inflamatoria en cáncer renal metastásico. Actas Urol Esp 2009;33(5):474-81.
- Jianhai T, Jian L, Long Z, Wei W, Shumao Z, Yiming W, et al. Vitamin D receptor gene polymorphisms and its interactions with environmental factors on renal cell carcinoma risk. Genes Environ 2021;43(1):1-6.
[Crossref] [Google scholar] [PubMed]
- Arjumand W, Ahmad ST, Seth A, Saini AK, Sultana S. Vitamin D receptor FokI and BsmI gene polymorphism and its association with grade and stage of renal cell carcinoma in North Indian population. Tumor Biol 2012;33(1):23-31.
[Crossref] [Google scholar] [PubMed]
- Karami S, Brennan P, Hung RJ, Boffetta P, Toro J, Wilson RT, et al. Vitamin D receptor polymorphisms and renal cancer risk in Central and Eastern Europe. J Toxicol Environ Health A 2008;71(6):367-72.
[Crossref] [Google scholar] [PubMed]
- Obara W, Suzuki Y, Kato K, Tanji S, Konda R, Fujioka T. Vitamin D receptor gene polymorphisms are associated with increased risk and progression of renal cell carcinoma in a Japanese population. Int J Urol 2007;14(6):483-7.
[Crossref] [Google scholar] [PubMed]
- Ikuyama T, Hamasaki T, Inatomi H, Katoh T, Muratani T, Matsumoto T. Association of vitamin D receptor gene polymorphism with renal cell carcinoma in Japanese. Endocr J 2002;49(4):433-8.
[Crossref] [Google scholar] [PubMed]
- Southard EB, Roff A, Fortugno T, Richie JP, Kaag M, Chinchilli VM, et al. Lead, calcium uptake and related genetic variants in association with renal cell carcinoma risk in a cohort of male finnish smokers lead, calcium, vitamin D and renal cell carcinoma. Cancer Epidemiol Biomarkers Prev 2012;21(1):191-201.
- Chen J, Cheng M, Yi L, Jiang CB. Relationship between CYP1A1 genetic polymorphisms and renal cancer in China. Asian Pac J Cancer Prev 2011;12(9):2163-6.
[Google scholar] [PubMed]
- Longuemaux S, Deloménie C, Gallou C, Méjean A, Vincent-Viry M, Bouvier R, et al. Candidate genetic modifiers of individual susceptibility to renal cell carcinoma: A study of polymorphic human xenobiotic-metabolizing enzymes. Cancer Res 1999;59(12):2903-8.
[Google scholar] [PubMed]
- Wang G, Hou J, Ma L, Xie J, Yin J, Xu D, et al. Risk factor for clear cell renal cell carcinoma in Chinese population: A case-control study. Cancer Epidemiol 2012;36(2):177-82.
[Crossref] [Google scholar] [PubMed]
- Ahmad ST, Arjumand W, Seth A, Nafees S, Rashid S, Ali N, et al. Risk of renal cell carcinoma and polymorphism in phase I xenobiotic metabolizing CYP1A1 and CYP2D6 enzymes. Urol Oncol 2013;31(7):1350-57.
[Crossref] [Google scholar] [PubMed]
- Wang GP. Association of genetic polymorphisms in CYP1A1 and NAT2 with susceptibility to renal cancer: A case control study. Acad J Second Mil Med Univ 2010:1147-52.
- Nunes IF, Cavalcante AA, Alencar MV, Carvalho MD, Sarmento JL, Teixeira NS, et al. Meta-analysis of the association between the rs228570 vitamin D receptor gene polymorphism and arterial hypertension risk. Adv Nutr 2020;11(5):1211-20.
[Crossref] [Google scholar] [PubMed]