- *Corresponding Author:
- G. Pandi
Department of Fish Processing Technology, Dr. J. Jayalalithaa Fisheries University, Thoothukudi, Tamil Nadu 628008, India
E-mail: ganesanresearch15@gmail.com
| Date of Received | 15 August 2023 |
| Date of Revision | 08 April 2024 |
| Date of Acceptance | 20 August 2024 |
| Indian J Pharm Sci 2024;86(4):1469-1479 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The present study compared aqueous and ethanolic solvents to evaluate the in vitro anticancer, anti-elastase and anti-collagenase activity of Hypnea pannosa seaweed extracts. Additionally, the study evaluated the extract's preliminary phytochemical composition, total phenolic and flavonoid content, antioxidant and antibacterial activity. The analysis revealed the presence of several phytochemicals and the range of total phenolic (11.14±0.17 mg gallic acid equivalents/g and flavonoid (5.83±0.09 mg quercetin equivalent/g) content in the ethanolic extract. The ethanolic extract showed the highest antioxidant activity against 2,2-diphenyl-1- picrylhydrazyl (half-maximal inhibitory concentration=6.32±0.04 μg/ml) and the most potent anticancer effect against COLO 829 melanoma cancer cell lines (half-maximal inhibitory concentration=95.70 μg/ml). It also exhibited the most potent anti-elastase and anti-collagenase effect against human melanoma cells, with halfmaximal inhibitory concentration values of 48.53 and 48.76 μg/ml, respectively. The ethanol extract showed maximum antibacterial activity and the highest inhibition zone of 14.5 and 14 mm against Escherichia coli and Pseudomonas aeruginosa, respectively. Fourier Transform Infrared Spectroscopic analysis shows that many polyphenols are present in ethanol extract, which has received importance in cosmeceutical perspectives (3925- 3893 cm-1). The study hence provides evidence that the ethanol extract of Hypnea pannosa has significant potential for antioxidants, anticancer, antibacterial, anti-elastase and anti-collagenase and may lead to further cosmeceutical applications through the isolation and purification of bioactive compounds.
Keywords
Antioxidant, antibacterial, anticancer, anti-elastase, anti-collagenase, 2,2-diphenyl-1- picrylhydrazyl, phenols, flavonoids, Fourier transform infrared spectroscopy
Skin ageing is a complex process influenced by various factors, both intrinsic and extrinsic. Intrinsic factors are related to the passage of time, while extrinsic factors mainly arise from exposure to the sun[1]. When exposed to Ultraviolet (UV) radiation, the skin changes complex pathways, eventually releasing Reactive Oxygen Species (ROS), Matrix Metalloproteinases (MMPs) and elastase[2]. ROS is responsible for skin aging by causing oxidative damage to the skin's lipids, proteins and DNA[3,4] and consequently induces severe human diseases, including chronic inflammation, atherosclerosis, cancer, cardiovascular disorders and aging[5]. Fortunately, recent developments in marine biotechnology have opened up new avenues for research in skin aging, inflammation and degradation. Marine bioactive compounds can positively impact the treatment of skin disorders[6]. Marine algae, also known as seaweed has become an essential component of the cosmeceutical industry, accounting for 40 % of the world's hydrocolloid market[7]. Seaweed has become increasingly popular due to its bioactive properties, such as potent antioxidant, antibacterial, antiviral, antifungal, anti-inflammatory and anticancer agent[8].
Red seaweed is being considered by the cosmetic industry as a source of bio-sustainable components for skin care due to its high concentration of biologically active compounds like agar and carrageenan, which can be used as effective skin care agents[9]. Hypnea has recently grown significantly among red algae due to its biological properties, such as anticancer, antiaging and antioxidant activities[10]. Algal extracts have also been utilized in dietary supplements, cosmetics, and alternative medicines recommended for skin-related diseases[11]. Plant extract such as Hypnea pannosa (H. pannosa) has discovered their ability to scavenge free radicals and exhibit anti-collagenase and anti-elastase activities. The extract from Hypnea positively impacts the skin as it aids in repairing and calming any damage caused by exposure to UV rays or sunburn[12]. Studies have shown that marine algae have properties that protect against cancer cells. Different types of algae have been found to have cytotoxic and antitumor activities[13,14]. UV B radiation is also the main factor in initiating and developing melanoma and non-melanoma skin cancers[15].
Polyphenolic compounds extracted from different algal species have many biological activities, including their role in inhibiting melanogenesis[16-18], elastase and collagenase enzymes[19] are essential in cosmeceutical product development. Collagenase is crucial in remodelling the Extracellular Matrix (ECM), especially in breaking down collagen. Elastase, on the other hand is a serine proteinase that is responsible for breaking down elastin in the ECM. The depletion of collagen and elastin in the skin can lead to undesired wrinkles and aging skin, as they are essential for maintaining the structural integrity and elasticity of the skin[20].
Currently, researchers are focusing on discovering phytochemicals, such as phenols and flavonoids, from marine algae, which have potent antioxidant effects. Seaweed has been identified as an essential source of these phytochemicals. The present study examined and compared the total phenolic and flavonoid content of the aqueous and ethanolic H. pannosa extract while determining it’s in vitro antioxidant, antibacterial, anticancer, anti-collagenase and anti-elastase properties. The study also examined the presence of functional groups of compounds by the FT-IR spectroscopic method. The results suggest that crude H. pannosa extracts possessing high bioactivity could be used as natural anti-aging ingredients, which can be incorporated into cosmetic products.
Materials and Methods
Plant material:
The red seaweed, H. pannosa was collected along the coast of Mandapam and washed thoroughly to remove all traces of sand and epiphytic organisms. The dried seaweed was then ground to a fine powder using a pulverizer equipped with a 3 mm perforated grill (Centertech, Madurai) and stored in the refrigerator until use.
Extraction:
H. pannosa was subjected to extraction using two solvents viz. ethanol and water, following the procedure outlined by Cho et al.[21]. Initially, 1 g of a powdered sample was added to 10 ml of ethanol and agitated using a magnetic stirrer at 720 rpm at ambient temperature for 24 h. The sample was centrifuged at 2090 rpm for 10 min and collected. The extraction procedure was repeated twice to obtain the residue, which was subsequently combined with the prior extract. H. pannosa was extracted using water as a solvent in a similar manner. The mixture was then filtered through Whatman filter paper (125 mm) and concentrated using a rotary evaporator. The concentrate was then dried at 37° and stored refrigerated until use.
Preliminary phytochemical screening:
All freshly prepared crude extracts of seaweed were evaluated qualitatively for the identification of different classes of active phytochemical constituents, such as terpenoids[22], tannin[23], saponin[24] and alkaloids[25] using standard techniques. The presence or absence of these compounds in the crude extracts tested was revealed during these general reactions.
Test for terpenoids (Salkowski test):
5 ml of each extract was added with 2 ml chloroform and 3 ml of concentrated sulphuric acid (H2SO4). The formation of a reddish-brown colour at the interface indicates the presence of terpenoids in the sample extracts[22].
Test for tannins:
3 ml of extract was mixed with a few drops of 1 % hydrochloric acid (HCl) and observed for precipitation. A red precipitate showed positive results for tannins in the crude extract[23].
Test for saponin (frothing test):
About 3 ml of each extract was treated with 3 ml of distilled water and the sample was shaken vigorously for a stable, persistent froth for about 1 min. The frothing was added with 3 drops of olive oil and shaken vigorously, then observed for emulsion formation, indicating the presence of saponins in the extracts[24].
Test for alkaloids:
By adding 1 ml of Dragendorff”s reagent to 2 ml of extracts, an orange-red precipitate was formed, indicating the presence of alkaloids[25].
Total phenolic content:
The total phenolic content determined in seaweed extract was carried out according to the method of Slinkard et al.[26]. 0.5 ml of sample extract was mixed with 0.5 ml of Folin-Ciocalteu (5 ml of Folin-Ciocalteu reagent diluted with 50 ml of distilled water). After 5 min, 2.5 ml of sodium carbonate (Na2CO3) (2 %) was added. After 40 mi, the absorbance was measured at 725 nm using a double-beam spectrophotometer (Model UV- 1800, Shimadzu, Kyoto, Japan). The total phenolic content was determined using a calibration curve derived from gallic acid and the results were expressed as mg of Gallic Acid Equivalents (GAE)/g of crude extract.
Total flavonoid content:
The determination of flavonoid content in seaweed extracts was analysed according to Kumar et al.[27]. 4 ml of distilled water, 0.3 ml of 10 % aluminum chloride (AlCl3) and 0.3 ml of 5 % NaNO2 were added to 0.5 ml of crude extract. After 6 min, 2.5 ml of distilled water and 2 ml of 1 N NaOH were added to the mixture. At 506 nm, the absorbance was then determined. Total flavonoid concentration in the extracts was calculated using a calibration curve using quercetin and the results were expressed as mg Quercetin (QE)/g of crude extract.
In vitro antioxidant activity using 2,2-Diphenyl-1- Picrylhydrazyl (DPPH):
The antioxidant properties were determined by DPPH scavenging potential, in which ascorbic acid was used as a positive control, according to Blois et al.[28]. A 2 ml aliquot of 0.16 mM DPPH solution in methanol was mixed with aliquots of extract (2 ml) and then incubated in the dark at room temperature for 30 min. The same procedure was followed for positive standard containing ascorbic acid. At 517 nm, the absorbance of the samples and standards was measured.
DPPH radical scavenging activity (%) = Abscontrol-Abssample/Abscontrol×100
In vitro antibacterial activity:
The disc diffusion method performed the in vitro antibacterial activity of the H. pannosa extracts. The test bacteria were inoculated in peptone water and incubated for 3-4 h at 35°. Mueller Hinton agar was prepared and poured into sterile petri plates. 0.1 ml of bacterial culture was inoculated on the Mueller Hinton agar plates' surface and spread using L-rod. The inoculated plates were allowed to dry for 5 min. The disk loaded with a sample concentration of 1000 μg/ml was placed on the surface of inoculated petri plates using a sterile technique. The plate was incubated at 37° for 18-24 h. The plate was then examined for the inhibitory zone and the zone of inhibition was measured in mm[29].
In vitro anticancer activity:
The 3-(4, 5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide (MTT) cytotoxic assay was performed on a COLO-829 malignant melanoma (human) cancer cell line to evaluate the anticancer activity of H. pannosa extracts. Before being treated with the H. pannosa extract, the cancer cells were cultured in Rosewell Park Memorial Institute media for 24 h. 100 μl of the extracts were diluted with Dimethyl Sulfoxide (DMSO) and added at three different concentrations (100 μg/ml, 200 μg/ml and 300 μg/ml), along with a negative control of DMSO. After treatment, the cells were incubated for 24 and 48 h before determining the effect of the different concentrations of the seaweed extract on the cancer cell line[30,31].
In vitro anti-elastase activity:
In vitro anti-elastase activity protocol by Senior et al.[32] and Sallenave et al.[33] was adopted with the required modifications in the present assay. SKMEL (15 000 cells/well) were seeded on 12 well plates and allowed to acclimate to the culture conditions, such as 37° and 5 % CO2 environment,in the incubator for 24 h. The test samples were prepared in Dulbecco's Modified Eagle Medium (DMEM) (100 mg/ml) and filtered and sterilized using a 0.2 μm Millipore syringe filter. The samples were diluted in DMEM media and added to the wells containing cultured cells at final concentrations of 6.25, 12.5, 25, 50, 100 and 200 μg/ml respectively. Untreated wells were kept in control. All the experiments were done in triplicate and average values were taken to minimize errors. After treatment with the test samples, the plates were further incubated for 24 h. After the incubation period, the media from the wells were aspirated and discarded. The anti-elastase activities of the extracts were finally expressed as half-maximal Inhibitory Concentration (IC50) value.
The percentage of elastase inhibition activity was calculated using the following equation;
Elastase inhibition (%)=(1-enzyme activity in the presence of the inhibitor/enzyme activity in absence of the inhibitor)×100
In vitro anti-collagenase activity:
In vitro anti-collagenase activity protocol by Park et al.[34] and Shanura Fernando et al.[35], was adopted with the required modifications in the present assay. 1 mg of azo dye-impregnated collagen (A4341, Sigma) was measured in test tubes and homogenized in 800 μl of 0.1 M Tris-HCl (pH 7.0). It was then mixed respectively with 400 μl of the sample cell lysates (control-6.25, 12.5, 25, 50, 100 and 200 μg/ml). Collagenase (C0130, Sigma) prepared at a concentration of 200 units ml-1 was rapidly incorporated into the reaction mixture. All the tubes were then incubated at 43° for 1 h. The contents were centrifuged at 3000 rpm for 10 min and the absorbance of the supernatant was measured at 550 nm. The anti-collagenase activities of the extracts were finally expressed as an IC50 value
The percentage of collagenase inhibition activity was calculated using the following equation;
Inhibitory rate of collagenase (%) = [(Absorbance of control-Absorbance of sample)/Absorbance of control]×100.
Fourier Transform-Infrared Spectroscopy (FT-IR):
FT-IR Spectroscopy was carried out by using FT-IR Spectrophotometer (ATR-FT-IR, Model P-4600, Thermo Scientific, USA). The dried seaweed extracts (aqueous and ethanol) were mixed with Potassium Bromide powder (KBr) in a ratio of 1:9 and ground well. After assembling the die, the powder was added to the collar and given a hydraulic press of about 100 kg/m3 to form the pellet. The pellet was taken out from the collar after disassembling the die. The spectrum was then recorded after placing the pellet on the sample holder and the signal was obtained in 32 scans at a resolution of 4 cm-1 from 600-4000 cm-1.
Statistical analysis:
All data were recorded, processed and entered using Statistical Package for Social Sciences (SPSS) statistics Version 20 (Chicago, IL, USA). The data obtained were expressed in average±Standard Deviation (SD).
Results and Discussion
The extraction yield depended solely on the solvent polarity, pH, extraction time and sample composition[36]. The most critical stages in isolating bioactive compounds from plant material are mainly the extraction methods, significantly optimizing the most suitable solvent for the extraction from the plant material[37]. The yield of aqueous and ethanol solvent extracts of H. pannosa was reported as (10.26±0.03 %) and (8.26±0.02 %) respectively (Table 1). The variation in extraction yield among different solvents may be attributed to the polarities of different compounds present in the seaweeds[38,39].
| Solvent extraction | Extraction yield (%) | Total phenolic content (mg GAE/g of extract) | Total flavonoids content (mg QE/g of extract) | DPPH (IC50 mg/ml) |
|---|---|---|---|---|
| Aqueous extract | 10.26±0.03 % | 8.03±0.28 | 3.20±0.01 | 9.45±0.03 |
| Ethanol extract | 8.26±0.02 % | 11.14±0.17 | 5.83±0.09 | 6.32±0.04 |
Note: All the data were provided in mean±standard deviation of three replicates (n=3)
Table 1: Antioxidant Properties of H. Pannosa Solvent Extracts
The presence or absence of phytoconstituents depends on the extraction solvent and the seaweed's physiological characteristics. The essential bioactive compounds in seaweed can be examined using standard methods involving various solvents and ambient conditions[40]. This study used ethanol and water extracts for phytochemical screening, confirming the presence of active chemical components such as terpenoids, saponin, tannin, alkaloids, phenolics, and flavonoids (Table 2). Eight out of ten tests were positive, while two were negative. However, Rafiquzzaman et al.[41], identified some phytochemicals in the red algae Hypnea musciformis (H. musciformis) collected in Bangladesh. The study results indicate that ethanol extract contains more phytochemicals than aqueous extract, which has implications for cosmeceutical properties.
| Name of the extracts | Terpenoids | Saponin | Tannin | Alkaloids | Phenols | Flavonoids |
|---|---|---|---|---|---|---|
| Aqueous | - | + | - | + | + | + |
| Ethanol | + | + | + | + | + | + |
Note: (-): Absence of the components and (+): Presence of the components
Table 2: Preliminary Phytochemical Screening of H. Pannosa Extracts
Polyphenolics are secondary metabolites primarily found in plants, with widespread deposition in the cell wall. These compounds possess several biological and redox properties responsible for their antioxidant capacity. The total phenolic content of the extracts was estimated using the Folin-Ciocalteu reagent method. It was found that the aqueous and ethanolic extracts had 8.03±0.28 and 11.14±0.17 mg GAE/g extract, respectively (Table 1). The study results indicate that the ethanolic extract contained more total polyphenols, identified as the primary antioxidant compounds. The solubility of phenolics increases with the polarity of the solvent, which increases the phenolic content[42]. The total phenolic content can vary based on the polarity of the solvent used for extraction, so ethanol is more effective than methanol in extracting the total phenolic content due to its higher polarity[43]. Earlier reports substantiate our present observation that solvent extracts of Hypnea sp. and other red seaweeds are good resources for polyphenolic compounds[44,45]. Thus, the study proved that ethanol extracts increase total phenolic content and provide good antioxidant properties.
Flavonoids are polyphenolic compounds that act as antioxidants in the body, preventing damage caused by oxidation. They have high redox potential, chelate metals, and donate hydrogen[46]. Their antioxidant capacity comes from the conjugation between flavonoid rings[47]. The total flavonoid content showed a significant difference among aqueous extract (3.20±0.01 mg QE/g) compared to ethanol extract (5.83±0.09 mg QE/g) as illustrated in Table 1. The ethanol extraction showed the highest total flavonoid content in Gracilaria sp.[48]. The variation in flavonoids may be due to changes in physiochemical characteristics such as salinity. Thus, the study proved that ethanolic extracts contribute to increased flavonoid content and positively correlate with phenolic content.
DPPH is a free radical that remains stable. Antioxidants can eliminate it by donating an electron or hydrogen atom to the DPPH radical, which transforms into a stable molecule, causing a colour change from purple to yellow[49]. In the present study, the DPPH assays were represented as IC50 value and the ethanolic extract exhibited rich scavenging effects on DPPH (6.32±0.04 mg/ ml) when compared to aqueous extract with an IC50 value of 9.45±0.03 mg/ml respectively (Table 1). The differences in antioxidant activity among the aqueous and ethanolic seaweed extract might be due to the changes in their chemical compositions, resulting in a significant difference in antioxidant activity. The stronger radical scavenging activity is linked to the ethanolic extract's phenolic content.
Similarly, Siriwardhana et al.[50] and Liu et al.[51], reported a high correlation between DPPH radicalscavenging activities and total polyphenolics. Components, such as polysaccharides, pigments, proteins or peptides in seaweed, also impart antioxidant activity. The study found that the use of ethanolic solvent extraction resulted in the highest level of antioxidant activity with a similar correlation to the levels of phenolic compounds.
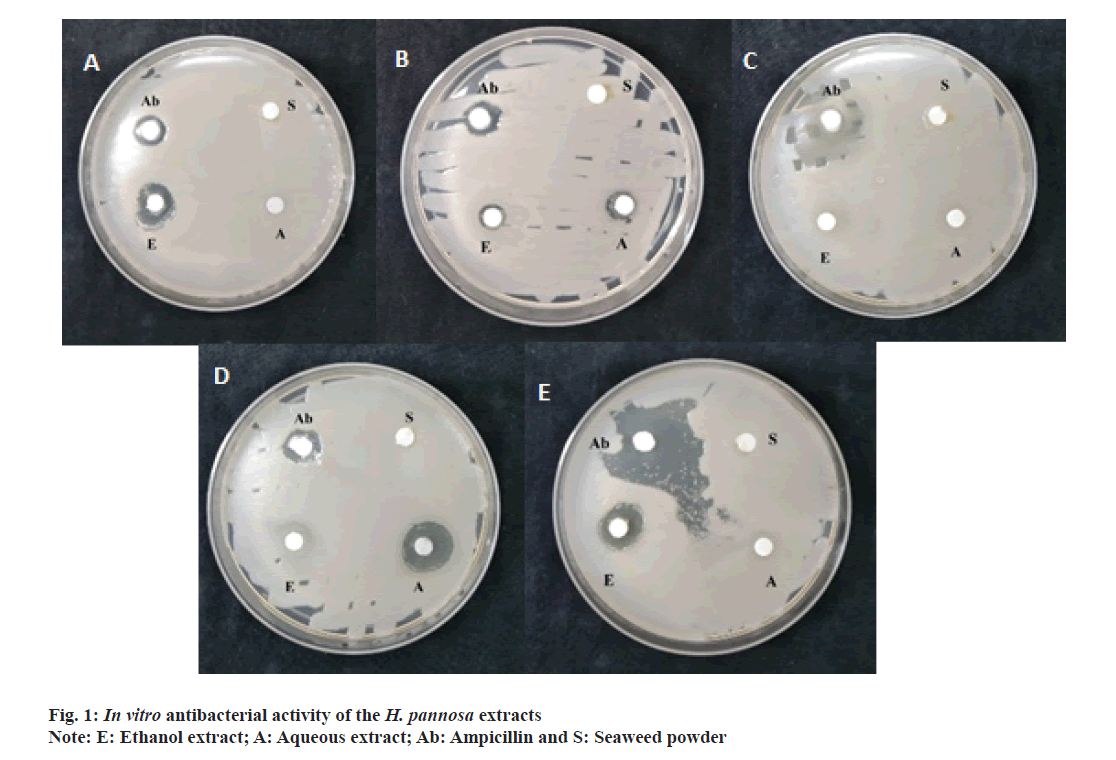
The present study evaluated the antibacterial properties of H. pannosa extracts using the disc diffusion method (Table 3 and fig. 1a-fig. 1e). The results showed that the extracts had significant antibacterial activity against the tested bacteria (Staphylococcus aureus, Escherichia coli, Bacillus subtilis, Bacillus cereus and Pseudomonas aeruginosa), indicating that the seaweed powder used in the extraction contained antimicrobial principles. The zone of inhibition was calculated for both aqueous and ethanolic extracts, with the ethanol extract exhibiting a more significant inhibition zone than the aqueous extract. The ethanolic extract was especially effective against Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa, with inhibition zones of 8 mm, 14.5 mm and 14 mm respectively. The aqueous extract was effective against Staphylococcus aureus (9 mm) and Bacillus cereus (15 mm). However, the extracts were less potent than the standard ampicillin.
| Bacteria | Zone of inhibition in mm | ||
|---|---|---|---|
| Ab | Aqueous extract | Ethanolic extract | |
| E. coli | 10 | - | 14.5 |
| Staphylococcus aureus | 11 | 9 | 8 |
| Bacillus subtilis | 8 | - | - |
| Bacillus cereus | 11 | 15 | - |
| Pseudomonas aeruginosa | 15 | - | 14 |
Note: Ab: Ampicillin used as a positive control
Table 3: In vitro antibacterial activity of H. Pannosa extracts
Moreover, some plant extracts could not exhibit antibacterial activity against tested bacterial strains[52]. Further, the antimicrobial activity of seaweed species is linked to the presence of phenols and flavonoids[53]. The study demonstrated that the ethanol extract was effective against skin bacteria and could be used as a cosmeceutical application to treat acne and bacterial skin infections.
The MTT cytotoxicity assay is used to evaluate the cytotoxicity of seaweed extracts. An active compound with anticancer activity is represented by an IC50 value (μg/ml) of less than 100. A weak anticancer activity is represented by an IC50 value ranging from 100 to 300, while an inactive compound has an IC50 value over 300[54]. The present study showed that the ethanol extract had an IC50 value of 95.70, while the aqueous extract had an IC50 value of 110.70 μg/ml (Table 4). The results of this assay suggest that the ethanol extract has the potential cytotoxic activity against COLO 829 cancer cell line. The anticancer activity of seaweeds may be related to the presence of various therapeutic compounds that can induce apoptosis through different pathways and molecular mechanisms[55]. In vitro cytotoxic capacity of the ethanolic extract might be due to its antioxidant activity. In the present study, the cytotoxic effect of the ethanolic extract was probably due to the flavonoids that scavenge free radicals and prevent oxidative damage, which have a powerful anticancer effect and protect cells against all stages of carcinogenesis[56]. The present study proved that ethanolic extract of H. pannosa are a rich source of anticancer agents as the presence of bioactive properties is mainly due to the alkaloids, tannins, sterols and terpenoids.
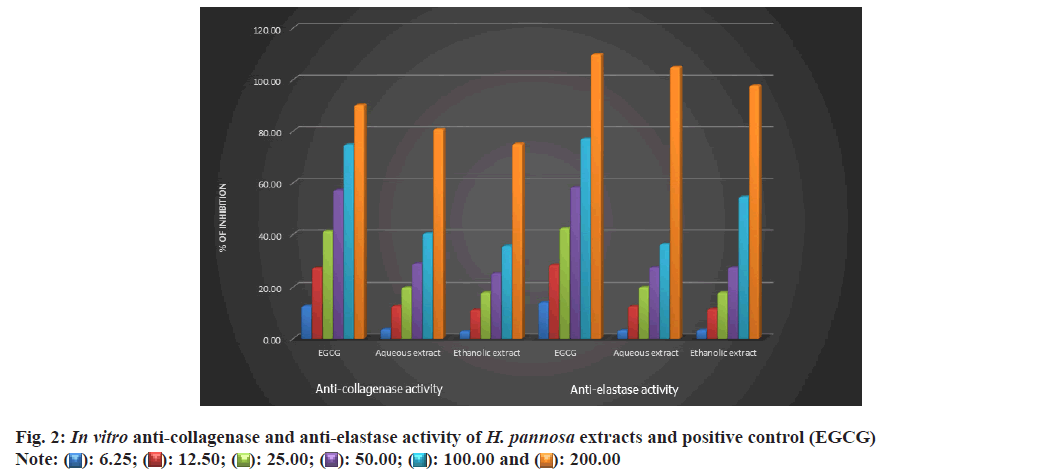
The present study was conducted to test the elastase inhibitory effects of H. pannosa extract using the SKMEL human melanoma cell line. The results showed that the extract inhibited elastase in a dose-dependent manner. It was noted that the ethanol extract was more effective compared to the aqueous extract, with an IC50 value of 48.53 and 48.65 μg/ml, respectively (Table 4, fig. 2 and fig. 3a). However, it was observed that both extracts were found to be more potent than the standard catechin, Epigallocatechin Gallate (EGCG) (49.63 μg/ml). Studies have shown that plant extracts have been identified as elastase inhibitors[57], but there have been limited investigations on the same from algae resources. Research has indicated that polyphenols extracted from plants possess strong elastase activity[58]. Elastase activity might be significantly inhibited by phenols such as epicatechin, catechin and catechol[45] and flavonoids such as kaempferol, quercetin, and myricetin[59]. Therefore, the study proved that the ethanolic extracts of H. pannosa could be used to develop cosmetic agents to prevent skin aging.
| Bioactive properties | IC50 value (µg/ml) of aqueous extract | IC50 value (µg/ml) of ethanol extract |
|---|---|---|
| Anti-cancer | 110.70 | 95.70 |
| Anti-elastase | 48.65 | 48.53 |
| Anti-collagenase | 49.32 | 48.76 |
Table 4: IC50 values of Anti-cancer , Anti-cancer and Anti-collagenase activity of H. Pannosa extracts
The degradation of collagen occurs with ageing due to collagenase activity resulting in wrinkles on the skin[60]. The present study investigated the effects of H. pannosa extracts on collagenase inhibition using the SKMEL human melanoma cell line. Results suggest that the extracts inhibit collagenase in a dose-dependent manner. The ethanol extract proved more effective than the aqueous extract, with an IC50 value of 48.76 compared to 49.32 μg/ml (Table 4, fig. 2 and fig. 3b). However, both extracts were more potent than the standard catechin, EGCG (49.74 μg/ ml). The presence of polyphenols in the seaweed extract may be responsible for its ability to inhibit collagenase activity. Based on these findings, the ethanolic extracts of H. pannosa show promising natural bioactive agents for future formulations aimed at reducing wrinkles and skin sagging.
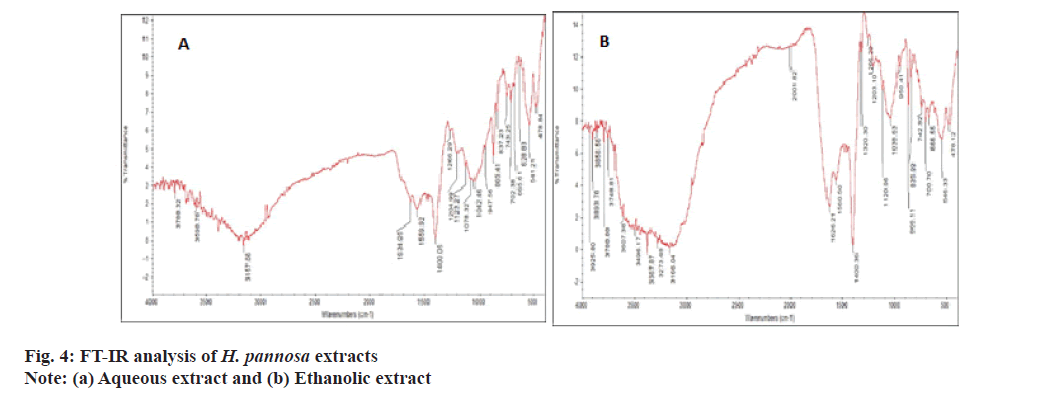
FT-IR analysis was conducted to identify and characterize the functional groups present in the extracts. FT-IR spectra can provide valuable information for identifying samples with specific functional ingredients[61]. The results of FTIR analysis confirmed the presence of phenols, carboxylic acids, aromatics, ketones, ethers, amides/amines and sulfonates compounds at varying concentrations. The ethanol extract exhibited wave patterns 3925, 3893, 3856, 3788 cm-1 corresponding to OH stretching (Hydroxy) compounds, while the aqueous extract exhibited wave patterns 3788.32, 3598.78 cm-1 corresponding to OH stretching (carboxylic acid) (fig. 4). However, Rafiquzzaman et al.[23], also used FT-IR to identify several phytochemicals such as phenols, carboxylic acids, ketones, ethers, aromatics, amides and sulfonates from H. musciformis. Therefore, it was concluded that the ethanolic extract contains high levels of phenolic compounds, making them valuable for cosmeceutical applications.
In conclusion, it is clear from the results presented here that the type of seaweed used for extraction and the solvent choice substantially impact the availability of functionally bioactive compounds, as demonstrated by in vitro screening and FT-IR analysis. The results showed that seaweed extracts possess various phytochemical substances and potent antioxidant properties, with varying levels of total phenolics, flavonoids and DPPH activity. Among the solvents, the ethanolic extract was the most effective in obtaining an antioxidant-rich extract with high phenolics compared to the aqueous extract, possibly due to high polyphenols, flavonoids and polysaccharides. The bioactive properties of the extract, including its in vitro antibacterial, anticancer, anti-elastase and collagenase activity, were also tested. The ethanol extract had good antibacterial, anti-ageing and anticancer properties compared to the aqueous extract. FT-IR analysis revealed that ethanolic extract is abundant in polyphenolic compounds, which have implications for cosmeceuticals. Using ethanol extract as an ingredient in cosmetic products could boost their efficacy. Further research should investigate the purification of each extract and the effects of different combinations. Finally, the nutrient content, health benefits and chemical composition of seaweed can differ depending on its origin, with factors such as water temperature, salinity and environmental pollutants playing a role. It is important to choose seaweed harvested from safe and sustainable sources. The present study has revealed that the red seaweed H. pannosa, sourced from the Mandapam coast, boasts advantageous cosmeceutical properties. Notably, the seaweed's ethanolic extract could serve as a natural antioxidant in cosmetics and pharmaceuticals.
Acknowledgments:
The authors thank the support of Tamil Nadu Dr. J. Jayalalithaa Fisheries University, Nagapattinam, Tamil Nadu to carry out this work.
Conflict of interests:
The authors declared no conflict of interests.
References
- Assaf H, Adly MA, Hussein MR. Aging and intrinsic aging: Pathogenesis and manifestations. Textbook of aging skin. 2010:129-38.
- Demeule M, Brossard M, Pagé M, Gingras D, Béliveau R. Matrix metalloproteinase inhibition by green tea catechins. Biochim Biophys Acta 2000;1478(1):51-60.
[Crossref] [Google Scholar] [PubMed]
- Fisher GJ, Kang S, Varani J, Bata-Csorgo Z, Wan Y, Datta S, et al. Mechanisms of photoaging and chronological skin aging. Arch Dermatol 2002;138(11):1462-70.
[Crossref] [Google Scholar] [PubMed]
- Sim GS, Lee BC, Cho HS, Lee JW, Kim JH, Lee DH, et al. Structure activity relationship of antioxidative property of flavonoids and inhibitory effect on matrix metalloproteinase activity in UVA-irradiated human dermal fibroblast. Arch Pharm Res 2007;30:290-8.
[Crossref] [Google Scholar] [PubMed]
- Ruberto G, Baratta MT, Biondi DM, Amico V. Antioxidant activity of extracts of the marine algal genus Cystoseira in a micellar model system. J Appl Phycol 2001;13:403-7.
- Pereira L. Seaweeds as source of bioactive substances and skin care therapy-cosmeceuticals, algotheraphy and thalassotherapy. Cosmetics 2018;5(4):68.
- Ferdouse F, Holdt SL, Smith R, Murua P, Yang Z. The global status of seaweed production, trade and utilization. Food and Agriculture Organization of the United Nations; 2018.
- Brunt EG, Burgess JG. The promise of marine molecules as cosmetic active ingredients. Int J Cosmet Sci 2018;40(1):1-5.
[Crossref] [Google Scholar] [PubMed]
- Couteau C, Coiffard L. Seaweed application in cosmetics. In: Seaweed in health and disease prevention; 2016:423-41.
- Khan AS, Ahmad B, Jaskani MJ, Ahmad R, Malik AU. Foliar application of mixture of amino acids and seaweed (Ascophylum nodosum) extract improve growth and physicochemical properties of grapes. Int J Agric Biol 2012;14(3):383-8.
- Kharkwal H, Joshi DD, Panthari PR, Pant MK, Kharkwal AC. Algae as future drugs. Asian J Pharm Clin Res 2012;5(4):1-4.
- Hjerpe LS. US Patent and Trademark Office: Patent Information. Legal Reference Services Quarterly 2003;22(1):53-65.
- Senousy HH, Abd Ellatif S, Ali S. Assessment of the antioxidant and anticancer potential of different isolated strains of cyanobacteria and microalgae from soil and agriculture drain water. Environ Sci Pollut Res 2020;27:18463-74.
[Crossref] [Google Scholar] [PubMed]
- Zandi K, Tajbakhsh S, Nabipour I, Rastian Z, Yousefi F, Sharafian S, et al. In vitro antitumor activity of Gracilaria corticata (a red alga) against Jurkat and molt-4 human cancer cell lines. Afr J Biotechnol 2010;9(40):6787-90.
- Katiyar SK, Pal HC, Prasad R. Dietary proanthocyanidins prevent ultraviolet radiation-induced non-melanoma skin cancer through enhanced repair of damaged DNA-dependent activation of immune sensitivity. Semin Cancer Biol 2017;46:138-45.
[Crossref] [Google Scholar] [PubMed]
- Ding Y, Kim SH, Lee JJ, Hong JT, Kim EA, Kang DH, et al. Anti-melanogenesis activity of Ecklonia cava extract cultured in tanks with magma seawater of Jeju Island. Algae 2019;34(2):177-85.
- Manandhar B, Wagle A, Seong SH, Paudel P, Kim HR, Jung HA, et al. Phlorotannins with potential anti-tyrosinase and antioxidant activity isolated from the marine seaweed Ecklonia stolonifera. Antioxidants 2019;8(8):240.
[Crossref] [Google Scholar] [PubMed]
- Namjooyan F, Farasat M, Alishahi M, Jahangiri A, Mousavi H. The anti-melanogenesis activities of some selected brown macroalgae from Northern coasts of the Persian Gulf. Brazil Arch Biol Technol 2019;62:e19180198.
- Riani MK, Anwar E, Nurhayati T. Antioxidant and anti-collagenase activity of Sargassum plagyophyllum extract as an anti-wrinkle cosmetic ingredient. Pharmacogn J 2018;10(5):932-6.
- Pientaweeratch S, Panapisal V, Tansirikongkol A. Antioxidant, anti-collagenase and anti-elastase activities of Phyllanthus emblica, Manilkara zapota and silymarin: An in vitro comparative study for anti-aging applications. Pharm Biol 2016;54(9):1865-72.
[Crossref] [Google Scholar] [PubMed]
- Cho SH, Kang SE, Cho JY, Kim AR, Park SM, Hong YK, et al. The antioxidant properties of brown seaweed (Sargassum siliquastrum) extracts. J Med Food 2007;10(3):479-485.
[Crossref] [Google Scholar] [PubMed]
- Harborne JB. Textbook of phytochemical methods, 1st Edn, Champraan and Hall Ltd. London 1973:110-113.
- Rafiquzzaman SM, Ahmad MU, Lee JM, Kim EY, Kim YO, Kim DG, et al. Phytochemical composition and antioxidant activity of edible red alga Hypnea musciformis from Bangladesh. J Food Process Preserv 2016;40(5):1074-83.
- Trease GE, Evans WC. Pharmacognosy. Brailliar Tiridel Can Macmillian Publishers. 1989.
- Kancherla N, Dhakshinamoothi A, Chitra K, Komaram RB. Preliminary analysis of phytoconstituents and evaluation of anthelminthic property of Cayratia auriculata (in vitro). Maedica 2019;14(4):350.
[Crossref] [Google Scholar] [PubMed]
- Slinkard K, Singleton VL. Total phenol analysis: Automation and comparison with manual methods. Am J Enol Vitic 1977;28:49-55.
- Kumar LR, Treesa Paul P, Anas KK, Tejpal CS, Chatterjee NS, Anupama TK, et al. Screening of effective solvents for obtaining antioxidant-rich seaweed extracts using principal component analysis. J Food Process Preserv 2020;44(9):e14716.
- Blois MS. Antioxidant determinations by the use of a stable free radical. Nature 1958;181(4617):1199-1200.
- Bauer AW, Kirby WMM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol 1966;45: 493-6.
[PubMed]
- Alley MC, Scudiere DA, Monks A, Czerwinski M, Shoemaker R, Boyd MR. Validation of an automated Microculture Tetrazolium Assay (MTA) to assess growth and drug sensitivity of human tumor cell lines. Proc Am Assoc Cancer Res 1986; 27(1):389-98.
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods 1983;65(1-2):55-63.
[Crossref] [Google Scholar] [PubMed]
- Senior RM, Campbell EJ, Landis JA, Cox FR, Kuhn C, Koren HS. Elastase of U-937 monocytelike cells: Comparisons with elastases derived from human monocytes and neutrophils and murine macrophagelike cells. J Clin Invest 1982;69(2):384-93.
[Crossref] [Google Scholar] [PubMed]
- Sallenave JM, Xing Z, Simpson AJ, Graham FL, Gauldie J. Adenovirus-mediated expression of an elastase-specific inhibitor (elafin): A comparison of different promoters. Gene Ther 1998;5(3):352-60.
[Crossref] [Google Scholar] [PubMed]
- Park KJ, Park SH, Kim JK. Anti-wrinkle activity of Acanthopanax senticosus extract in Ultraviolet B (UVB)-induced photoaging. J Korean Soc Food Sci Nutr 2010; 39: 42-46.
- Shanura Fernando IP, Asanka Sanjeewa KK, Samarakoon KW, Kim HS, Gunasekara UK, Park YJ, et al. The potential of fucoidans from Chnoospora minima and Sargassum polycystum in cosmetics: Antioxidant, anti-inflammatory, skin-whitening, and antiwrinkle activities. J Appl Psychol 2018;30:3223-32.
- Do QD, Angkawijaya AE, Tran-Nguyen PL, Huynh LH, Soetaredjo FE, Ismadji S, et al. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J Food Drug Anal 2014;22(3):296-302.
[Crossref] [Google Scholar] [PubMed]
- Azwanida NN. A review on the extraction methods use in medicinal plants, principle, strength and limitation. Med Aromat Plants 2015;4(196):1-6.
- Cho M, Lee HS, Kang IJ, Won MH, You S. Antioxidant properties of extract and fractions from Enteromorpha prolifera, a type of green seaweed. Food Chem 2011;127(3):999-1006.
[Crossref] [Google Scholar] [PubMed]
- Larsen DB, Farvin S, Jacobsen C. Antioxidant effect of seaweed extracts in vitro and in food emulsion systems enriched with fish oil. Am Oil Chem Soc Annual Meet Expo 2013; 2013.
- Ganesan P, Kumar CS, Bhaskar N. Antioxidant properties of methanol extract and its solvent fractions obtained from selected Indian red seaweeds. Bioresour Technol 2008;99(8):2717-23.
[Crossref] [Google Scholar] [PubMed]
- Rafiquzzaman SM, Kim EY, Kim YR, Nam TJ, Kong IS. Antioxidant activity of glycoprotein purified from Undaria pinnatifida measured by an in vitro digestion model. Int J Biol Macromol 2013;62:265-72.
[Crossref] [Google Scholar] [PubMed]
- Balange A, Benjakul S. Enhancement of gel strength of bigeye snapper (Priacanthus tayenus) surimi using oxidised phenolic compounds. Food Chem 2009;113(1):61-70.
- Naczk M, Shahidi F. Phenolics in cereals, fruits and vegetables: Occurrence, extraction and analysis. J Pharm Biomed Anal 2006;41(5):1523-42.
[Crossref] [Google Scholar] [PubMed]
- Pavia H, Åberg P. Spatial variation in polyphenolic content of Ascophyllum nodosum (Fucales, Phaeophyta). In Fifteenth International Seaweed Symposium: Proceedings of the Fifteenth International Seaweed Symposium held in Valdivia, Chile, in January 1995. 1996;199-203.
- Yoshie Y, Wang WE, Petillo D, Suzuki T. Distribution of catechins in Japanese seaweeds. Fisheries Sci 2000;66(5):998-1000.
- Velioglu Y, Mazza G, Gao L, Oomah BD. Antioxidant activity and total phenolics in selected fruits, vegetables and grain products. J Agri Food Chem 1998;46(10):4113-7.
- Zeka K, Ruparelia K, Arroo RR, Budriesi R, Micucci M. Flavonoids and their metabolites: prevention in cardiovascular diseases and diabetes. Diseases 2017;5(3):19.
[Crossref] [Google Scholar] [PubMed]
- Sasadara MM, Wirawan IG. Effect of extraction solvent on total phenolic content, total flavonoid content, and antioxidant activity of Bulung Sangu (Gracilaria sp.) Seaweed. OP Conf Ser Earth Environ Sci 2021;712(1):12005.
- Prior RL, Wu X, Schaich K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J Agric Food Chem 2005;53(10):4290-4302.
[Crossref] [Google Scholar] [PubMed]
- Siriwardhana N, Lee KW, Jeon YJ, Kim SH, Haw JW. Antioxidant activity of Hizikia fusiformis on reactive oxygen species scavenging and lipid peroxidation inhibition. Food Sci Technol Int. 2003;9(5):339-346.
- Liu F, Ng TB. Antioxidative and free radical scavenging activities of selected medicinal herbs. Life Sci 2000;66(8):725-35.
[Crossref] [Google Scholar] [PubMed]
- Schwarz ST, Noble WC. Aspects of bacterial resistance to antimicrobials used in veterinary dermatological practice. Vet Dermatol 1999;10(3):163-76.
[Crossref] [Google Scholar] [PubMed]
- Sandsdalen E, Haug T, Stensvag K, Styrvold OB. The antibacterial effect of a polyhydroxylated fucophlorethol from the marine brown alga, Fucus vesiculosus. World J Microbiol Biotechnol 2003;19:777-782.
- Arsianti A, Fadilah F, Wibisono LK, Kusmardi S, Putrianingsih RI, Murniasih TU, et al. Phytochemical composition and anticancer activity of seaweeds Ulva lactuca and Eucheuma cottonii against breast MCF-7 and colon HCT-116 cells. Asian J Pharm Clin Res 2016;9(6):115-9.
- Moghadamtousi SZ, Karimian H, Rouhollahi E, Paydar M, Fadaeinasab M, Kadir HA. Annona muricata leaves induce G1 cell cycle arrest and apoptosis through mitochondria-mediated pathway in human HCT-116 and HT-29 colon cancer cells. J Ethnopharmacol 2014;156:277-89.
[Crossref] [Google Scholar] [PubMed]
- Cook NC, Samman S. Flavonoids-chemistry, metabolism, cardioprotective effects, and dietary sources. J Nutr Biochem. 1996;7(2):66-76.
- Thring TS, Hili P, Naughton DP. Anti-collagenase, anti-elastase and anti-oxidant activities of extracts from 21 plants. BMC Complement Altern Med 2009;9:1-27.
[Crossref] [Google Scholar] [PubMed]
- Mutripah S, Meinita MD, Kang JY, Jeong GT, Susanto AB, Prabowo RE, et al. Bioethanol production from the hydrolysate of Palmaria palmata using sulfuric acid and fermentation with brewer?s yeast. J Appl Phycol 2014;26:687-93.
- Yoshie-Stark Y, Hsieh YP, Suzuki T. Distribution of flavonoids and related compounds from seaweeds in Japan. J Tokyo Univ Fish 2003;89:1-6.
- Castejón N, Thorarinsdottir KA, Einarsdóttir R, Kristbergsson K, Marteinsdóttir G. Exploring the potential of icelandic seaweeds extracts produced by aqueous pulsed electric fields-assisted extraction for cosmetic applications. Mar Drugs 2021;19(12):662.
[Crossref] [Google Scholar] [PubMed]
- Lingegowda DC, Kumar JK, Prasad AD, Zarei M, Gopal S. FTIR spectroscopic studies on cleome gynandra-Comparative analysis of functional group before and after extraction. Rom J Biophys 2012;22(3-4):137-43.
 : 6.25;
: 6.25;  : 12.50;
: 12.50;  : 25.00;
: 25.00;  : 50.00;
: 50.00;  : 100.00 and
: 100.00 and  : 200.00
: 200.00