- *Corresponding Author:
- Baolin Sh
Department of Neurology, Weifang People’s Hospital, Weifang, Shandong 261000, China
E-mail: 15965096500@163.com
| This article was originally published in a special issue, “Transformative Discoveries in Biomedical and Pharmaceutical Research” |
| Indian J Pharm Sci 2023:85(4) Spl Issue “29-35” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
This study aimed to detect associations of bile acid profile with white matter hyperintensity and quantify the risk of cerebral small vessel disease with high level of bile acid among cognitively normal individuals. All data were obtained from the patients with Alzheimer’s disease neuroimaging initiative with a 10 y follow-up. Multivariate linear regressions were performed with variable selection using the least absolute shrinkage and selection operator method and the linear mixed model was used to analyze longitudinal relationship between non-zero coefficient variables and white matter hyperintensity volume. Four predictors (age, deoxycholic acid, glucose, total intracranial volume) were identified by least absolute shrinkage and selection operator regression analysis and multivariate linear regressions. Age (p value<0.001, mean difference, 1.03 cm3 [95 % interval: 1.02, 1.04]) and deoxycholic acid (p value<0.05, mean difference, 1.05 cm3 [95 % confidence interval: 1.00, 1.10]) were significantly associated with white matter hyperintensity. When divided into two groups by the median deoxycholic acid level (1.38 μM), participants with higher deoxycholic acid levels had a higher white matter hyperintensity volume at the 4th y than those with lower deoxycholic acid levels (mean difference, 0.1 points [95 % confidence interval: -0.17, -0.03]; p<0.05). We found that increased deoxycholic acid levels may lead to the progression of white matter hyperintensity, which suggest that higher deoxycholic acid burden may play a possible role in the pathogenesis of cerebral small vessel disease in cognitively normal older adults. Besides, the study indicates a possible role for inflammation in the pathogenesis of cerebral small vessel disease.
Keywords
Deoxycholic acid, white matter hyperintensity, cerebral small vessel disease, Alzheimer’s disease neuroimaging initiative, magnetic resonance imaging
White Matter Hyperintensity (WMH) is the main imaging feature of Cerebral Small Vessel Disease (CSVD). WMH can be detected on Magnetic Resonance Imaging (MRI) in 90 % of elderly individuals[1-3]. WMH volume is related to cognitive decline and an increased risk of dementia[4]. Bile Acids (BAs) can regulate intestinal microbes and are also an indicator of intestinal malnutrition and intestinal flora disorders[5,6]. Peripheral BA is passively diffused into the brain through the Blood- Brain Barrier (BBB) and increases the permeability of the BBB[7-9]. An increasing number of studies have found that gut microbes play a role in central nervous system diseases, that is, the gut-brain axis affects the central nervous system[10].
This study showed that WMH was associated with cognitive decline and an increased risk of dementia in the general population[11] and changes in BA were associated with cognitive impairment in individuals with Alzheimer’s disease[9]. The relationship between altered BA profiles and CSVD is not yet known. The purpose of this study was to find the correlation between the BA profile and WMHs among cognitively normal older adults.
Materials and Methods
Participants:
Since 2005, the Alzheimer’s Disease Neuroimaging Initiative (ADNI) has conducted research on people with normal cognition or diagnosed with cognitive impairment of varying degrees[12]. The ADNI battery includes a series of assessments by neuroimaging, testing of cerebrospinal fluid and other biomarkers, and performing clinical and neuropsychological assessments. In the present study, BAs and WMH were analyzed in subjects with normal cognition or subjective memory impairments. Participants could self-report subjective memory problems or through a research partner or clinician[13]. The ADNI recruited participants between the ages of 55 y and 90 y, with a Hachinski score≤4 and in good physical condition. The participants were recruited at 83 locations in the United States and Canada. After providing informed consent, participants will undergo a series of initial tests that will be repeated periodically over the next few years, including clinical assessment (blood pressure, height, weight, etc.), neuropsychological testing, genetic testing, lumbar puncture and MRI and Positron Emission Tomography (PET) scans.
Processing of images:
Following a standardized protocol for cross-platform verification, MRI (1.5 Tesla) was performed across sites. A high-resolution T1-weighted volume magnetization fast gradient echo sequence in the sagittal direction was obtained. The axial proton density/T2-weighted fast spin echo sequence was obtained. Sites included in the ADNI protocol must pass rigorous scanner verification tests. The data were transferred to the University of California, Davis, for WMH analysis.
Our WMH measurement method is based on the Bayesian method for segmentation of high-resolution three Dimensional (3D) T1 and Fluid-Attenuated Inversion Recovery (FLAIR) sequences. Briefly, non-brain tissue was first removed from T1-weighted and FLAIR images, MRI artifacts were removed, the images were spatially aligned and then, the sorted image was placed in the standard template space, according to the signal intensity of the voxels in the MRI. The signal strength of adjacent voxels and the prior probability of WMH existence were assessed, WMHs were identified and WMH volume was automatically estimated through a fully automated method. The volumes of gray matter, white matter and cerebrospinal fluid were divided and recorded by using the new SPM8 segmentation toolbox and the total volume of the brain was estimated automatically.
Sample collection and quantification of BAs:
The concentration of 20 BA metabolites in the serum samples from the ADNI cohort was measured by targeted metabolomics analysis. Baseline fasting serum samples were collected and referenced according to ADNI standard operating procedures. BAs were assessed by Liquid Chromatography- Tandem Mass Spectrometry (LC-MS/MS) and quantified by Biocrates Life Science AG (Innsbruck, Austria) according to the manufacturer’s instructions (BAs, abbreviations and their levels in the diagnostic group are shown in Table 1).
| Variables | Overall | DCA<1.38 µM | DCA>1.38 µM | p value |
|---|---|---|---|---|
| Number | 177 | 88 | 89 | |
| Age (mean (SD)) | 73.375 (6.335) | 73.690 (6.270) | 73.064 (6.418) | 0.5127 |
| Sex (%) | 0.9424 | |||
| Female | 92 (51.98) | 45 (51.14) | 47 (52.81) | |
| Male | 85 (48.02) | 43 (48.86) | 42 (47.19) | |
| Education (mean (SD)) | 16.565 (2.509) | 16.477 (2.482) | 16.652 (2.546) | 0.645 |
| Ethnicity (%) | 0.5656 | |||
| Hispanic/Latino Americans | 11 (6.21) | 6 (6.82) | 5 (5.62) | |
| Not Hispanic/Latino | 165 (93.22) | 81 (92.05) | 84 (94.38) | |
| Unknown | 1 (0.56) | 1 (1.14) | 0 (0.00) | |
| Race (%) | 0.8679 | |||
| Indian/Alaskan | 1 (0.56) | 1 (1.14) | 0 (0.00) | |
| Asian | 5 (2.82) | 2 (2.27) | 3 (3.37) | |
| Black | 15 (8.47) | 7 (7.95) | 8 (8.99) | |
| More than one | 2 (1.13) | 1 (1.14) | 1 (1.12) | |
| White | 154 (87.01) | 77 (87.50) | 77 (86.52) | |
| Marital status at baseline (%) | 0.0811 | |||
| Divorced | 26 (14.69) | 10 (11.36) | 16 (17.98) | |
| Married | 119 (67.23) | 64 (72.73) | 55 (61.80) | |
| Never married | 8 (4.52) | 6 (6.82) | 2 (2.25) | |
| Widowed | 24 (13.56) | 8 (9.09) | 16 (17.98) | |
| Total cholesterol (mean (SD)) | 3.797 (0.679) | 3.760 (0.623) | 3.834 (0.732) | 0.4714 |
| Esterified cholesterol (mean (SD)) | 2.658 (0.491) | 2.632 (0.452) | 2.683 (0.529) | 0.4901 |
| Free cholesterol (mean (SD)) | 1.139 (0.189) | 1.128 (0.172) | 1.150 (0.204) | 0.4249 |
| Serum total triglycerides (mean (SD)) | 1.109 (0.404) | 1.037 (0.364) | 1.181 (0.430) | 0.0168 |
| GLC (mean (SD)) | 3.949 (0.666) | 3.822 (0.614) | 4.075 (0.694) | 0.0111 |
| Systolic BP (median [Interquartile Range (IQR)]) | 132 [121, 142] | 130 [120, 143] | 133 [122, 142] | 0.4463 |
| Diastolic BP (median [IQR]) | [68, 80] | 73 [68, 80] | 73 [68, 81] | 0.8314 |
| BMI (mean (SD)) | 27.289 (4.470) | 25.809 (3.203) | 28.753 (5.047) | <0.0001 |
| 1387.34 | 1396.665 | 1376.23 | ||
| Total brain volume (median [IQR]) | 1286.030, 1478.160 | 1288.298, 1485.753 | 1279.320, 1469.590 | 0.5702 |
| WMH (mean (SD)) | 0.489 (0.544) | 0.473 (0.541) | 0.505 (0.550) | 0.7012 |
Note: DCA: Deoxycholic Acid; GLC: Glucose; BP: Blood Pressure; BMI: Body Mass Index and WMHs: White Matter Hyperintensities
Quality Control (QC) of BA profiles:
The diagnostic and pathological data of all studies were unknown to the metabolomics laboratory staff. At ADNI, after uncovering and releasing the data, the metabolite map was checked and data preprocessing was performed by QC, including batch adjustment, missing assignment and logarithmic transformation (supplementary method). After QC correction, the data set included 15 BAs (5 BAs did not pass the QC standard) from 177 subjects. The preprocessed BA value after QC was directly used in the subsequent association analysis or adjusted according to the effect of the drug on the level. All analyses were performed using both adjusted and unadjusted BA levels and the results from the adjusted drug data and the adjustment process are described in the supplementary method and the accompanying tables.
Statistical methods:
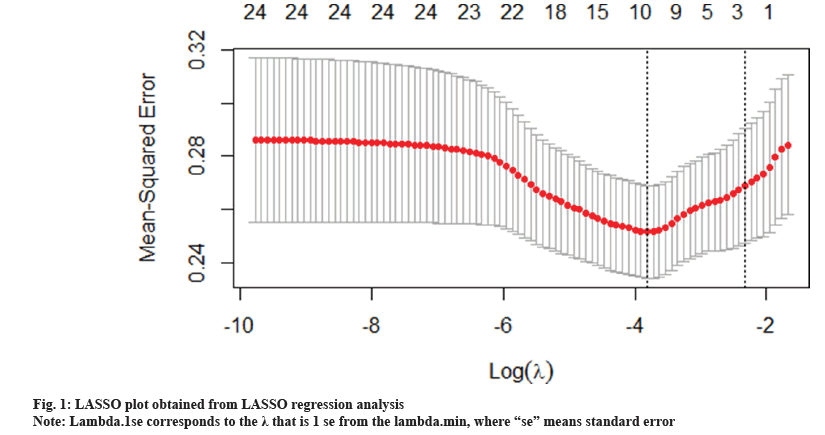
An analysis was performed to develop a multimarker predictive model with the goal of determining a panel of compounds that might predict WMH. The chosen method was multivariate linear regression with variable selection using the Least Absolute Shrinkage and Selection Operator (LASSO) 14, because our data have more variables than the observed data, so we used the free GLMNET package to select and reduce the data using LASSO regression analysis. LASSO is an improved form of least square regression that uses a regularization parameter lambda (λ) to penalize a complex model. Regularization is the process of adding constraints to a problem to avoid overfitting. Regularization in GLMNET is achieved by generating the path of the tuning parameter (λ) and solving the following equations in the range of λ to identify the optimal λ. GLMNET uses crossvalidation to identify the optimal penalty term (λ), which minimizes the average cross-validation error of our model and prevents the third type of error (the assumptions already made in the test data). We use the K=10-fold cross validation method. Then we report the non-zero coefficient, the regression coefficient and the R squared value which was determined in the model calculation and analysis based on the Akaike Information Criterion (AIC) multivariable linear regression model. All non-normal continuous distribution variables are transformed logarithmically before entering the linear mixed effect model (fig. 1).
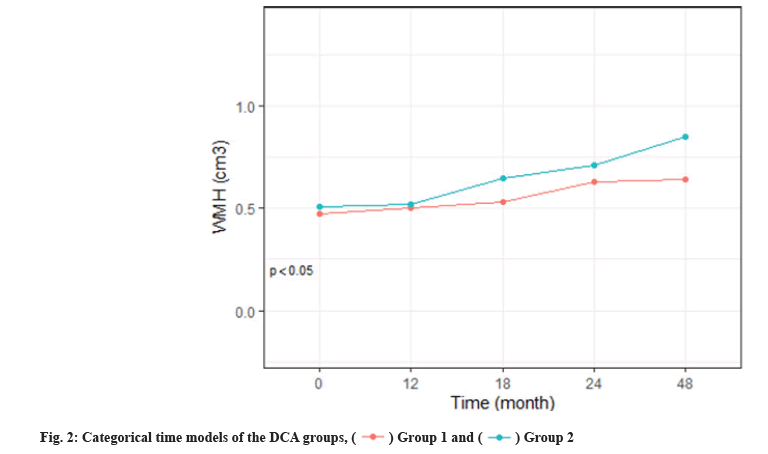
We calculated the descriptive statistics of characteristics, WMH, mean and Standard Deviation (SD) of continuous variables and distribution of classified variables (%) by the median of Deoxycholic Acid (DCA) levels. To summarize the characteristics of the high and low DCA groups, a t-test of 2 samples or Pearson’s Chi-squared (χ2) test were used to test the difference. A linear mixed effect model was used to analyze the progress of ADNI participants with normal DCA cognition, the original analysis treated time was continuous. With the support of the AIC, an objective model selection tool, the quadratic term time is increased. The additional model is the same as the main model, except that time is considered to be categorical (fig. 2). These classification time models do not make any assumptions about the shape of the time trend and are therefore useful for evaluating the shape assumptions imposed by continuous time models (fig. 3).
Age is a known factor associated with WMH burden and is included as a covariable in all models. The correlation between baseline GLC, diastolic blood pressure, Body Mass Index (BMI) and total brain volume was used as a cross-validation model and AIC was used to select additional covariates. Baseline WMH indicators are not included as covariables because they are modeled as response variables. The overall effect of the DCA group was tested by the likelihood ratio test. The point estimate for 4 y was emphasized because of the paucity of data available at subsequent study visits. A multiple imputation approach was applied that was robust to the missing at random assumption. All the tests were 2-sided and a p value less than 0.05 was considered to be statistically significant. Models were fit and plotted using R (version 4.0.0, https://www.R-project.org/).
Results and Discussion
Key variables by DCA group are reported in Table 1. There was no significant difference in age between the elevated DCA group and the reduced DCA group. Overall, 93 (63 %) out of 177 participants remained active in the study and the rest were not interviewed (n=84). The overall DCA group had a significant influence on WMH volume by the likelihood ratio test (p<0.001).
The WMH volume was larger in the elevated DCA group at 4 y. The mean volume of the principals was 0.47 (95 % Confidence Interval (CI), 0.36 to 0.59) at baseline and 0.63 (95 % CI, 0.52-0.73) in the low DCA group in 4 y and 0.51 (95 % CI, 0.39-0.63) at baseline and 0.71 (95 % CI, 0.60-0.68) in the high DCA group in 4 y (mean difference, 0.1 point [95 % CI, -0.17 to -0.03]; p<0.05). Fig. 2 shows the proportion of progress events over time in the AIC selected covariant DCA group. The likelihood ratio of the whole-amyloid group association was significant at p<0.01, of all 4 progression events when time was considered continuous.
DCA group is classified by median DCA level (1.38 μM). Because ten predictors together optimized the model fit using the minimum λ identified by cross-validation (fig. 1). This minimum value λ corresponds to the cost of reaching the minimum Mean Squared Error (MSE). As shown in fig. 1, plot of non-zero variable fit after cross-validation. Representation of the 10-fold cross-validation performed in LASSO that chooses the optimal λ. Lambda.min corresponds to the λ that minimizes the MSE and was used for variable selection and lambda.1se corresponds to the λ that is 1 se from the lambda.min, where “se” means standard error. Four of the predictors is BA profile (DCA, Glycine- Conjugated Bile Acid Glycochenodeoxycholic Acid (GCDCA), Taurolithocholic Acid (TLCA), Ursodeoxycholic Acid (UDCA)), one is age, one is clinical variables (free cholesterol, GLC, diastolic Blood Pressure (BP), BMI) and one is academic imaging variables (total intracranial volume) as shown in Table 2. The R square of the LASSO regression model and intercept-only regression model was 0.1544, which showed that the model can explain 15.44 % of the variance in WMHs. Age and DCA were significantly associated with WMHs in the AIC of standard leave-one-out multivariable linear regression model analyses (Table 2). The associations of age and DCA with WMHs retained significance after correction for total brain (adjusted p value<0.001 and 0.03, respectively). Every 1 % increase in age and DCA levels was related to a 1.03 cm3 (95 % CI: 1.02, 1.04) and 1.05 cm3 (95 % CI: 1.00, 1.10) increase in WMHs, respectively (Table 1). Since the average relative abundance was low for these taxa, we also expressed the results using a one SD (1-SD) increase in the relative abundance. The standard regression coefficient showed that as age and DCA value increased, the WMH value increased correspondingly. So we divided them into two groups by the median DCA level (1.38 μM).
| Variables | Standard error | t value | Beta (β)-selection | p value |
|---|---|---|---|---|
| Age | 0.006 | 4.71 | 1.03 | <0.001 |
| Total brain volume | 0.0003 | 2.81 | 1.00 | <0.001 |
| DCA | 0.02 | 2.15 | 1.052 | <0.05 |
| GLC | 0.06 | -1.52 | 0.92 | 0.12 |
Note: Deoxycholic Acid (DCA) and Glucose (GLC)
As shown in fig. 2 and fig. 3, linear mixed-effects models were controlled for age and other baseline covariates and are conditional on covariates as follows: Age, DCA, Glucose (GLC), total intracranial volume, likelihood ratio tests comparing models (elevated amyloid group vs. normal) were used for p values. Shaded regions indicate 95 % CIs. DCA groups 1 and 2 were classified by median DCA level (1.38 μM).
In this study, we found that the blood BA content and WMH volume were positively correlated in elderly individuals over 60 y old, so the DCA content in the blood of elderly individuals was independently correlated with WMH volume. WMH is one of the imaging markers of CSVD[3]. We can conclude that the increase in blood BA content in elderly patients is significantly correlated with the increase in WMH volume, which promotes the occurrence of CSVD.
Cholesterol is metabolized in the liver to produce primary BAs. Primary BAs such as Cholic Acid (CA) and Chenodeoxycholic Acid (CDCA) are converted into secondary BAs, such as DCA and Lithocholic Acid (LCA), through the intestinal microbiota, and of the secondary BAs, DCA and LCA are produced at the highest levels[14,15]. The normal physiological level of BA can counteract the intestinal flora and regulate the composition of the intestinal microbiota.
Studies have shown that normal physiological levels of DCA can inhibit the growth of Escherichia coli and other bacteria, regulate blood lipids, inhibit fatty acid uptake in the liver and reduce triglyceride production[5,16-18].
BAs can cross the BBB and cause potential neurotoxicity[18]. In vitro and in vivo experiments[19] verified the role of DCA in blood vessels and found that it is essentially hydrophobic and toxic. Zebrafish embryos have been observed to have abnormal vascular development under DCA treatment, which is lethal at the highest concentration of 25 μm. BAs can not only cross the BBB but can also cross and destroy the blood-testis barrier. Studies have shown that mice fed with a CA diet initially experience germ cell shedding and blood-testicular barrier rupture and later, sperm cell apoptosis is observed, leading to testicular defects and reduced fertility[18].
In obese mice fed with a high-fat diet, elevated serum levels of BAs can destroy vascular smooth muscle cells, leading to the occurrence and development of atherosclerosis[20]. In clinical studies, patients with Coronary Artery Disease (CAD) have significantly lower BA excretion than non-CAD patients, so the impaired ability to excrete cholesterol may be considered another independent risk factor for the development of CAD. It is also associated with the risk of stroke[21,22].
There may be unpredictable selection bias in the ADNI data. Participants were recruited from memory clinics and advertisements, and the inclusion criteria for people with abnormal cognition are highly selective, so the research group cannot represent the general population. This study was restricted to participants with a smaller WMH burden, which excluded patients with Hachinski scores>0, resulting in a relatively low correlation. This was an observational study and the results may include confounding biases. For example, diet, lifestyle, exposure and other factors can cause changes in the gut. The ADNI cohort and other large studies did not collect fecal matter and therefore excluded direct analysis of changes in the microbiome across the disease trajectory.
Studies have shown that increasing hemicellulose and pectin in the diet can increase the excretion of BAs and reduce cholesterol[23]. Therefore, in addition of using statins to lower lipids, diet and other related drugs can also regulate cholesterol metabolism, increase BA excretion and reduce the content of BAs in the blood to prevent or reduce CSVD and atherosclerosis was found. This study provides new directions for future clinical research, diagnosis and prevention.
Acknowledgements:
The study was funded by Weifang City Science and Technology Development Project Plan (No. 2022YX010 and 2020YX003).
Conflict of interests:
The authors declared no conflict of interest.
References
- Wardlaw JM, Smith C, Dichgans M. Small vessel disease: Mechanisms and clinical implications. Lancet Neurol 2019;18(7):684-96.
[Crossref] [Google scholar] [PubMed]
- Pantoni L. Cerebral small vessel disease: From pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol 2010;9(7):689-701.
[Crossref] [Google scholar] [PubMed]
- Park JH, Seo SW, Kim C, Kim GH, Noh HJ, Kim ST, et al. Pathogenesis of cerebral microbleeds: In vivo imaging of amyloid and subcortical ischemic small vessel disease in 226 individuals with cognitive impairment. Ann Neurol 2013;73(5):584-93.
[Crossref] [Google scholar] [PubMed]
- Wang YL, Chen W, Cai WJ, Hu H, Xu W, Wang ZT, et al. Associations of white matter hyperintensities with cognitive decline: A longitudinal study. J Alzheimers Dis 2020;73(2):759-68.
[Crossref] [Google scholar] [PubMed]
- Islam KS, Fukiya S, Hagio M, Fujii N, Ishizuka S, Ooka T, et al. Bile acid is a host factor that regulates the composition of the cecal microbiota in rats. Gastroenterology 2011;141(5):1773-81.
[Crossref] [Google scholar] [PubMed]
- Camilleri M, Gores GJ. Therapeutic targeting of bile acids. Am J Physiol Gastrointest Liver Physiol 2015;309(4):209-15.
[Crossref] [Google scholar] [PubMed]
- Quinn M, McMillin M, Galindo C, Frampton G, Pae HY, de Morrow S. Bile acids permeabilize the blood brain barrier after bile duct ligation in rats via Rac1-dependent mechanisms. Dig Liver Dis 2014;46(6):527-34.
[Crossref] [Google scholar] [PubMed]
- Pan X, Elliott CT, McGuinness B, Passmore P, Kehoe PG, Hölscher C, et al. Metabolomic profiling of bile acids in clinical and experimental samples of Alzheimer’s disease. Metabolites 2017;7(2):28.
[Crossref] [Google scholar] [PubMed]
- MahmoudianDehkordi S, Arnold M, Nho K, Ahmad S, Jia W, Xie G, et al. Altered bile acid profile associates with cognitive impairment in Alzheimer's disease-an emerging role for gut microbiome. Alzheimers Dement 2019;15(1):76-92.
[Crossref] [Google scholar] [PubMed]
- Lalić-Popović M, Vasović V, Milijašević B, Goločorbin-Kon S, Al-Salami H, Mikov M. Deoxycholic acid as a modifier of the permeation of gliclazide through the blood brain barrier of a rat. J Diabetes Res 2013;2013:1-8.
[Crossref] [Google scholar] [PubMed]
- Wolters FJ, Zonneveld HI, Hofman A, van der Lugt A, Koudstaal PJ, Vernooij MW, et al. Cerebral perfusion and the risk of dementia: A population-based study. Circulation 2017;136(8):719-28.
[Crossref] [Google scholar] [PubMed]
- Weiner MW, Veitch DP, Aisen PS, Beckett LA, Cairns NJ, Cedarbaum J, et al. Impact of the Alzheimer's disease neuroimaging initiative, 2004 to 2014. Alzheimers Dement 2015;11(7):865-84.
[Crossref] [Google scholar] [PubMed]
- Donohue MC, Sperling RA, Petersen R, Sun CK, Weiner MW, Aisen PS, et al. Association between elevated brain amyloid and subsequent cognitive decline among cognitively normal persons. JAMA 2017;317(22):2305-16.
[Crossref] [Google scholar] [PubMed]
- Jiao NA, Baker SS, Chapa-Rodriguez A, Liu W, Nugent CA, Tsompana M, et al. Suppressed hepatic bile acid signalling despite elevated production of primary and secondary bile acids in NAFLD. Gut 2018;67(10):1881-91.
[Crossref] [Google scholar] [PubMed]
- Funabashi M, Grove TL, Wang M, Varma Y, McFadden ME, Brown LC, et al. A metabolic pathway for bile acid dehydroxylation by the gut microbiome. Nature 2020;582(7813):566-70.
[Crossref] [Google scholar] [PubMed]
- Wang S, Martins R, Sullivan MC, Friedman ES, Misic AM, El-Fahmawi A, et al. Diet-induced remission in chronic enteropathy is associated with altered microbial community structure and synthesis of secondary bile acids. Microbiome 2019;7(1):1-20.
[Crossref] [Google scholar] [PubMed]
- Nie B, Park HM, Kazantzis M, Lin M, Henkin A, Ng S, et al. Specific bile acids inhibit hepatic fatty acid uptake in mice. Hepatology 2012;56(4):1300-10.
[Crossref] [Google scholar] [PubMed]
- Huang F, Wang T, Lan Y, Yang L, Pan W, Zhu Y, et al. Deletion of mouse FXR gene disturbs multiple neurotransmitter systems and alters neurobehavior. Front Behav Neurosci 2015;9:70.
[Crossref] [Google scholar] [PubMed]
- Kundu S, Bansal S, Muthukumarasamy KM, Sachidanandan C, Motiani RK, Bajaj A. Deciphering the role of hydrophobic and hydrophilic bile acids in angiogenesis using in vitro and in vivo model systems. Medchemcomm 2017;8(12):2248-57.
- Shimizu H, Hagio M, Iwaya H, Tsuneki I, Lee JY, Fukiya S, et al. Deoxycholic acid is involved in the proliferation and migration of vascular smooth muscle cells. J Nutr Sci Vitaminol 2014;60(6):450-4.
[Crossref] [Google scholar] [PubMed]
- Ding L, Chang M, Guo Y, Zhang L, Xue C, Yanagita T, et al. Trimethylamine-N-oxide (TMAO)-induced atherosclerosis is associated with bile acid metabolism. Lipids Health Dis 2018;17:1-8.
[Crossref] [Google scholar] [PubMed]
- Charach G, Karniel E, Novikov I, Galin L, Vons S, Grosskopf I, et al. Reduced bile acid excretion is an independent risk factor for stroke and mortality: A prospective follow-up study. Atherosclerosis 2020;293:79-85.
[Crossref] [Google scholar] [PubMed]
- Huang CT, Gopalakrishna GS, Nichols BL. Fiber, intestinal sterols and colon cancer. Am J Clin Nutr 1978;31(3):516-26.
[Crossref] [Google scholar] [PubMed]
 ) Group 1 and (
) Group 1 and ( ) Group 2
) Group 2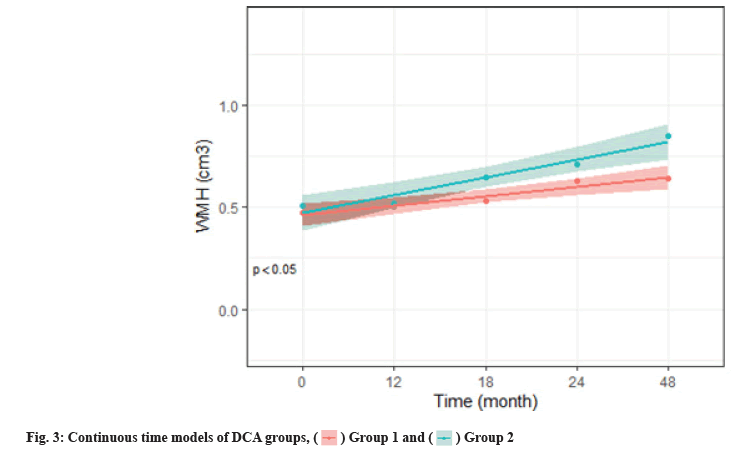
 ) Group 1 and (
) Group 1 and ( ) Group 2
) Group 2



