- *Corresponding Author:
- R. Kumar
Department of Pharmaceutical Sciences, Lovely Professional University, Phagwara, Punjab 144411, India
E-mail: rajesh.23035@lpu.co.in
| Date of Received | 19 December 2023 |
| Date of Revision | 24 September 2024 |
| Date of Acceptance | 30 December 2024 |
| Indian J Pharm Sci 2024;86(6):1910-1921 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Metal-organic frameworks are highly porous crystalline materials that enable molecular structural control. They were first described by Hoskins and Robson in 1989. In order to create metal-organic frameworks, coordinate bonds are used to link organic ligands with metal ions or clusters into two- or three-dimensional networks. Traditional synthesis, non-solvothermal, solvothermal, microwave synthesis, and sonochemical synthesis are the most commonly used techniques for the preparation of metal-organic frameworks. Nano-metal-organic frameworks, or nanoscale metal-organic frameworks, can be fabricated by carriers for biotherapeutics owing to their capability of undergoing size customisation. The metal ion catalytic activity and organic functional group properties of nano-metal-organic frameworks provide an excellent therapeutic technique. Nano-metal-organic frameworks have been studied a lot as drug delivery vehicles because they have a very large surface area, are very porous, and are easy to change chemically. They have been used to deliver a variety of therapeutic agents including anticancer drugs, antimicrobial agents, metabolic labelling molecules, anti-glaucoma drugs, and hormones. Recent research has demonstrated the use of metal-organic frameworks for better cellular intake, controlled drug release, and drug targeting. Drugs are frequently incorporated within metal-organic frameworks either through in situ encapsulation or post-synthetic adjustment. New opportunities for multifunctional drug delivery systems may be made possible by such a multimodal treatment system. However, there is a need to address a major challenge of toxicity linked with the biomedical use of metal-organic frameworks which can be attributed to the metal ion leaching. Considering that, the development of metal-organic frameworks-based safe, versatile, and efficient drug delivery system is a promising research area which is yet to be explored fully.
Keywords
Metallic organic frameworks, nano-carriers, drug delivery, biomedical
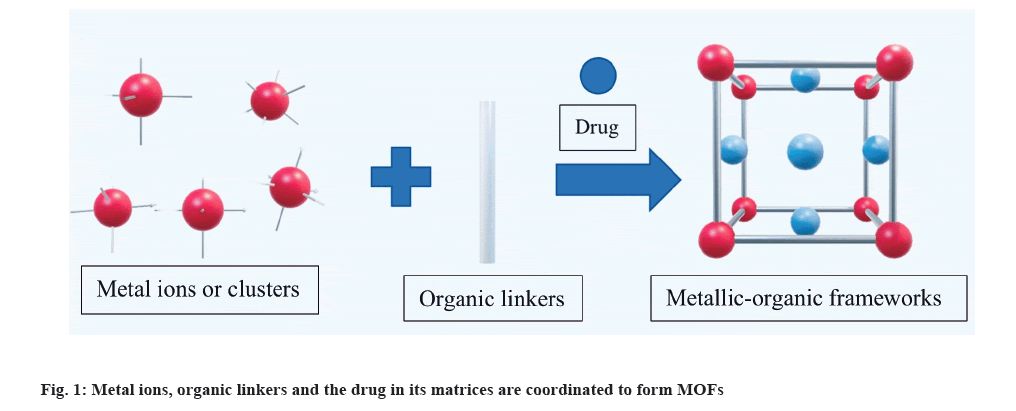
Metallic Organic Frameworks (MOFs) are a new trend in the research and scientific community of nanoparticles and have attracted a lot of interest in the last twenty years. MOFs are nano-porous materials that have gained interest in applications like gas storage and separation of gases. MOFs are also promising prospects for disease diagnosis, controlled drug delivery, and targeting. MOFs are networks of coordinated metal ions or clusters in two or three dimensions, with organic ligands functioning as linkers between the metal clusters as shown in fig. 1. They are often called metal organic polymers or Porous Coordination Networks (PCNs)[1]. MOFs have seen an increase in application as biological drug delivery systems during the past few years. Nano-MOFs (NMOFs) act as an efficient nano drug delivery agent for delivering drugs and imaging, chemotherapy, photo-thermal therapy. NMOFs are produced when MOFs are scaled down to the nanoscale.
MOFs are believed to be the best possible drug delivery choices due to their unique properties. MOFs have a huge number of active components that can be stored and that have the capacity to modify their physical and chemical characteristics by adjusting the concentration of organic clusters or ligands. Substrates can also be included in the void space and interact with other incorporated molecules in MOFs, and MOFs can be made biodegradable by reducing the strength of coordination bonds[2]. In MOFs, drugs can be chemically conjugated or physically incorporated by various interactions, like hydrogen bonding, Vander Waals forces, the π-π effect between aromatic rings, electrostatic interactions, covalent bonds, coordination bonds, etc. Biomolecules can also be encapsulated in MOFs in different ways, like surface attachment, covalent linkage, pore encapsulation, and in situ encapsulation, which leads to the formulation of bio-MOFs. MOFs' excellent encapsulation capacity makes them a better and different platform for drug loading. Many drugs like oxiplastin, 5-fluorouracil, beta-estradiol, and Nitric Oxide (NO) have been incorporated and tested in MOFs[3]. The synthesis of MOFs has been reported using a number of techniques, like traditional synthesis, non-solvothermal synthesis, solvothermal synthesis, microwave synthesis, sonochemical synthesis, etc. Each synthesis method offers advantages over the others for creating MOFs with various physiochemical characteristics, functionalizations, and scaling capacities[4]. Due to the large number of potential ions and ligands, MOFs have a variety of useful chemical and physical characteristics. By using reticular chemistry (structure-guided synthesis), MOF frameworks can be designed to meet the requirements of a certain function without requiring extensive empirical research[5].
Classification of MOFs
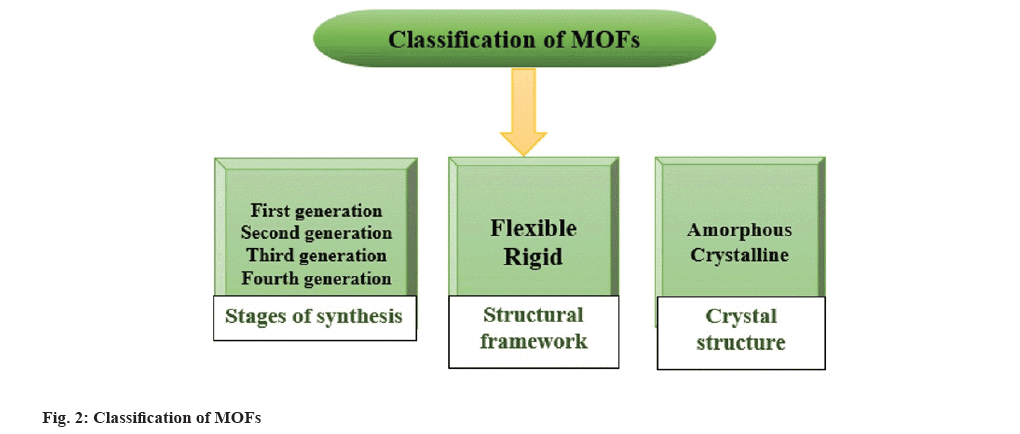
Specifically, MOFs are classified into three groups depending on the several synthesis steps that are in first generation (regular MOFs); second generation (functionalized MOFs); third generation (smart MOFs) and fourth generation.
First-generation MOFs have temporary porosity because of detachable host-guest dependence; it is frequently seen in MOFs whose pores are filled with counter ions. First-generation MOFs collapse when guests are detached from them. The second generation has a permanent relationship with the host and possesses surface modification through a chemical process. Third-generation MOFs, on the other hand, exhibit flexibility to encapsulate biomolecules such as drugs, cations, bioactives, toxins, etc. Fourth-generation MOFs have the ability to undergo post-synthetic modification and are also called post-processing MOFs[6]. Additionally, depending on how robust the structural framework is, MOFs can alternatively be categorised as flexible or rigid MOFs. Molecular inclusion, temperature, and pressure are examples of external stimuli that flexible MOFs can respond to by changing their structural conformation. Rigid MOF frames, in contrast, do not alter their conformation in the response of outside stimuli[7] as demonstrated in fig. 2.
MOFs can also be classified depending on how their crystal structures are arranged. A highly regular infinite arrangement of a solid porous framework is present in crystalline MOFs. The constantly reproducing structures offer crystalline MOFs with an irregular porous architecture that is advantageous for their physicochemical sorption properties. As opposed to crystalline MOFs, amorphous MOFs preserve the same core building blocks and connections but exhibit a long-range periodic organising order in their structural framework[8].
Materials used in MOFs
Metal ions:
When it comes to selecting a metal ion for the synthesis of MOFs, the options are virtually limitless. MOFs should be biocompatible and non-toxic when used in the medical field for drug delivery. Metals and linkers should be chosen considering the above factors. Copper, iron, potassium, zirconium and zinc-based MOFs remain the most commonly used for drug delivery for various disorders like cancer, diabetes, neurological disorders, etc.,[9].
Organic linkers:
The choice of linkers yields distinctive characteristics and MOFs applications. The Three Dimensional (3D) supramolecular structure and physicochemical characteristics of the MOFs are impacted by the organic linkers to a substantial extent. Carboxylates and other organic anions, like sulfonate, phosphonate, and heterocyclic compounds, are the most popular types of organic linkers[10].
Synthesis of MOFs
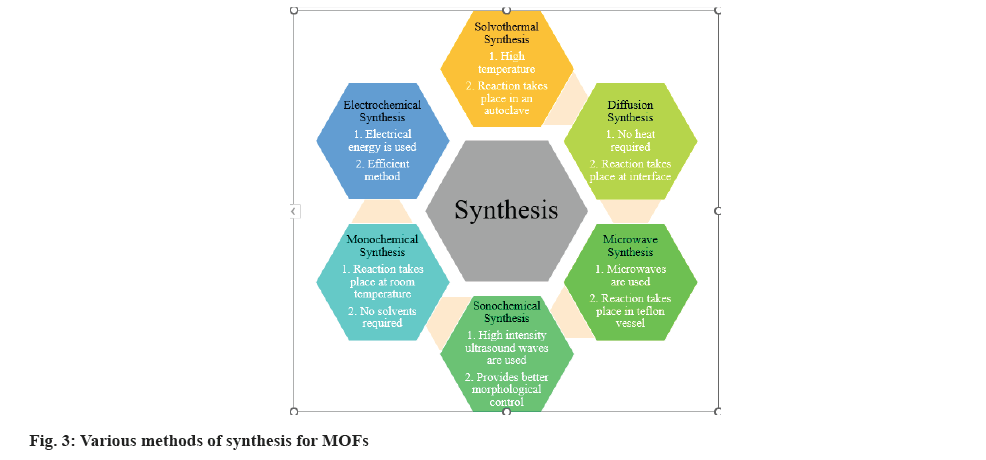
MOFs provide benefits such a flexible surface to incorporate active ingredients, freedom in size and shape modification, and simple surface adjustments. The size of the substance or drug delivery component limits its application in drug delivery. Particle size is the chief property required to obtain stable formulations like patches, and tablets. Decreasing MOFs' size to the nanoscale level is a very good plan of action for increasing its application in drug delivery systems[11]. Certain therapeutic routes require extremely specific sizes of nanoparticles while administration, such as intravenous injection, where the size of the particles should be smaller than 200 nm to provide high stability suspensions in aqueous medium and free circulation within the smallest capillaries without any aggregation. So it's important to synthesise stable and uniform nanoscale MOFs[12]. The conditions in which MOFs are synthesised can affect their porosity, size, shape, and crystallinity. So, choosing a proper synthesis method is a major challenge as it controls the physiochemical characteristics of the formed product. In addition, economic and environmental concerns must be taken into account, especially in large-scale synthesis. Depending on the desired frameworks and characteristics, a wide range of different synthetic techniques can be used to produce MOFs. Solvothermal, diffusion, microwave, sonochemical, electrochemical and mechanochemical synthesis are some of these approaches as shown in fig. 3[13].
Solvothermal synthesis:
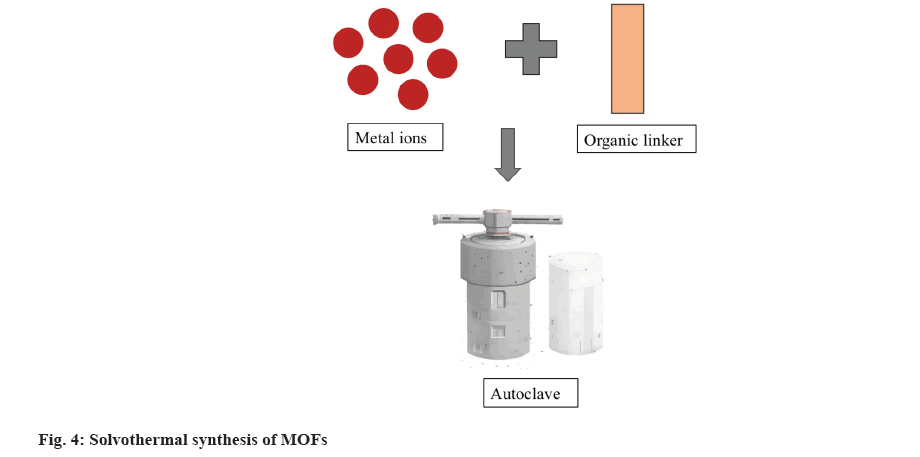
Among the many different synthetic techniques for the synthesis of MOFs that have been found so far, the solvothermal method is still the one that is most regularly used[14]. Any chemical reaction involving a solvent in supercritical or nearly supercritical conditions is referred to as solvothermal[15]. Solvothermal synthesis of MOFs is typically regarded as an effective method. Solvothermal preparation is used to produce the majority of MOFs[16]. In the solvothermal method, a closed vessel (an autoclave as depicted in fig. 4) is used to carry out a reaction at temperatures between 50° and 270°, and this reaction is lengthy (hours and sometimes days).
Teflon-lined autoclaves are employed in these processes when the temperature is higher than 400°. The temperature of the processes may be increased if kinetically inert ions are used to promote bond formation and ensure proper crystallisation. The crystalline structure is also influenced by change in temperature, and long reaction time may cause the final product to disintegrate[17]. The basic principle of the solvothermal approach is to combine solvent solutions containing metal ions, salts, and organic ligands, and to keep the reaction mixture at a higher temperature (>100°) for a predetermined time. MOFs are synthesised at high temperatures to get a high yield of MOFs in a required amount of time. Temperature, pH, and the chemical make-up of the reactants are the factors in the synthesis that affect the final product. Surfactants or molecular templates are typically used in the formulation process to produce a nano-sized material[18]. In a recent research study, HF-free MIL-100(Fe) MOFs were prepared by the solvothermal method. The cubic crystal structure of solvothermal synthesised MOFs, which have a high surface area and pore volume, allows for the best drug loading[19].
Diffusion synthesis:
In general, metal salt and organic ligand solutions cannot be linked without heating to produce MOFs. The formation of microcrystalline powder as a result of the mixing of metal ions with ligand solution does occur in some specific situations, and this is undesirable for single crystal XRD studies. Slow solvent diffusion is used in an effort to overcome the issue of polycrystalline powder material formation[20].
Gel diffusion, gas phase diffusion, and liquid phase diffusion are the three basic subdivisions of the diffusion method. Gel diffusion produces MOFs by combining metal ions and organic ligands in gel for a specified period of time. In gas phase diffusion, an organic ligand solution is employed as the solvent[21]. Liquid-phase diffusion is a method that uses three layers of materials. In this method, one layer is composed of metal ions and the other is composed of organic linkers; these two layers are separated by a layer of solvent, and the reaction takes place at the interface[22].
Microwave synthesis:
The microwave method emits electromagnetic radiation to heat the solution or mixture. Between radio waves and infrared wavelengths, these electromagnetic waves are located. Microwave techniques have been successfully used in the synthesis of MOFs[23]. This method is advantageous due to its quick crystallisation as well as its phase selectivity, limited particle size distribution, and easy shape control. Microwaves that are available in the market are equipped with fiber-optic temperature controllers, pressure controllers, and adjustable power outputs. In microwave synthesis, a mixture of substrates is transferred to a Teflon vessel, locked, and heated for the needed period of time at the particular temperature. The solvent is then drained away. The microwave method causes quick heating of the liquid phase by coupling an electric field that is being applied that oscillates in response to the molecules' permanent dipole moments in the synthesis medium[14].
Sonochemical synthesis:
MOFs synthesised with a sonochemical method have uniform nucleation and significantly shorter crystallisation times than MOFs synthesised by heating in an oven. The primary process of sonochemical synthesis or sonochemistry is the formation, expansion, and collapse of an acoustic cavitation, a bubble that forms in liquid and produces extraordinarily high local temperatures (5000-25 000 K), pressures, and heating and cooling rates. High-intensity ultrasound is often used as a stoichiometric reagent to make metals more reactive. This is a common synthetic method for many kinds of chemical and organometallic processes[24]. In modern research, the use of ultrasound-based sonochemical synthesis has helped to obtain morphological control, phase selectivity, and faster reaction times in MOFs[25].
Electrochemical synthesis:
The electrochemical method is a precise, controlled, less complicated and feasible method. The key benefits of this approach are its efficiency and great purity because mixtures do not contain counter ions like nitrate, percolate, or chloride from salts of metals. In this method, metal ions are put at the anode in place of metal salts, while organic linkers are positioned at the cathode and conducting salt is added to the electrochemical cell[26]. The electrochemical method can be used to synthesise thin films of MOFs[27].
Mechanochemical synthesis:
Mechanochemistry has become a very promising approach for the synthesis of MOFs and even large-scale production of MOFs. In conventional methods of synthesis, metal ions or clusters were mixed with organic ligands in a solution, and high heat was provided. Now, mechanochemical organic synthesis has made it possible for MOFs to be synthesised solvent-free or in the solid state without any harmful solvents[28]. In mechanochemical synthesis, chemical reactions are started by mechanical force. The reaction can take place at room temperature. Temperature can alter the reaction rates, and it has been observed that minor changes in temperature can cause changes in the reaction mechanism. Broad use of mechanochemical synthesis has been observed in the synthesis of several types of MOFs like ZIFs, MOF-5, MIL-100, MOF-74, HKUST-1, and UiO-66[29]. Quantitative yields are observed in solid-solid reactions. This unique approach gives the product directly in powder form. As a result, the materials do not undergo any time-consuming processes and are ready for use in a variety of applications[30-39] (Table 1).
| S. No. | Name of MOF | Method of preparation | Application | Reference |
|---|---|---|---|---|
| 1 | Fe(III)-MOF | Solvothermal | Antimicrobial | [31] |
| 2 | MIL-101(Fe) | Solvothermal | Anticancer | [32] |
| 3 | UiO-66-NH2 | Sonochemical | Anticancer | [33] |
| 4 | Ca-BDC | Microwave | Controlled delivery of curcumin | [34] |
| 5 | ZnBDP-MOF | Microwave | Drug loading | [35] |
| 6 | Zn2(1,4-bdc)2 | Diffusion | Drug loading | [36] |
| 7 | g-cyclodextrin | Diffusion | Drug adsorption | [37] |
| 8 | Zn(1,3-bdc)0.5(bzim) | Electrochemical | Ibuprofen adsorption | [27] |
| 9 | UiO-66-NH2 | Electrochemical | Fluorescence detection | [38] |
| 10 | CD-MOFs | Mechanochemical | Cosmetic ingredient | [39] |
Table 1: List of MOFs reported via different methods of synthesis by various researchers.
MOFs Drug Release Approaches
MOFs can be upgraded as smart materials. The development of MOFs has made stimuli-sensitive drug delivery systems much stronger. The drug stored in these systems is released by both internal and external stimuli. Localised inside the tumour microenvironment are intrinsic stimuli. Tumour micro-environments differ from healthy tissues in that they have higher temperature and have a lower pH, which can be used to create internal drug delivery systems that respond to stimuli like pH and ATP. Extrinsic stimuli drug release mechanisms regulate the body's ability to release drugs from the outside. The accumulation of nano-carriers at the target site can be seen using extrinsic stimulation methods like magnetic fields and ions, and these nano-carriers can be activated from outside of the body[40].
Special attention has been given to MOFs in developing them as controlled-release systems. Different internal and external stimuli, such as temperature, pH, light, Reactive Oxygen Species (ROS), and chemical agents, have been put to use to release drugs incorporated in MOF[41].
pH responsive:
Normal living cells gain energy through oxidative phosphorylation, but malignant cells gain energy through a process that is not dependent on oxygen (oxygen-independent glycolysis), which leads to the release of an abnormal amount of lactate along with excess carbon dioxide and protons. This extra release turns the environment of the cell acidic and changes the pH to 6.5-6.7[42]. The decrease in pH can be used to initiate faster drug release at the target site. In a recent research study, cationic porous MOF ZJU-101 (Zhejiang University) has been formulated by coordinating zirconium with 2,2’-Bipyridine-5,5’-Dicarboxylate (BPYDC). Diclofenac sodium is the drug utilised, which is a non-steroidal anti-inflammatory drug. Anionic diclofenac gets easily into cationic MOFs by ion exchange. Since the ion concentration is larger at lower pH than it is at neutral pH, diclofenac sodium is released in inflammatory tissues (pH=5.4) at a faster pace. Half of the drug dose is estimated to have been released from diclofenac sodium ZJU-101 at 15, 42 and 65 h, respectively, at Phosphate Buffer Saline (PBS) with pH values of 5.4, 6.5, and 7.4[40].
Changes in the pH of the cell can trigger processes that are helpful for regulating the release of drugs from MOFs, such as dissociation, disassembly, size change, and even tuning of surface charges and shape. The crucial step in creating a pH-responsive MOF is to trigger intrinsic protonation, which will disrupt the coordination links. In low pH conditions, these deprotonated chemical groups become protonated, resulting in the breakdown of the MOF, the breaking of coordination bonds between the metal and ligand, and the release of the drug-encapsulated molecule to the target site[43]. For example, Zn-MOF-74 was used to deliver arsenic trioxide, which is an anticancer drug. It exhibited a pH-triggered release in a pH range suitable for cancer treatment. Unlike pH 7.4, which reflects the microenvironment of healthy cells, the drug was released faster at pH levels that are lower, which are characteristic of malignant tissues[44].
Redox responsive:
Drug release from MOFs in cancerous cells can be achieved with precise targeting by taking advantage of the difference between the microenvironment of a cancerous cell and a normal cell environment. Previous research has shown that tumour cells contain more Glutathione (GSH) vs. normal cells. Since GSH has reducing characteristics, oxygen-reduction chemicals might potentially be employed to make nano-MOFs. First, MOFs with disulphide bonds are added into the organic chains of nano-MOFs for drug-loading therapy of malignant cells because the disulphide bond is easily reduced. The extra GSH in the tumours weakens the disulphide bond, causing the core of nano-MOFs to collapse and the release of the drug[45].
There have been created MOFs based on GSH-triggered Drug Delivery Systems (DDS) that release anticancer drugs intracellularly in response to redox conditions. 6-mercaptopurine was incorporated in UiO-66-(SH)2 MOFs. Drug was released at a much faster rate in the presence of GSH, and very little drug was released in its absence. This proved the excellence of this approach. To verify the safety of the formulation, it was tested in normal cells, and >90 % of living cells were reported, proving its biocompatibility[46].
Temperature response:
Certain MOFs are heat sensitive, and this property of some MOFs can be exploited to deliver drugs. MOFs can release drugs when hyperthermia is applied to the body. Drugs are released because MOFs degrade as temperatures rise and the force between MOFs decreases under hyperthermia conditions[47]. A study was done to demonstrate temperature-controlled release of drugs from MOFs. The ZJU-801 MOFs were formulated with diclofenac sodium. Negligible drug release was seen at temperatures of 25°-30°. When the temperature reached 45°, drug release increased, and at 60°, drug release was especially rapid. Drug release was 10.3 times higher at 60° as compared to 25°[48].
Ion responsive:
A novel drug delivery method has been introduced via ion-responsive MOFs. The frameworks of these drug carriers have strong electrostatic interactions with the drugs that control drug release and dispersion[49]. Because the physiological environment in the body varies, the different ions in tissue fluid can contribute to release of drug from nano-MOFs. The cationic drug carrier MOF-74-Fe(III), which was created by oxidising neutral MOF-74-Fe, was described by Hu et al.[50] An MTT test revealed that the cytotoxicity of this positively charged MOF against PC12 cells is relatively low. Using ion exchange and salt penetration techniques, ibuprofen anions were loaded into the cationic MOF with an effective encapsulating capacity (15.9 wt%). The drug release approach involves two techniques with different drug release rates. Ibuprofen sodium and other coordinated loose anions were the primary compounds that were initially released, and the rate of release was controlled by the rate of diffusion or ion exchange between the drug-loaded MOF and PBS solution. Phosphate (PO43-) anions, which preferentially coordinate with iron cations, started the process for the subsequent release of the coordinated drug. These two separate kinetic mechanisms make drug release administration more versatile and flexible.
Applications of MOFs in Delivery of Drug
MOFs have undergone frequent, thorough examination and assessment for several applications. They display strong chemical and thermal stability, are crystalline, and have flexible and rigid frameworks. Many things may be accomplished with MOFs, such as gas storage and separation, catalysis, sensing and other applications. They are also being employed for drug delivery[51]. Many negative side effects have been linked with uncontrolled and generalised drug distribution by direct ingestion of a free drug inside the body. There have been studies and research undertaken to create formulations and methods that can target and provide controlled drug delivery systems. This issue has a quick and effective remedy owing to nano particles. Dendrimers, liposomes, and other organic nanoparticles, as well as inorganic nanoparticles including metal oxide and metal nanoparticles, quantum dots, and others, have shown promise as drug delivery systems. Once inside the body, the Reticuloendothelial system (RES) can easily catch organic nano particles like liposomes since they are less stable. It has been noted that inorganic nano particles such gold, silver, and silica nanoparticles are cytotoxic. Porous NMOFs are an example of an inorganic-organic hybrid nanoparticles that has several benefits compared to their pure organic and inorganic equivalents and has proven to be the ideal technique of drug administration[52].
MOFs in drug delivery for diabetes:
Diabetes is a metabolic and endocrine condition brought on by insufficient insulin production in the pancreas. Diabetes causes hyperglycaemia, or an increase in blood glucose. If untreated, glucose levels could rise to a fatal level. In addition to other drugs, insulin is used to treat diabetes[53]. Insulin is the drug that is most frequently used to treat diabetes, and regulating a patient's blood sugar levels depends on how well insulin is delivered in vivo[54]. Insulin has strong hydrophilicity and has <2 % bioavailability by the oral route as it is degraded in gastrointestinal fluid by proteolytic enzymes. This degradation hinders the passage of insulin from the intestinal epithelium to the systemic circulation. Nanocarriers are required, which can prevent the degradation of insulin in gastrointestinal fluid[55]. NU-1000 was designed to encapsulate and deliver insulin without it degrading. It uses zirconium-based mesoporous MOFs. The NU-1000 crystals were loaded with insulin, which was distributed evenly throughout them. Insulin@NU-1000 MOFs were tested in a simulation fluid that was created to mimic the conditions of GIT fluid. The results were more than satisfactory, with only 10 % of insulin released after 60 min. The remaining insulin began to release after 1 h when the MOF's structure began to degrade. Insulin@NU-1000 was shown to be a viable insulin carrier for oral administration because the majority of the insulin carried its action[56].
A MOF with its unique anti-diabetic action was reported. Using 5-aminotetrazole and methyl-2-amino-4-isonicotinate anionic ligands, a novel zinc-based MOF was created by hydrothermal technique. Developed MOFs underwent cytotoxicity and in vivo tests. Studies on cytotoxicity were carried out using HEK 293 cells. Cell viability measurements of the tested MOFs and regular control cells showed no significant differences. Wistar rats were used for in vivo studies. Glycaemia levels during fasting were lower in rats treated with MOFs compared to the untreated diabetic group of rats[57].
MOFs in drug delivery for wound healing:
Skin accounts for approximately 15 % of our body weight. It is the largest organ and has a complicated, multi-layered structure. The prevalence of accidents and pathophysiological problems has increased; making skin wounds a significant healthcare concern. In order to prevent wounds from worsening and resulting in more tissue damage and scarring, they should be treated as soon as possible[58]. A Diabetic Foot Ulcer (DFU) is one of the most common diabetes complications, and if the infection becomes worse, the patient may need amputation. A MOF-based hydrogel system containing metformin hydrochloride and curcumin embedded in copper-based MOFs has been developed to treat DFU. The system made of hydrogel is termed as Cur/MH/HKUST-1@Gel. As a result, drug release was found to be appropriate in the pH environment that was prepared, like that of a wound. Metformin helped create the right blood glucose environment, and the release of curcumin produced an antimicrobial environment. The release of copper ions from MOFs promoted the healing of wounds as well[59].
The first choice for wound healing has traditionally been patches. Researchers have created a unique porous MOF micro-needle patch that enables photo thermal-responsive NO delivery for accelerating healing of wound caused by diabetes (DFU). The formulated MOFs were termed NO@HKUST-1@Graphene Oxide (GO). GO, in which the MOFs are encapsulated. Animal tests revealed that the patch could, particularly in hyperglycaemic diabetic wounds, encourage the migration and proliferation of endothelial cells. The outcomes strongly suggested that formulations based on MOF have a wide range of applications in wound healing[60].
MOFs in drug delivery for cancer:
Cancer is one of the leading causes of death in the globe. Chemotherapy, surgical procedures, and radiotherapy are currently the main cancer treatments. Chemotherapy causes side effects because it partially destroys cancerous cells while simultaneously affecting healthy, normal cells. MOFs can be smart carriers of drugs, limiting the effect only to the cancerous cells by various approaches like pH responsiveness, temperature control, etc.,[61]. Recently, two drugs were loaded onto MOF-808 those were associated with zirconium-based MOFs. MOFs were loaded with carboplatin and fluorouridine. To target malignant cells, these nanoparticles were functionalized with polyacrylic acid-mannose acrylamide glycopolymers. Both drugs therapeutic effects were improved by MOF-808. Fluxuridine and carboplatin had a synergistic effect. Polymer enhanced nanoparticle absorption in cancer cells. The effect of nanoparticles was seen in all cell lines as the cell viability decreased after 72 h of incubation[62].
In a different work, copper sulphide and oxaliplatin were incorporated into the pores using zirconium-based UiO-66-NH2 MOFs. To increase oxaliplatin's anticancer activity, copper sulphide was added. The OXA-CuS@UiO-66-NH2 formulation was created to treat colorectal cancer. In the cytotoxic assay, it was found that copper sulphide had a synergistic effect on the drug. The prepared formulation was successful in eradicating the malignant cells. This research broaden the potential therapeutic and diagnostic applications of MOFs and demonstrate that they can even precisely target malignant cells[63].
MOFs in drug delivery of antimicrobial agents:
According to the World Health Organization (WHO), widespread bacterial resistance is now a severe public health issue due to the indiscriminate use of antibiotics. Because microbes are now routinely resistant to numerous drugs, there are fewer therapeutic alternatives and, consequently nosocomial infections are becoming more serious[64]. The development of antibiotic resistance in bacteria produced a new surge in the incidence of infectious diseases and the demand for new antimicrobial drugs. A new way to combat this resistance of microbes is to use metal-based nanoparticles[65]. Researchers have developed Fe(III)MOF, which exhibit outstanding antibacterial properties in bacteria, fungus, and yeast. The interaction between ferric ions, which is present in Fe(III)-MOFs, and the cell walls of microorganisms could be the mechanism of action for the formulation's anti-microbial activity. The antibacterial activity of Fe(III)-MOF in vitro was tested against Gram-positive/negative bacteria, fungus, and yeast. In the results, it was shown that the antimicrobial activity was compared to that of common drugs in nutrient agar petri dishes. Inhibition zones for MOF were bigger than those for conventional drugs[31].
MOFs with the tetracycline drug have been prepared by scientists. Titanium dioxide (TiO2)@Chitosan@ZIF-8 MOFs were prepared to incorporate the drug. Staphylococcus aureus (S. aureus) and Escherichia coli (E. coli) bacteria were tested with the formed MOFs. Agar healthy diffusion was used to test the antibacterial activity of MOFs. The results were more than satisfactory and were able to kill the bacteria by destroying their cell walls. MOF with the drug was able to create an inhibition zone >30 mm[66-76] (Table 2).
| S. No. | Patent Title | Patent number | Publication Year | Reference |
|---|---|---|---|---|
| 1 | 2-periodic MOFs as Super-Molecular Building Layers (SBLs) for making targeted 3-periodic MOFs | US 9139599B1 | 2015 | [67] |
| 2 | Oral drug delivery system and method for fabricating thereof | US2021/05945A1 | 2021 | [68] |
| 3 | Host-guest metal organic framework systems | US10947321B2 | 2021 | [69] |
| 4 | Metal-organic framework functionalized polymeric compositions | US 2019/0358618A1 | 2019 | [70] |
| 5 | Cyclodextrin-based metal organic framework material and preparation method therefor | US202/0282046A1 | 2022 | [71] |
| 6 | MOF composite materials, methods, and uses thereof | US2020/0197901A1 | 2020 | [72] |
| 7 | Polymer-metal organic framework materials and methods of using the same | US10201803B2 | 2019 | [73] |
| 8 | Metal-organic framework supported on porous polymer | US 9375678B2 | 2016 | [74] |
| 9 | Compositions and medical devices for controlled release of nitric oxide and methods of production thereof | US9.216198B2 | 2015 | [75] |
| 10 | Nanoparticle for protein delivery | US2021/021295A1 | 2021 | [76] |
Table 2: Patents related to MOFs.
Limitations and Future Perspectives
The search for the perfect carrier for drug delivery is at its peak. Finding a stable and non-toxic carrier for controlled and targeted drug delivery is a big challenge[77]. MOFs have the potential to be efficient drug delivery systems. MOFs perform precisely necessary tasks such as drug loading and protection, targeting, and stimuli responsive delivery. MOFs also allow modifications after drugs have been loaded[78]. MOFs have various advantages, but certain concerns obstruct their potential use in drug delivery. Toxicity is the main concern with MOFs. Toxic reagents such as insoluble catalysts, chelating agents, and organic solvents may be used in the formulation of MOFs. Another reason that limits the use of MOFs in drug delivery is the formation of by-products. Metal oxides are primarily formed as by-products of the MOF formation process. Copper oxide, for example, has been seen as a by-product in the synthesis of copper-containing MOFs, although only at high temperatures[79].
There is currently no dose or exposure limit for MOFs. A toxic dose of IRMOF-3 was found in PC12 cells, according to recent toxicological research. In PC12 cells, the dose at a low concentration (25 μg/ml) exhibited barely detectable toxic effects. Morphological changes in PC12 cells were seen at higher dosages (100-400 μg/ml). In cells, Zn was released, which led to toxicity and, at very high doses, cell death[80]. The toxicity of MOFs is impacted by their physical and chemical properties. MOFs must first undergo toxicity testing before being used in biological applications[81]. MOF toxicity is also affected by their internal composition. Most MOFs have excellent biocompatibility but lack in-depth research in terms of toxicology.
Over the past two decades, MOFs have transformed the science of drug delivery. The remarkable potential of MOFs for delivery of drug has been emphasised in this review. The future of MOFs is undoubtedly optimistic in terms of drug administration, but recent research has shown that MOFs can also be utilised to identify biomarkers in biological fluids like blood and urine. This can increase the early diagnosis of diseases[82]. Metabolism and excretion were not well understood until now. Existing MOF-based bioimaging and theranostic applications are far from meeting therapeutic needs. These concerns are the areas of research, and MOFs emerge as the most suitable carriers as long as the research being done is going in the right direction[83].
Conclusion
MOFs have significantly improved drug delivery. These hybrid systems give the flexibility for customisation of a huge number of metal centres and organic building blocks to create novel materials with desirable properties as drug carriers. MOFs can be formulated according to their needs through various synthesis techniques. Stimuli-responsive drug delivery is also advantageous in that it allows for targeted drug delivery and dose dumping in malignant cells. The encapsulating nature of MOFs protects drugs from harsh environments, like the encapsulation of insulin, preventing its degradation in GIT fluid. MOFs have the ability to image and distribute the drug. MOFs' compositions sometimes provide an extra hand by rewarding them with other properties, like antimicrobial. MOFs have been reported to increase the efficacy of drugs loaded into them. Little data on metabolism and excretion makes it difficult to be used extensively. Another problem is toxicity, which is brought on by metal ions. At the end, research gaps are meant to be filled; these areas of toxicity and other studies will be solved in the coming years as the capabilities of MOFs have been proven in the medical field and in several other fields.
Conflict of interests:
The authors declared no conflict of interests.
References
- Kush P, Kaur M, Sharma M, Madan J, Kumar P, Deep A, et al. Investigations of potent biocompatible metal-organic framework for efficient encapsulation and delivery of Gemcitabine: Biodistribution, pharmacokinetic and cytotoxicity study. Biomed Phys Eng Express 2020;6(2):025014.
[Crossref] [Google Scholar] [PubMed]
- Sun Y, Zheng L, Yang Y, Qian X, Fu T, Li X, et al. Metal-organic framework nanocarriers for drug delivery in biomedical applications. Nanomicro Lett 2020;12:1-29.
[Crossref] [Google Scholar] [PubMed]
- Wang Y, Yan J, Wen N, Xiong H, Cai S, He Q, et al. Metal-organic frameworks for stimuli-responsive drug delivery. Biomaterials 2020;230:119619.
[Crossref] [Google Scholar] [PubMed]
- Beg S, Rahman M, Jain A, Saini S, Midoux P, Pichon C, et al. Nanoporous metal organic frameworks as hybrid polymer-metal composites for drug delivery and biomedical applications. Drug Discov Today 2017;22(4):625-37.
[Crossref] [Google Scholar] [PubMed]
- Yaghi OM, O'Keeffe M, Ockwig NW, Chae HK, Eddaoudi M, Kim J. Reticular synthesis and the design of new materials. Nature 2003;423(6941):705-14.
[Crossref] [Google Scholar] [PubMed]
- Liu J, Chen L, Cui H, Zhang J, Zhang L, Su CY. Applications of metal-organic frameworks in heterogeneous supramolecular catalysis. Chem Soc Rev 2014;43(16):6011-61.
- Horcajada P, Serre C, Maurin G, Ramsahye NA, Balas F, Vallet-Regí M, et al. Flexible porous metal-organic frameworks for a controlled drug delivery. J Am Chem Soc 2008;130(21):6774-80.
[Crossref] [Google Scholar] [PubMed]
- Fonseca J, Gong T, Jiao L, Jiang HL. Metal-Organic Frameworks (MOFs) beyond crystallinity: Amorphous MOFs, MOF liquids and MOF glasses. J Mater Chem A 2021;9(17):10562-611.
- He S, Wu L, Li X, Sun H, Xiong T, Liu J, et al. Metal-organic frameworks for advanced drug delivery. Acta Pharm Sin B 2021;11(8):2362-95.
[Crossref] [Google Scholar] [PubMed]
- Rocío-Bautista P, Taima-Mancera I, Pasán J, Pino V. Metal-organic frameworks in green analytical chemistry. Separations 2019;6(3):33.
- Sun CY, Qin C, Wang XL, Su ZM. Metal-organic frameworks as potential drug delivery systems. Expert Opin Drug Deliv 2013;10(1):89-101.
[Crossref] [Google Scholar] [PubMed]
- Horcajada P, Gref R, Baati T, Allan PK, Maurin G, Couvreur P, et al. Metal-organic frameworks in biomedicine. Chem Rev 2012;112(2):1232-68.
[Crossref] [Google Scholar] [PubMed]
- Al Sharabati M, Sabouni R, Husseini GA. Biomedical applications of metal-organic frameworks for disease diagnosis and drug delivery: A review. Nanomaterials 2022;12(2):277.
[Crossref] [Google Scholar] [PubMed]
- Lee YR, Kim J, Ahn WS. Synthesis of metal-organic frameworks: A mini review. Korean J Chem Eng 2013;30:1667-80.
- Jeyaseelan C, Jain P, Soin D, Gupta D. Metal organic frameworks: An effective application in drug delivery systems. Inorg Nano Met Chem 2022;52(12):1463-75.
- Qian Y, Zhang F, Pang H. A review of MOFs and their composites-based photocatalysts: Synthesis and applications. Adv Funct Mater 2021;31(37):2104231.
- Raptopoulou CP. Metal-organic frameworks: Synthetic methods and potential applications. Materials 2021;14(2):310.
[Crossref] [Google Scholar] [PubMed]
- Pan Y, Heryadi D, Zhou F, Zhao L, Lestari G, Su H, et al. Tuning the crystal morphology and size of zeolitic imidazolate framework-8 in aqueous solution by surfactants. CrystEngComm 2011;13(23):6937-40.
- Simon MA, Anggraeni E, Soetaredjo FE, Santoso SP, Irawaty W, Thanh TC, et al. Hydrothermal synthesize of HF-free MIL-100 (Fe) for isoniazid-drug delivery. Sci Rep 2019;9(1):16907.
- Gangu KK, Maddila S, Mukkamala SB, Jonnalagadda SB. A review on contemporary metal-organic framework materials. Inorg Chim Acta 2016;446:61-74.
- Bian Y, Xiong N, Zhu G. Technology for the remediation of water pollution: A review on the fabrication of metal organic frameworks. Processes 2018;6(8):122.
- Safaei M, Foroughi MM, Ebrahimpoor N, Jahani S, Omidi A, Khatami M. A review on metal-organic frameworks: Synthesis and applications. TrAC Trends Anal Chem 2019;118:401-25.
- Khan NA, Jhung SH. Synthesis of Metal-Organic Frameworks (MOFs) with microwave or ultrasound: Rapid reaction, phase-selectivity, and size reduction. Coord Chem Rev 2015;285:11-23.
- Mai Z, Liu D. Synthesis and applications of Isoreticular Metal-Organic Frameworks (IRMOFs)-n (n=1,3,6,8). Cryst Growth Des 2019;19(12):7439-62.
- Yu K, Lee YR, Seo JY, Baek KY, Chung YM, Ahn WS. Sonochemical synthesis of Zr-based porphyrinic MOF-525 and MOF-545: Enhancement in catalytic and adsorption properties. Microporous Mesoporous Mater 2021;316:110985.
- Mandani S, Rezaei B, Ensafi AA, Rezaei P. Ultrasensitive electrochemical molecularly imprinted sensor based on AuE/Ag-MOF@ MC for determination of hemoglobin using response surface methodology. Anal Bioanal Chem 2021;413(19):4895-906.
- de Lima Neto OJ, de Oliveira Frós AC, Barros BS, de Farias Monteiro AF, Kulesza J. Rapid and efficient electrochemical synthesis of a zinc-based nano-MOF for Ibuprofen adsorption. New J Chem 2019;43(14):5518-24.
[Crossref] [Google Scholar] [PubMed]
- Wang Z, Li Z, Ng M, Milner PJ. Rapid mechanochemical synthesis of metal-organic frameworks using exogenous organic base. Dalton Transactions 2020;49(45):16238-44.
- Chen D, Zhao J, Zhang P, Dai S. Mechanochemical synthesis of metal-organic frameworks. Polyhedron 2019;162:59-64.
- Klimakow M, Klobes P, Thunemann AF, Rademann K, Emmerling F. Mechanochemical synthesis of metal-organic frameworks: A fast and facile approach toward quantitative yields and high specific surface areas. Chem Mater 2010;22(18):5216-21.
- Sheta SM, Salem SR, El-Sheikh SM. A novel Iron (III)-based MOF: Synthesis, characterization, biological and antimicrobial activity study. J Mater Res 2022;37(14):2356-67.
- Xie W, Zhou F, Li X, Liu Z, Zhang M, Zong Z, et al. A surface architectured metal-organic framework for targeting delivery: Suppresses cancer growth and metastasis. Arab J Chem 2022;15(3):103672.
- Gholami M, Hekmat A, Khazaei M, Darroudi M. OXA-CuS@ UiO-66-NH2 as a drug delivery system for Oxaliplatin to colorectal cancer cells. J Mater Sci Mater Med 2022;33(3):26.
[Crossref] [Google Scholar] [PubMed]
- George P, Das RK, Chowdhury P. Facile microwave synthesis of Ca-BDC metal organic framework for adsorption and controlled release of curcumin. Microporous Mesoporous Mater 2019;281:161-71.
- Rojas S, Carmona FJ, Maldonado CR, Horcajada P, Hidalgo T, Serre C, et al. Nanoscaled zinc pyrazolate metal-organic frameworks as drug-delivery systems. Inorg chem 2016;55(5):2650-63.
[Crossref] [Google Scholar] [PubMed]
- Motakef-Kazemi N, Shojaosadati SA, Morsali A. In situ synthesis of a drug-loaded MOF at room temperature. Microporous Mesoporous Mater 2014;186:73-9.
- Liu B, Li H, Xu X, Li X, Lv N, Singh V, et al. Optimized synthesis and crystalline stability of γ-cyclodextrin metal-organic frameworks for drug adsorption. Int J Pharm 2016;514(1):212-9.
[Crossref] [Google Scholar] [PubMed]
- Wei JZ, Gong FX, Sun XJ, Li Y, Zhang T, Zhao XJ, et al. Rapid and low-cost electrochemical synthesis of UiO-66-NH2 with enhanced fluorescence detection performance. Inorg Chem 2019;58(10):6742-7.
[Crossref] [Google Scholar] [PubMed]
- Kang HJ, Choi YH, Joo IW, Lee JE. Mechanochemical synthesis of CD-MOFs and application as a cosmetic ingredient. Bull Korean Chem Soc 2021;42(5):737-9.
- Cai W, Wang J, Chu C, Chen W, Wu C, Liu G. Metal-organic framework-based stimuli-responsive systems for drug delivery. Adv Sci 2019;6(1):1801526.
[Crossref] [Google Scholar] [PubMed]
- Chen WH, Luo GF, Vázquez-González M, Cazelles R, Sohn YS, Nechushtai R, et al. Glucose-responsive metal-organic-framework nanoparticles act as “smart” sense-and-treat carriers. ACS Nano 2018;12(8):7538-45.
- Feng L, Dong Z, Tao D, Zhang Y, Liu Z. The acidic tumor microenvironment: A target for smart cancer nano-theranostics. Nat Sci Rev 2018;5(2):269-86.
- Shen X, Pan Y, Sun Z, Liu D, Xu H, Yu Q, et al. Design of metal-organic frameworks for pH-responsive drug delivery application. Mini Rev Med Chem 2019;19(20):1644-65.
[Crossref] [Google Scholar] [PubMed]
- Schnabel J, Ettlinger R, Bunzen H. Zn-MOF-74 as pH-responsive drug-delivery system of arsenic trioxide. ChemNanoMat 2020;6(8):1229-36.
- Yang J, Wang H, Liu J, Ding M, Xie X, Yang X, et al. Recent advances in nanosized metal organic frameworks for drug delivery and tumor therapy. RSC Adv 2021;11(6):3241-63.
[Crossref] [Google Scholar] [PubMed]
- Gong M, Yang J, Li Y, Gu J. Glutathione-responsive nanoscale MOFs for effective intracellular delivery of the anticancer drug 6-mercaptopurine. Chem Commun 2020;56(47):6448-51.
- Cai M, Chen G, Qin L, Qu C, Dong X, Ni J, et al. Metal organic frameworks as drug targeting delivery vehicles in the treatment of cancer. Pharmaceutics 2020;12(3):232.
[Crossref] [Google Scholar] [PubMed]
- Jiang K, Zhang L, Hu Q, Zhang Q, Lin W, Cui Y, et al. Thermal stimuli-triggered drug release from a biocompatible porous metal-organic framework. Chemistry 2017;23(42):10215-21.
[Crossref] [Google Scholar] [PubMed]
- Wu MX, Yang YW. Metal-Organic Framework (MOF)-based drug/cargo delivery and cancer therapy. Adv Mater 2017;29(23):1606134.
[Crossref] [Google Scholar] [PubMed]
- Hu Q, Yu J, Liu M, Liu A, Dou Z, Yang Y. A low cytotoxic cationic metal-organic framework carrier for controllable drug release. J Med Chem 2014;57(13):5679-85.
[Crossref] [Google Scholar] [PubMed]
- Maranescu B, Visa A. Applications of metal-organic frameworks as drug delivery systems. Int J Mol Sci 2022;23(8):4458.
[Crossref] [Google Scholar] [PubMed]
- Mittal A, Roy I, Gandhi S. Drug delivery applications of Metal-Organic Frameworks (MOFs). InDrug Carriers 2022.
- Rahman MM, Islam MR, Shohag S, Hossain ME, Rahaman MS, Islam F, et al. The multifunctional role of herbal products in the management of diabetes and obesity: A comprehensive review. Molecules 2022;27(5):1713.
[Crossref] [Google Scholar] [PubMed]
- Tong PH, Zhu L, Zang Y, Li J, He XP, James TD. Metal-Organic Frameworks (MOFs) as host materials for the enhanced delivery of biomacromolecular therapeutics. Chem Commun 2021;57(91):12098-110.
- Wang M, Wang C, Ren S, Pan J, Wang Y, Shen Y, et al. Versatile oral insulin delivery nanosystems: From materials to nanostructures. Int J Mol Sci 2022;23(6):3362.
[Crossref] [Google Scholar] [PubMed]
- Chen Y, Li P, Modica JA, Drout RJ, Farha OK. Acid-resistant mesoporous metal-organic framework toward oral insulin delivery: Protein encapsulation, protection and release. J Am Chem Soc 2018;140(17):5678-81.
[Crossref] [Google Scholar] [PubMed]
- Briones D, Fernández B, Calahorro AJ, Fairen-Jimenez D, Sanz R, Martínez F, et al. Highly active anti-diabetic metal-organic framework. Cryst Growth Des 2016;16(2):537-40.
- Tabriz AG, Douroumis D. Recent advances in 3D printing for wound healing: A systematic review. J Drug Deliv Sci Technol 2022;74:103564.
- Yang L, Liang F, Zhang X, Jiang Y, Duan F, Li L, et al. Remodeling microenvironment based on MOFs-Hydrogel hybrid system for improving diabetic wound healing. Chem Eng J 2022;427:131506.
- Yao S, Wang Y, Chi J, Yu Y, Zhao Y, Luo Y, et al. Porous MOF microneedle array patch with photothermal responsive nitric oxide delivery for wound healing. Adv Sci 2022;9(3):2103449.
[Crossref] [Google Scholar] [PubMed]
- Falsafi M, Saljooghi AS, Abnous K, Taghdisi SM, Ramezani M, Alibolandi M. Smart metal organic frameworks: Focus on cancer treatment. Biomater Sci 2021;9(5):1503-29.
- Demir Duman F, Monaco A, Foulkes R, Becer CR, Forgan RS. Glycopolymer-functionalized MOF-808 nanoparticles as a cancer-targeted dual drug delivery system for carboplatin and floxuridine. ACS Appl Nano Mater 2022;5(10):13862-73.
[Crossref] [Google Scholar] [PubMed]
- Gholami M, Hekmat A, Khazaei M, Darroudi M. OXA-CuS@ UiO-66-NH2 as a drug delivery system for Oxaliplatin to colorectal cancer cells. J Mater Sci Mater Med 2022;33(3):26.
[Crossref] [Google Scholar] [PubMed]
- Mammari N, Lamouroux E, Boudier A, Duval RE. Current knowledge on the oxidative-stress-mediated antimicrobial properties of metal-based nanoparticles. Microorganisms 2022;10(2):437.
[Crossref] [Google Scholar] [PubMed]
- Gudkov SV, Serov DA, Astashev ME, Semenova AA, Lisitsyn AB. Ag2O nanoparticles as a candidate for antimicrobial compounds of the new generation. Pharmaceuticals 2022;15(8):968.
[Crossref] [Google Scholar] [PubMed]
- Akbari M, Sadeghi ME, Ghasemzadeh MA. Controlled delivery of tetracycline with TiO2@ Chitosan@ ZIF-8 nanocomposite and evaluation of their antimicrobial activities. Research on Chemical Intermediates 2022;48(9):3971-85.
- Eddaoudi M, Eubank JF. 2-periodic Metal-Organic Frameworks (MOFs) as Supermolecular Building Layers (SBLs) for making targeted 3-periodic MOFs. United States patent US 9139599; 2015.
- Sung HW, Miao YB, Chen KH. Oral drug delivery system and method for fabricating thereof; 2021.
- Liang K, Ricco R, Doherty CM, Falcaro P, inventors; Industrial Research Organization CSIRO, assignee. Host-guest metal organic framework systems. United States patent US 10947321; 2021.
- Reynolds MM, Harding JL, Neufeld MJ, inventors; Colorado State University Research Foundation, assignee. Metal-organic framework functionalized polymeric compositions. United States patent application US 16/527457; 2019.
- Li X, Chengdeng CH, Chen L, inventors; South China University of Technology SCUT, assignee. Cyclodextrin-based metal organic framework material and preparation method therefor. United States patent application US 17/605249; 2022.
- Qingye LU, Omar MA, Song P, inventors; UTI LP, assignee. Metal Organic Framework (MOF) composite materials, methods, and uses thereof. United States patent US 11135565; 2021.
- Cohen SM, Zhang Z, inventors. Polymer-metal organic framework materials and methods of using the same. United States patent US 10201803; 2019.
- Nair S, Brown A, Jones CW, inventors; Georgia Tech Research Corp, assignee. Metal-organic framework supported on porous polymer. United States patent US 9375678; 2016.
- Balkus Jr KJ, Liu HA, inventors; University of Texas System, assignee. Compositions and medical devices for controlled release of nitric oxide and methods of production thereof. United States patent US 9216198; 2015.
- Zheng S, Cheng G. Nanoparticle for protein delivery; 2021.
- Sreenivasulu A, Selvam JD, Sajith S, Vasumathy M, Barwant MM, Alagarsamy S, et al. A comprehensive revision on the nanocarrier drug delivery systems with special reference to artificial intelligence. Int J Health Sci 2022(III):7163-93.
- Lawson HD, Walton SP, Chan C. Metal-organic frameworks for drug delivery: A design perspective. ACS Appl Mater Interfaces 2021;13(6):7004-20.
[Crossref] [Google Scholar] [PubMed]
- Salehipour M, Rezaei S, Rezaei M, Yazdani M, Mogharabi-Manzari M. Opportunities and challenges in biomedical applications of metal-organic frameworks. J Inorg Organomet Polym Mater 2021;31(12):1-20.
- Ren F, Yang B, Cai J, Jiang Y, Xu J, Wang S. Toxic effect of zinc nanoscale metal-organic frameworks on rat Pheochromocytoma (PC12) cells in vitro. J Hazard Mater 2014;271:283-91.
[Crossref] [Google Scholar] [PubMed]
- Wagner A, Liu Q, Rose OL, Eden A, Vijay A, Rojanasakul Y, et al. Toxicity screening of two prevalent metal organic frameworks for therapeutic use in human lung epithelial cells. Int J Nanomed 2019:7583-91.
[Crossref] [Google Scholar] [PubMed]
- Zhao W, Deng J, Ren Y, Xie L, Li W, Wang Q, et al. Antibacterial application and toxicity of metal-organic frameworks. Nanotoxicology 2021;15(3):311-30.
[Crossref] [Google Scholar] [PubMed]
- Mendes RF, Figueira F, Leite JP, Gales L, Paz FA. Metal-organic frameworks: A future toolbox for biomedicine? Chem Soc Rev 2020;49(24):9121-53.