- *Corresponding Author:
- Lei Qi
Department of Orthopaedics, The Fourth Affiliated Hospital of Nanjing Medical University, Nanjing, Jiangsu Province 210000, China
E-mail: shenhong999@163.com
| Date of Received | 02 December 2022 |
| Date of Revision | 30 June 2023 |
| Date of Accepted | 10 May 2024 |
| Indian J Pharm Sci 2024;86(3):1083-1090 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
This study examined the effects of artesunate on pain and inflammatory response of chondrocytes in osteoarthritis in rats and its effect on toll-like receptor 4/myeloid differentiation response gene 88/nuclear factor kappa B pathway. Thirty specific-pathogen free-grade Sprague-Dawley rats were collected and divided into 3 groups. The knee osteoarthritis model was established by intra-articular injection of papain in both osteoarthritis and artesunate groups. After 6 w of modeling in each group, the artesunate group was treated with 60 mg/kg of artesunate intraperitoneal injection, while the Sham and osteoarthritis groups were injected with an equal amount of saline for 4 w consecutively, once in a day. After 10 w of modeling, foot printing experiments were performed to analyze the behavior of murine pain hypersensitivity reaction in each group, and enzyme-linked immunosorbent assay was performed to determine the interleukin-1 beta, interleukin-6, interleukin-10 and tumor necrosis factor-alpha. The degree of articular cartilage destruction was observed under the microscope of hematoxylin and eosin staining. Reverse transcription polymerase chain reaction was used to detect toll-like receptor 4/myeloid differentiation response gene 88/nuclear factor kappa B messenger ribonucleic acid while Western blotting was used to detect the proteins related to toll-like receptor 4/myeloid differentiation response gene 88/nuclear factor kappa B signaling pathway. In rats with osteoarthritis, artesunate can reduce pain and chondrocyte inflammatory response which may be attributed to the control of the toll-like receptor 4/myeloid differentiation response gene 88/nuclear factor kappa B pathway.
Keywords
Artesunate, toll-like receptor 4/myeloid differentiation response gene 88/nuclear factor kappa B, osteoarthritis, anti-inflammation
Osteoarthritis is a progressive degenerative disease of the skeleton characterized by inflammatory cartilage degeneration in older adults[1], which leads to chronic joint pain resulting in significant reduction of quality of life, thereby increasing the socioeconomic burden. However, long-term use of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) for arthritis may lead to cardiovascular and gastrointestinal side effects[2]. Arthritis aims to relieve clinical symptoms rather than treating the underlying cause. Therefore, there is an urgent need for more effective and safer treatments to minimize chronic pain and restore the associated function in patients with arthritis. In the field of Traditional Chinese Medicine (TCM), Chinese medicines have a long history of treating osteoarthritis and have been found to be effective in improving clinical symptoms with fewer side effects. Studies have shown that Artesunate (ART) can exert antimalarial, antioxidant, anti-inflammatory and other biological activities, and reduce the inflammatory response of chondrocytes by inhibiting the Janus Kinase (JAK)/Signal Transducer and Activator of Transcription (STAT) pathway[3]. At the same time, it was found that ART inhibitd osteoarthritis development through the Osteoprotegerin (OPG)/ Receptor Activator of Nuclear factor Kappa-Β Ligand (RANKL)/RANK signaling pathway[4]. The above studies have partially elucidated the pharmacological mechanism of ART, suggesting that its pharmacological effects may be realized through the synergistic action of multiple components regulating to multiple pathways. However, the pharmacological mechanism of ART and its interaction with osteoarthritis-related targets and pathways have not yet been elucidated, and further studies are needed. Previous studies have found that the Toll-Like Receptor 4 (TLR4)/Myeloid Differentiation response gene 88 (MyD88)/Nuclear Factor Kappa B (NF- κB) pathway is involved in the development of osteoarthritis, but it is not clear whether ART acts through this pathway. The present research was intended to investigate the effects of ART on osteoarthritis pain and inflammatory response of chondrocytes in rats and to clarify the possible mechanisms.
Materials and Methods
General information:
Materials: Liaoning University of TCM facility animal center, laboratory animal License No: SYXK (Liao) 2021-0009, furnished 30 Specific- Pathogen Free (SPF) grade Sprague-Dawley (SD) rats, which were chosen and housed in an SPF grade animal facility with regular food and water. The applicable requirements of the regulations on the administration of laboratory animals were followed during the experimental procedures involving the experimental animals. The experimental drug, ART, was produced by Guilin Nanzhu Pharmaceutical Co., Ltd.
Methods:
Rats were randomized into three groups namely Sham, OA, and ART groups. In the OA and ART groups, intra-articular papain injections were used to create the knee osteoarthritis model. In contrast to the typical Sham group, rats in the OA group had swollen joints, decreased mobility and lameness, while the Sham group was left untreated. After 6 w of modeling, rats in each group were treated with 60 mg/kg of ART intraperitoneal injection in the ART group, and equal amount of saline was injected into the Sham group and OA group, consecutively for 4 w once in a day.
Observation indices:
Pain hypersensitivity reaction: After 10 w, the behavior of pain hypersensitivity reaction of rats in each group was analyzed by foot printing experiments, in which rats were charted with brownish-yellow and navy blue dyes on the front and hind paws respectively, and then placed at the beginning of the experimental runway. The end point was used as a node to obtain the rat’s foot prints. Each rat was performed 5×, and its footprint map was scanned; percentage of ipsilateral contact area of the right hind paw was calculated.
Inflammatory response assay: After 10 w of modeling, the Enzyme-Linked Immunosorbent Assay (ELISA) kit instructions were strictly followed to measure Interleukin-1 Beta (IL-1β), IL-6, IL-10 and Tumor Necrosis Factor-Alpha (TNF-α) levels.
Hematoxylin and Eosin (H&E) staining: After 10 w of modeling, the knee joint tissues of rats in each group were killed, and the degree of damage to articular cartilage in each group was evaluated by using OA histological scoring. The articular cartilage tissue sections of each group were taken and placed in xylene for two times, stained with hematoxylin for 30 s and then rinsed with tap water twice, each time for 3 min, and then rinsed with 1 % hydrochloric acid alcohol for 5 min. Subsequently, cells were stained with solidified green stain for 7 min, and then rinsed with 1 % acetic acid. Finally, the samples were stained with saffron O for 3 min, then dehydrated and transparent was collected.
Real-time Polymerase Chain Reaction (PCR) to detect TLR4/MyD88/NF-κB messenger Ribonucleic Acid (mRNA): Cartilage tissue was taken from each group of rats after 10 w of modeling, 50 mg was weighed and grinded into a powder, and then it was gathered into an Eppendorf (EP) tube. 1 m1 of RNA lysate was taken and it was allowed to stand at room temperature for 5 min, centrifuged for 5 min. Then the supernatant was removed by adding 200 ml of chloroform, and it was shaken vigorously for 15 s. After 15 min of centrifugation at 4°, the supernatant was removed and it was allowed to centrifuge in a tube.
After thoroughly mixing with an equal amount of isopropanol, it was left undisturbed for 10 min. Then it was washed with 1 ml and allowed to dry. The precipitate was dissolved in 20 μl of RNAfree water. Complementary Deoxyribonucleic Acid (cDNA) was reverse-transcribed by takala plus tailed reverse transcription kit, amplified by cDNA, and reverse-transcribed. Data was analyzed using 2-ΔΔCt method.
Detection of protein expression by Western blotting: Cartilage tissue was collected from each group of rats which weighed 50 mg after 10 w of modeling. It was grinded into powder. 10 times the weight of Radioimmunoprecipitation Assay (RIPA) lysis buffer was added and homogenized on ice. Further, it was vortexed for 30 s at 5 min intervals. Then, 30 min of lysis at room temperature and centrifugation were performed at 4° for 15 min, and the supernatant was collected. It was lysed for 30 min at room temperature and centrifuged at 4° for 15 min. The complete protein solution is found in the supernatant. To ascertain the protein content, the Bicinchoninic Acid (BCA) technique was applied. The gel was dispensed, electrophoresed, transferred, antigen-antibody reaction and color development were performed.
Statistical analysis:
The statistical data was analyzed using the Statistical Package for the Social Sciences (SPSS) version 23.0 software package. Comparisons of the measurement data that fit into a normal distribution were expressed (x±s). ap<0.05 and bp<0.05, were found to be statistically significant.
Results and Discussion
Normal Sham group rats had normal diet, normal water intake, flexible body reaction and normal defecation. After modeling, the rats in OA and ART groups showed reduced activity, redness, swelling and lameness of the joints of the affected limbs, of which the rats in ART group showed milder symptoms than those in OA group.
H&E staining revealed that the joint space in Sham group was obvious, the joint surface was flat and smooth, and the chondrocytes were neatly arranged. While in OA group, the joint surface showed missing tide lines, with chaotic hierarchy cell structure, the joint space was narrowed, and the cartilage layer was filled with fibroblasts, with a large number of inflammatory cells infiltrating. ART group presented visible joint space with elevated inflammatory infiltration.
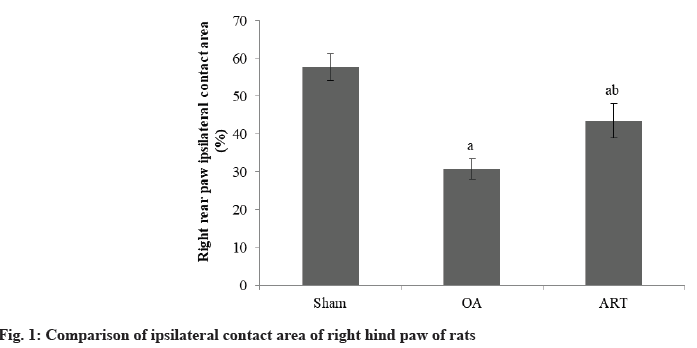
Rats in the OA group had a reduced proportion of ipsilateral contact area in their right hindpaw compared to the Sham group. The ART group had a larger proportion of ipsilateral contact area in their right hindpaw compared to OA group as shown in Table 1 and fig. 1.
| Group | n | Contact area (%) |
|---|---|---|
| Sham | 16 | 57.68±3.56 |
| OA | 16 | 30.75±2.68a |
| ART | 16 | 43.57±4.52ab |
| F | 165.18 | |
| p | <0.001 |
ap<0.05 and bp<0.05, respectively
Table 1: Comparison of Ipsilateral Contact Area of Right Hind Paw of Rats (X̄±S)
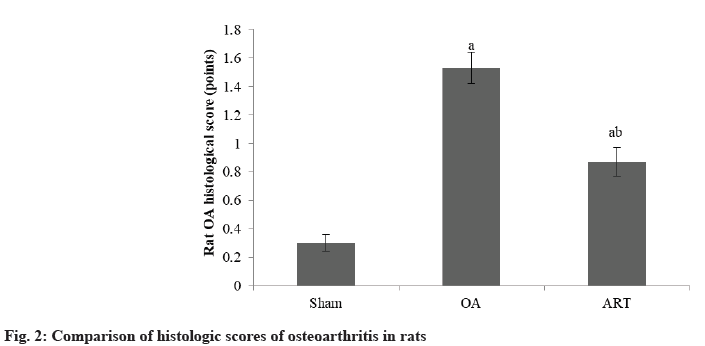
The histological score of osteoarthritis in rats in ART group was lower than OA group as shown in Table 2 and fig. 2.
| Group | n | Scores |
|---|---|---|
| Sham | 16 | 0.30±0.06 |
| OA | 16 | 1.53±0.11a |
| ART | 16 | 0.87±0.10ab |
| F | 173.69 | |
| p | <0.001 |
ap<0.05 and bp<0.05, respectively
Table 2: Comparison of Histologic Scores of Osteoarthritis in Rats (X̄±S)
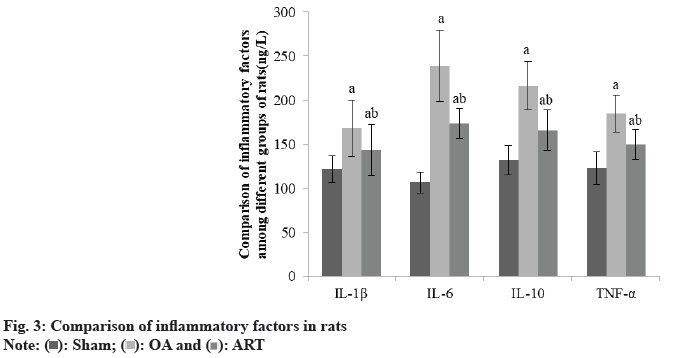
Compared to the Sham group, OA and ART groups had greater IL-1β, IL-6, IL-10, and TNF-α, whereas ART group had lower amounts of these substances than OA group as shown in Table 3 and fig. 3.
| Group | IL-1β | IL-6 | IL-10 | TNF-α |
|---|---|---|---|---|
| Sham | 121.52±14.86 | 106.57±11.85 | 131.87±16.86 | 122.68±18.45 |
| OA | 167.96±32.45a | 238.94±40.57a | 216.49±27.34a | 184.57±20.85a |
| ART | 143.52±28.91ab | 173.52±16.89ab | 165.78±22.96ab | 149.55±16.85ab |
| F | 16.589 | 89.656 | 123.005 | 18.695 |
| p | <0.001 | <0.001 | <0.001 | <0.001 |
ap<0.05 and bp<0.05, respectively
Table 3: Comparison of Inflammatory Factors in Rats (X̄±S, Ng/L)
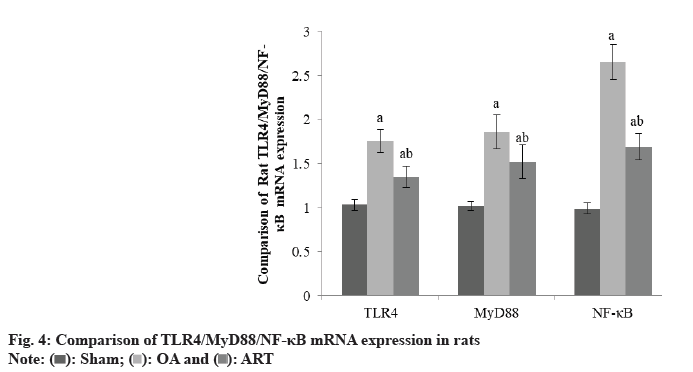
TLR4/MyD88/NF-κB mRNA levels were higher in the OA group and ART group had Sham group of rats, although TLR4/MyD88/NF-κB mRNA levels were lower in the ART group than OA group as shown in Table 4 and fig. 4.
| Group | TLR4 | MyD88 | NF-κB |
|---|---|---|---|
| Sham | 1.03±0.06 | 1.02±0.05 | 0.99±0.06 |
| OA | 1.76±0.13a | 1.86±0.19a | 2.65±0.20a |
| ART | 1.35±0.12ab | 1.52±0.19ab | 1.69±0.15ab |
| F | 4.896 | 6.537 | 8.654 |
| p | <0.001 | <0.001 | <0.001 |
ap<0.05 and bp<0.05, respectively
Table 4: Comparison of TLR4/MYD88/NF-κB(X̄±S)
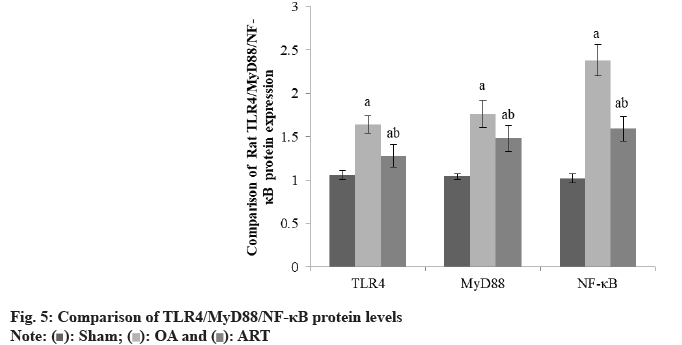
Rats in the OA group and ART group had greater TLR4/MyD88/NF-κB protein levels than the Sham group, and rats in the ART group had lower TLR4/ MyD88/NF-κB protein levels than the OA group as shown in Table 5 and fig. 5.
| Group | TLR4 | MyD88 | NF-κB |
|---|---|---|---|
| Sham | 1.06±0.05 | 1.04±0.03 | 1.02±0.05 |
| OA | 1.64±0.10a | 1.76±0.16a | 2.38±0.18a |
| ART | 1.28±0.13ab | 1.48±0.15ab | 1.59±0.14ab |
| F | 3.568 | 5.025 | 7.354 |
| p | <0.001 | <0.001 | <0.001 |
ap<0.05 and bp<0.05, respectively
Table 5: Comparison of TLR4/MYD88/NF-κB Protein (X̄±S)
Besides having anti-malarial effects, ART has inhibitory effects on a variety of cell lines such as neuroblastoma, breast cancer cells and Non-Small Cell Lung Cancer (NSCLC) cells. Several studies have shown that ART attenuates the biological characteristics of tumor cells through cell cycle inhibition of cell proliferation, disruption of cell invasiveness, angiogenesis inhibition and modulation of nuclear receptor responsiveness[5]. ART has been reported to promote RANKLinduced osteoclastogenesis, bone clasp proteinspecific gene expression, and bone resorption[6]. Specifically, ART was able to achieve this by decreasing RAKL levels and restoring OPG levels. In addition, ART effectively modulates Transforming Growth Factor-Beta (TGF-β)/ Suppressor of Mother Against Decapentaplegic (SMAD) signaling and inhibits pulmonary fibrosis[7]. Furthermore, ART inhibits Vascular Endothelial Growth Factor (VEGF) and down regulates mesenchymal progenitor cell’s osteogenic differentiation during placental growth factor-induced bone remodeling[8]. It can be seen that ART seems to show potential effects against multiple pathologic changes in OA. The proportion of the right hind paw’s ipsilateral contact area in the rats in the OA group was shown to be lower than in the Sham group, according to this study. Rats in the ART group had a greater proportion of ipsilateral contact area in their right hind paw than did the rats in the OA group. ART group had reduced osteoarthritis histology scores compared to the OA group. This indicates that ART can effectively relieve osteoarthritic pain and reduce OA histological score in rats.
IL-1β, IL-6, and TNF-α, are involved in the regulation of connective tissue metabolism, as demonstrated by Yao et al.[9]. and Sanchez et al.[10], who showed that the expression of IL-1β in cartilage and synovial tissues of patients with OA is significantly increased, while the expression of its receptor antagonist, IL-1 Receptor Antagonist (RA) protein, is significantly decreased. In addition, IL-1β can promote the expression of many Matrix Metalloproteinase (MMP) and deintegrin metalloproteinase, leading to matrix degradation, and can also synergize with IL-6 to induce the expression of MMP. TNF-α has a similar effect as IL-1β, with elevated expression in cartilage and synovial tissues of patients with OA. It can also induce the expression of MMP and block the synthesis of proteoglycans. IL-6 is a proinflammatory cytokine, and Villal et al.[11]. found that IL-1β and TNF-α induced IL-6 synthesis in chondrocytes and synovial cells of OA patients. We used rhSMOC2 to stimulate rat primary chondrocytes and found that except for TNF-α, the expression of IL-1β and IL-6 were increased, whereas the IL-1ra was decreased, and the expression showed concentration dependence on rhSMOC2. Inducible Nitric Oxide Synthase (iNOS) is an inducible Nitric Oxide (NO) synthase, and in the development of OA. During the development of OA, inflammatory factors can induce iNOS expression in chondrocytes and synoviocytes to promote elevated NO release, thereby accelerating chondrocyte apoptosis and blocking matrix synthesis. Li et al.[12]. found that the levels of iNOS in serum and joint fluid of OA patients were significantly increased. Cyclooxygenase-2 (COX-2) is a key rate-limiting enzyme for synthesizing prostaglandins, which promotes inflammatory response, and its content in cartilage tissue and joint fluid of OA patients was increased, which can be an important target for OA drug therapy. In this investigation, we discovered that the OA and ART groups had greater IL-1β, IL-6, IL-10, and TNF-α than Sham group, and the ART group had lower levels than OA group.
TLR4 is overexpressed in osteoarthritic cartilage, and it is important in the cartilage degeneration[13]. TLR4 and the associated ligand MyD88 work together to activate NF-κB and cause phosphorylated NF- κB to translocate to the nucleus, which promotes transcription of inflammation-associated genes and activation of a validation cascade. TLR signaling has a role in NF-κB signaling activation[14]. In rheumatoid arthritis, Lipopolysaccharide (LPS) promotes the degradation of Extracellular Matrix (ECM) proteins by binding to TLR4[15]. TLR4 mediates the activation of NF-κB, which serves as a downstream transcription factor associated with the progression of OAA as a major catabolic signaling. NF-κB activation is achieved through the mediation of the adaptor protein MyD88 by the process as MyD88 and activates Inhibitor Kappa B (IκB) kinase by removing the cytoplasmic transcription factor p65 subunit chelate, which triggers the nuclear translocation of the associated protein[16]. Previous studies have identified the NF-κB pathway as a major catabolic signaling pathway of OA[17]. NF-κB interacts with IκB-α, an intracytoplasmic inhibitory protein, while it is in the inactivated state. The nuclear translocation of activated NF-κB is triggered by oxidative stress and inflammatory mediator stimulation. This in turn promotes catabolic factor production, cartilage inflammation, and OA chondrocyte death via controlling the expression of many genes linked to inflammation, including as INO, COX-2, NO, Prostaglandin E2 (PGE2), MMP-9, and Gomez et al.[14]. Thus, specific suppression of NF-κB signaling might be beneficial for the management of osteoarthritis. The study’s findings demonstrated that the ART group’s TLR4/MyD88/ NF-κB mRNA and protein levels were larger than those of the Sham group and lower than those of the OA group. It is evident that TLR4/MyD88/NF- κB mRNA was expressed and activated during the course of OA development[18].
In conclusion, ART reduced chondrocyte inflammatory response and alleviated osteoarthritis pain in rats. One possible explanation for its mechanism is the regulation of the TLR4/MyD88/ NF-κB pathway.
Conflict of interests:
The authors declared no conflict of interests.
References
- Peat G, Thomas MJ. Osteoarthritis year in review 2020: Epidemiology and therapy. Osteoarthritis Cartilage 2021;29(2):180-9.
[Crossref] [Google Scholar] [PubMed]
- Latourte A, Kloppenburg M, Richette P. Emerging pharmaceutical therapies for osteoarthritis. Nat Rev Rheumatol 2020;16(12):673-88.
[Crossref] [Google Scholar] [PubMed]
- Shalitanati U. Exploring the effect and mechanism of artesunate on osteoarthritis based on abnormal subchondral bone resorption and JAK/STAT pathway. Xinjiang Med Univ 2024;12:1-7.
- Li YC. Exploring the effects and mechanisms of artesunate on osteoarthritis based on NF-κB and OPG/RANKL/RANK signaling pathway. Xinjiang Med Univ 2020;7:1-8.
- Jiang W, Huang Y, Wang JP, Yu XY, Zhang LY. The synergistic anticancer effect of artesunate combined with allicin in osteosarcoma cell line in vitro and in vivo. Asian Pac J Cancer Prev 2013;14(8):4615-9.
[Crossref] [Google Scholar] [PubMed]
- Wei CM, Liu Q, Song FM, Lin XX, Su YJ, Xu J, et al. Artesunate inhibits RANKL‐induced osteoclastogenesis and bone resorption in vitro and prevents LPS‐induced bone loss in vivo. J Cell Physiol 2018;233(1):476-85.
[Crossref] [Google Scholar] [PubMed]
- Wang C, Xuan X, Yao W, Huang G, Jin J. Anti-profibrotic effects of artesunate on bleomycin-induced pulmonary fibrosis in Sprague Dawley rats. Mol Med Rep 2015;12(1):1291-7.
[Crossref] [Google Scholar] [PubMed]
- Zhao C, Liu Q, Wang K. Artesunate attenuates ACLT-induced osteoarthritis by suppressing osteoclastogenesis and aberrant angiogenesis. Biomed Pharmacother 2017;96:410-6.
[Crossref] [Google Scholar] [PubMed]
- Yao Y, Li ZY, Zhang H, Zheng YH, Mai LX, Liu WJ, et al. Synovial fluid‑derived synovial fragments represent an improved source of synovial mesenchymal stem cells in the temporomandibular joint. Int J Mol Med 2018;41(1):173-83.
[Crossref] [Google Scholar] [PubMed]
- Sanchez-Tirado E, Salvo C, Gonzalez-Cortes A, Yanez-Sedeno P, Langa F, Pingarrón JM. Electrochemical immunosensor for simultaneous determination of interleukin-1 beta and tumor necrosis factor alpha in serum and saliva using dual screen printed electrodes modified with functionalized double-walled carbon nanotubes. Analytica Chim Acta 2017;959:66-73.
[Crossref] [Google Scholar] [PubMed]
- Villal A, Larranaga-Vera A, Lamuedra A, Perez-Baos S, Lopez-Reyes AG, Herrero-Beaumont G, et al. Modulation of the inflammatory process by hypercholesterolemia in osteoarthritis. Front Med 2020:1-13.
[Crossref] [Google Scholar] [PubMed]
- Li M, Wang M. Significance of changes in serum and joint fluid nitric oxide and its synthase in patients with osteoarthritis. Anhui Med 2005;9(2):108-12.
- Cui Y, Wang Y, Zhao D, Feng X, Zhang L, Liu C. Loganin prevents BV‐2 microglia cells from Aβ1‐42‐induced inflammation via regulating TLR4/TRAF6/NF‐κB axis. Cell Biol Int 2018;42(12):1632-42.
[Crossref] [Google Scholar] [PubMed]
- Gomez R, Villalvilla A, Largo R, Gualillo O, Herrero-Beaumont G. TLR4 signalling in osteoarthritis-finding targets for candidate DMOADs. Nat Rev Rheumatol 2015;11(3):159-70.
[Crossref] [Google Scholar] [PubMed]
- Zhang Y, Zeng Y. Curcumin reduces inflammation in knee osteoarthritis rats through blocking TLR4/MyD88/NF‐κB signal pathway. Drug Dev Res 2019;80(3):353-9.
[Crossref] [Google Scholar] [PubMed]
- Lorenz W, Buhrmann C, Mobasheri A, Lueders C, Shakibaei M. Bacterial lipopolysaccharides form procollagen-endotoxin complexes that trigger cartilage inflammation and degeneration: Implications for the development of rheumatoid arthritis. Arthritis Res Ther 2013;15:1-7.
[Crossref] [Google Scholar] [PubMed]
- Bobacz K, Sunk IG, Hofstaetter JG, Amoyo L, Toma CD, Akira S, et al. Toll‐like receptors and chondrocytes: the lipopolysaccharide‐induced decrease in cartilage matrix synthesis is dependent on the presence of Toll‐like receptor 4 and antagonized by bone morphogenetic protein 7. Arthritis Rheum 2007;56(6):1880-93.
[Crossref] [Google Scholar] [PubMed]
- Lepetsos P, Papavassiliou KA, Papavassiliou AG. Redox and NF-κB signaling in osteoarthritis. Free Radic Biol Med 2019;132:90-100.
[Crossref] [Google Scholar] [PubMed]
 ): Sham; (
): Sham; ( ): OA and (
): OA and ( ): ART
): ART