- *Corresponding Author:
- W. Li*
Department of Nephrology, Nantong Hospital of Traditional Chinese Medicine
Affiliated Traditional Chinese Medicine Hospital of Nantong University
Nantong 226000, PR China
E-mail:shuding92597@163.com
| This article was originally published in a special issue, “XXXXXX” |
| Indian J Pharm Sci 2020:82(1)spl issue1;XX-XX |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The aim of the study is to provide evidence-based information for the efficacy, range of adaptation, dosage and course of treatment and adverse reactions for the treatment of chronic glomerulonephritis using Bailing capsule. Computer and manual retrieval was used to collect the clinical literature on treatment of chronic glomerulonephritis with Bailing capsule. A total of 661 patients (330 cases in the treatment group and 331 cases in the control group) in 8 reported studies were included as per inclusion criteria. RevMan 4.2 software was used to analyze the overall efficacy, the change of urine protein within 24 h before and after treatment, course of treatment, dosage, and adverse reactions. The total effect of Bailing capsule in the treatment of chronic glomerulonephritis was better than that in the control group. Compared to the control group, Bailing capsule exerted better control on urine protein without serious adverse reactions. There was a certain publication bias against the included cases. Bailing capsule brought urine protein from nephritis under control, but it is still necessary to make further investigation into the effect of Bailing capsule on different renal pathological changes of chronic glomerulonephritis, the best dosage and course of treatment.
Keywords
Bailing capsule, chronic glomerulonephritis, urine protein within 24 h, meta-analysis
Chronic glomerulonephritis (CGN) is a common disease frequently occurring in young and middle-aged people. Its clinical manifestations are proteinuria, hematuria, hypertension, edema[1,2]. The disease has a slow onset and as the disease progresses, the patients can have different degrees of renal injury and even develop into end-stage renal failure, therefore, timely and effective treatment is required. The pathogenic factors of primary CGN are complex, the disease develops slowly, and there is no obvious symptoms in the early stage of this disease. It can gradually develop into chronic renal failure[3]. Chinese medicine practitioners believe that CGN falls into the categories of labor deficiency, hematuria and edema and it is caused by the weakness of the internal organs and the invasion of foreign pathogens. Although western medicine can effectively improve the clinical symptoms of CGN, there are limitations to this treatment due to a large number of clinical drugs and different curative effects[4]. Following the principles of syndrome differentiation and treatment based on traditional Chinese medicine (TCM), treatment and control of the entire disease are very beneficial to the improvement of patients’ clinical symptoms and stability of the disease. Bailing capsule is separated from Cordyceps sinensis by bioengineering method and made by low temperature fermentation. It has a very similar chemical composition to Cordyceps sinensis, including vitamins, amino acids, adenosine and polysaccharides. With wide clinical application, it was found that Bailing capsule can reduce urinary N-acetyl-β-glucosaminidase levels in human body and can act on renal tubules to protect the activity of Na+/ K+-ATP, improve the secretion of epidermal growth factor in organism, repair the damaged epithelial cells and improve renal tubular function, thereby effectively inhibit renal interstitial fibrosis and renal tubular atrophy, and prevent further deterioration of renal function[5]. However, it is not yet clear what the clinical situation, dosage and course of treatment, and how to deal with the possible adverse reactions of Bailing capsule. In this paper, a meta-analysis of the clinical studies reported in Bailing capsule for the treatment of CGN was conducted to provide evidence for the efficacy, range of adaptation, dosage and course of treatment, adverse reactions and other evidencebased medical evidence for the treatment of CGN, so as to provide reference for the rational application of the drug and further research. It might cause mild side effects such as diarrhea, constipation, and abdominal discomfort.
VP information database of Chinese Sci-tech periodicals (2004-2017.12), Chinese Journal full text database (2004-2017.12), China RCTs network database, MEDLINE database (2004-2017.12) were retrieved by computer and traced the references included in the literature. The search terms included, CGN, Bailing capsule, randomised controlled trial. One time published randomized controlled or controlled studies, in which the subjects were CGN patients, who were in accordance with recognized clinical diagnostics, pathological diagnosis and efficacy evaluation criteria[6,7] were included. The age was between 15 to 72, regardless of sex. The treatment intervention was Bailing capsule, with a control group. Time to start treatment, course of treatment and dosage were not limited and the balance between groups was good.
Non-randomized controlled or controlled studies in which subjects with unclear diagnosis, or secondary kidney disease such as lupus nephritis, diabetic nephropathy, purpura nephritis, or patients with severe renal insufficiency were excluded. The research and design were not rigorous, such as nonstandard diagnostic and therapeutic standards, unclear interpretation of sample data, multiple treatment intervention and where the statistical method was not appropriate. Repeated publications, review articles, commentary, case reports were also excluded.
The quality of the methodology about included studies was evaluated according to the Cochrane system evaluator manual (4.2 edition). Using the Jadad score, including generation of random sequence, randomized hiding, blind method, withdrawal and exit situation. Scores 1 to 3 were divided into low-quality research and 4 to 7 were divided into high-quality research.
Revman 4.2 software provided by Cochrane Collaboration network was used. The X2 test was used to examine the heterogeneity of clinical research results. P>0.05 indicated there was no heterogeneity between groups and the fixed effect model was used to analyze the results. P<0.05 meant there was heterogeneity between groups. Subgroup analysis or sensitivity analysis was used to eliminate heterogeneity according to the possible heterogeneity factor. If heterogeneity still existed while clinical suggested of homogeneity, using the random effect model to analysis. The odds ratio (OR) and 95 % CI were used as effect size. Measurement data differed according to whether the unit of measurement was the same. When each clinical study used the same scale to measure the same curative effect, the weighted mean difference (WMD) and 95 % CI were calculated and the meta-analysis forest map was drawn according to the above results. Sensitivity analysis was performed after excluding studies that did not use blinding or blinding hidden protocols. The potential publication bias assessment was using inverse funnel plot analysis.
In the initial examination of 68 articles, 50 articles were excluded by reading the title, abstract and full text. After one by one screening and evaluation final 8 Chinese articles[8-15] were selected, with a total of 661 patients (330 in the treatment group and 331 in the control group) were included in the analysis. Pretreatment urinary protein level in all cases was less than 3.5 g per 24 h and the duration of the disease was 3 mo to 8 y. Treatments for the 3 studies (Liu and Cheng[8], Qian et al.[9], Dai et al.[10]) were Bailing capsule plus conventional treatment, compared to conventional treatment; three studies (Liu and Liu[11], Qi et al.[13], Zhou and Zhao[15]) using Bailing capsule plus telmisartan, compared to telmisartan and one study (Liu et al.[14]) using Bailing capsule plus alprostadil, compared to alprostadil.
One of the eight studies (Dai et al.[10]) mentioned the use of the random number table method, and the rest did not mention the specific randomization method or only the word random was used. None of the studies mentioned whether blinding was used. The Jadad scores of all 8 papers were 2 to 3 points.
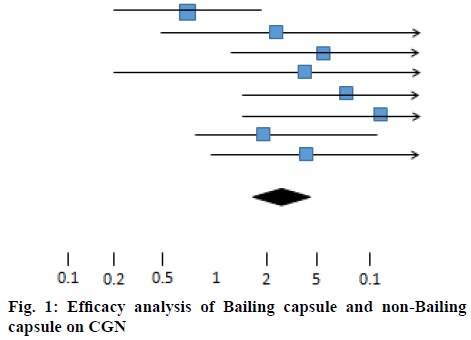
Heterogeneity test, X2=10.73, df=7, p>0.05, indicated that the inclusion studies were homogeneous, using the fixed effect model, OR=2.25, 95 % CI [1.43, 3.59]. The hypothesis test of effect combination value was Z=3.41, p<0.01. One horizontal line in figure 1 represented the CI of one test result. The shorter the CI was, the more accurate the results were, otherwise, the sample size was small and the conclusion was not accurate and reliable. The middle line represented OR=1 and the lowermost diamond symbol represented the combined results of the included studies. The short horizontal line/diamond symbol was in contact with or correlated with the midline, indicating that the difference was not statistically significant. The favorable outcome short horizontal line/diamond symbol on the right side of the midline indicated that the treatment group was effective, while on the left side suggested that the control group was effective, but the adverse outcome was the opposite. In this chart, the diamond symbol is on the right side of the midline, indicating that the clinical efficacy of Bailing capsule group and non-Bailing capsule group on CGN is different. Bailing capsule group has a better curative effect than the non-Baling capsule group (Table 1).
Table 1: Bailing Capsule Group Vs Non-Baling Capsule Group in Random Number Table Method
| Study or sub-category | Bailing capsule group (n/N) | Non bailing capsule group (n/N) | OR (fixed) 95 % Cl | Weight % | OR (fixed) 95 % Cl | |
|---|---|---|---|---|---|---|
| Liu and Cheng[8] | 15/30 | 16/30 | Favours Bailing capsule group | Favours non-Bailing capsule group | 39.47 | 0.56 [0.17,1.55] |
| Qian[9] | 21/30 | 21/30 | 7.03 | 2.02 [0.32,12.27] | ||
| Dai[10] | 26/30 | 21/30 | 11.71 | 3.66 [1.05,12.56] | ||
| Liu and Liu[11] | 53/60 | 43/60 | 2.74 | 2.79 [0.18,40.08] | ||
| Zhang et al.[12] | 44/47 | 41/48 | 9.36 | 4.48 [1.17,16.47] | ||
| Qi[13] | 26/30 | 19/30 | 5.32 | 6.37 [1.16,33.16] | ||
| Liu et al.[14] | 46/48 | 36/48 | 13.47 | 1.89 [0.49,7.03] | ||
| Zhou and Zhao[15] | 49/55 | 40/55 | 10.90 | 3.28 [0.86,12.15] | ||
| Total (95 % Cl) | 330 | 331 | 100.00 | 2.25 [1.43,3.59] | ||
Total events: 280 (Bailing capsule group), 237 (non-Bailing capsule group). Test for heterogeneity: Chi?=10.73, df=7(p=0.15), I?=35.4 %. Test for overall effect: Z=3.41(p=0.0005)
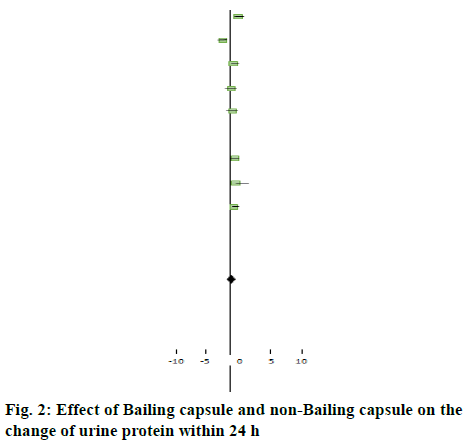
The change in urine protein level within 24 h after treatment was measured in the 8 studies. Heterogeneity test, X2=62.74, df=7, p<0.01, indicated that there was obvious heterogeneity in the study (figure 2). Because of the clinical homogeneity in each study, the baseline of sex, age and course of patients between groups was basically the same, so the effect size was combined with the random effect model. The results showed that there was a significant difference between groups [WMD=-0.93, 95 % CI (-0.68,-0.15), Z=2.86, p<0.01], indicating that there was a difference in the therapeutic effect of Bailing capsule group and non-Bailing capsule group on proteinuria. Bailing capsule had a good effect on the control of proteinuria (Table 2).
Table 2: Therapeutic Effect of Bailing Capsule Group and Non-Bailing Capsule Group on Proteinuria
| Study or sub-category | N | Bailing capsule group Mean (SD) | N | Non-Bailing capsule group Mean (SD) | OR (fixed) 95 % Cl | Weight % | OR (fixed) 95 % Cl | |
|---|---|---|---|---|---|---|---|---|
| Liu and Cheng[8] | 30 | 1.03 (0.37) | 30 | 1.03 (0.43) | Favors Bailing capsule group | Favors non -Bailing capsule group | 14.55 | 0.00 [-0.17,0.17] |
| Qian[9] | 30 | 1.18 (0.76) | 30 | 2.75 (0.64) | 11.47 | -1.63 [-2.14,-1.27] | ||
| Dai[10] | 30 | 0.77 (0.46) | 30 | 1.26 (0.73) | 13.78 | -0.48 [-0.74,-0.25] | ||
| Liu and Liu[11] | 60 | 0.94 (0.35) | 60 | 1.43 (0.49) | 11.48 | -0.49 [-0.92,-0.05] | ||
| Zhang et al.[12] | 47 | 1.07 (1.02) | 48 | 1.59 (1.36) | 8.71 | -0.51 [-1.14,0.11] | ||
| Qi[13] | 30 | 0.67 (0.42) | 30 | 1.14 (0.48) | 14.12 | -0.44 [-0.65,-0.18] | ||
| Liu et al.[14] | 48 | 1.25 (0.69) | 48 | 1.08 (0.77) | 10.96 | 0.25 [-0.24,0.67] | ||
| Zhou and Zhao[15] | 55 | 0.65 (0.29) | 55 | 0.76 (0.34) | 14.93 | -0.17 [-0.33,-0.04] | ||
| Total (95 % Cl) | 330 | 331 | 100 | -0.39 [-0.68,-0.15] | ||||
Test for heterogeneity: Chi?=62.74, df=7 (p=0.15), I?=87.4. Test for overall effect: Z=2.86 (p=0.003)
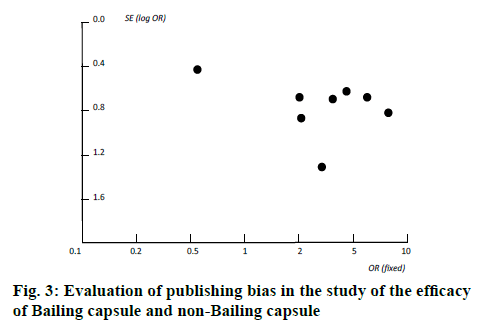
The course of treatment was usually 2-4 months. The dosage of Bailing capsule was a conventional dose, with 5 capsules each time (0.2 g/table), 3 times a day. Six studies did not describe the occurrence of adverse reactions, so the incidence of adverse reactions was not counted. Two studies found no serious adverse reactions. Since the quality of the 8 research methodologies included in the review was poor, the sensitivity of this system evaluation to high-quality research cannot be analyzed. In addition, all studies did not mention the blinding method, nor could exclude the sensitivity analysis without blinding. The evaluation and analysis of publication bias in the comparison of the efficacy of Bailing capsule group and non-Bailing capsule group were involved. The dissymmetry of the funnel plot and non-funnel plot distribution indicated that there was a certain publication bias in the study (figure 3).
Meta-analysis is a series of processes that comprehensively analyze multiple medical studies with the same research goals. It can combine a number of comparable individual research results for analysis, thereby enhancing both the strength of the preliminary conclusions of the analysis and evaluation of the strength of the effect. The introduction of meta-analysis into TCM research and scientific evaluation of its efficacy and problems can provide reliable evidence for clinical treatment and provide new ideas for further research in the future.
CGN is still the main cause of end-stage renal disease in China. Proteinuria is both an important clinical marker of CGN nephropathy and an independent risk factor for accelerating the progression of renal disease[16]. Therefore, effective control of proteinuria has become one of the main targets of CGN treatment. The main ingredient of Bailing capsule is fermented Cordyceps sinensis powder, which contains many trace elements. It has the functions of nourishing the kidney and nourishing spleen, replenishing qi and nourishing Yin, solidifying the body and nourishing yuan and so on[17]. The results of modern pharmacology show that[18], bailing capsule can inhibit the expression of interleukin (IL)-6, IL-10 and so on, so as to realize the role of bi-directional regulation of human immune function. It can improve the utilization of amino acids, promote protein synthesis and metabolism, and will not produce obvious adverse reactions.
In this meta-analysis, 8 clinical studies on the treatment of CGN with Bailing capsule were collected, including 661 patients with non-nephrotic syndrome. The results showed that conventional dose of Bailing capsule could improve the clinical effect of CGN and control proteinuria, and the curative effect was better than that of the non-Bailing capsule. No serious adverse reactions were found when using Bailing capsule.
Because of the small sample size of the 8 studies, most of the literature collected was Chinese literature, the inclusion and exclusion criteria were not fully reported, the curative effect criteria was not uniform enough, the methodological quality of the study was not evaluated sufficiently. The bias of the positive result papers by the author or the magazine, resulting in a lack of literature on negative results. All these reasons may increase the chances of selection bias and publication bias, resulting in meta-analysis overestimating the true therapeutic effect of Bailing capsule. In addition, some studies lacked relatively perfect renal pathological data, and some studies with pathological data have failed to analyze the curative effect of different pathology and also failed to analyze the effect of some factors that may make the condition worse, such as infection, hypertension and metabolic disorder and this will also interfere with the evaluation of the true efficacy of Bailing capsule.
Through this meta-analysis, the therapeutic effect of Bailing capsule on nephritis proteinuria can be clearly defined, but the course of treatment and the observation of adverse reactions of Bailing capsule need further discussion. In the future, high-quality, multi-center, randomized, blinded, and controlled clinical studies can be conducted on these issues.
Conflict of Interest
There was no conflict of interest.
Funding
Application basic research of Nantong Science and Technology Bureau,Project No: yyz15019.
Project Name
Clinical study on the immunomodulatory effect of moxibustion on patients with chronic glomerulonephritis.
References
- Zhou H, Chen M, Zhu Y, Wang B, Liu XN, Zuo Z, et al. Polymorphisms in NADPH oxidase CYBA gene modify the risk of ESRD in patients with chronic glomerulonephritis. Ren Fail 2016;38:262-7.
- Novak SM, Caraveo JM, Trowbridge AA. Progress in the treatment of chronic glomerulonephritis with traditional Chinese medicine. Cheminform 2005;36:3542-2.
- Gao JR, Qin XJ, Jiang H, Gao YC, Guo MF, Jiang NN. Potential role of lncRNAs in contributing to pathogenesis of chronic glomerulonephritis based on microarray data. Gene 2018;15:46-54.
- Fani A, Ghasedi M, Esmaeelion F, Alizadeh B. The effect of curcuma on improvement of clinical symptom of patients with irritable bowel syndrome. Ark Med Univ J 2010;113.
- Wang P, Wan S, Xinzhi DU, Xue Y, Gao H, Yan Y, et al. Study on the Correlation Between the?Plase of Diabetic Nephropathy and Bailing Capsules Combied with Salvianolate. China Pharmacist 2016;19:524-6.
- Wang SJ, Yang JY, Bai W. Therapies of warming kidney and draining dampness,and activating blood circulation and descending turbid used in 187 cases of chronic renal failure. J Beijing Uni Trad Chin Med 2009;32:782-5.
- Wang L, Zhao D, Yang N. Effect of Qi-strengthening and Blood-activating Method for Chronic Nephritis: An Observation of 60 Cases. J Guangzhou Univ Trad Chin Med 1999;23:122-6.
- Liu J, Cheng Y. Effect of Bailing Capsule on immunoglobulin and renal tubular function in patients with chronic nephritis. J Chin Physician 2004;6:275-6.
- Qian Y. Effect of Bailing Capsule on chronic glomerulonephritis. Shanxi J Trad Chin Med 2011;27:20-8.
- Dai H. Effect of Bailing Capsule on chronic glomerulonephritis. Mod Pract Med 2014;26:1212-3.
- Liu D, Liu C. Telmisartan in combination with Corbrin capsule on chronic glomerulonephritis amount Scr, 24 h urinary protein BUN, near future curative effect. Chin J Pract Int Med 2015;35:528-30.
- Zhang W, Li J, Chang W. Clinical observation of Shenfukang Capsules combined with Corbrin Capsules in treatment of chronic glomerulonephritis. Drugs Clinic 2016;31:823-5.
- Qi T. Bailing Capsule Combined with telmisartan in patients with chronic glomerulonephritis Influence of renal function. J Beth Med Sci 2017;15:251-2.
- Liu F, Ceng H, Miao H. Effect of Bailing Capsule Combined with alprostadil on urinary protein level and renal function in patients with primary chronic glomerulonephritis. Pract Clin J Integr Trad Chin West Med 2017;17:127-8.
- Zhou Y, Zhao D. Efficacy of Telmisartan Combined with Bailing Capsule in Treatment of Chronic Glomerulonephritis and Its Effect on Renal Function. Eval Anal Drug-use Hosp China 2017:1211-5.
- Kaliev R, Murkamilov IT, Fomin VV, Kaliev KP, Aver'ianova NI. Effect of erythropoietin and its combination with hypoxic altitude chamber training on the clinical and functional manifestations of chronic glomerulonephritis. Terapevt Arkh 2014;86:40-6.
- Wang L, Zhao D, Yang N. Effect of Qi-strengthening and Blood-activating Method for Chronic Nephritis: An Observation of 60 Cases. Trad Chin Med 2006;23:122-6.
- Song J, Li Y, Yang XD. Effect of combined therapy with bailing capsule and benazepril on urinary albumin excretion rate and C-reactive protein in patients with early diabetic nephropathy. Chin JIntegr Trad Westmed 2009;29:791-3.