- *Corresponding Author:
- Yan Hong
Department of Psychiatry, Tianjin Mental Health Institute, Tianjin Anding Hospital, Hexi, Tianjin 300222, China
E-mail: yanhong202112@sina.com
| This article was originally published in a special issue, “Current Trends in Pharmaceutical and Biomedical Sciences” |
| Indian J Pharm Sci 2022:84(5) Spl Issue “304-312” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The natural efficacy based trials of the atypical antipsychotics are required to yield extensive information concerning the efficiency, security, tolerance and safety among the patients suffering from schizophrenia who are treated in the hospital-based outpatient care centre based practice. In this study, the men and women were taken with the age range of 14 to 90 y with recognition of schizophrenia. There were a total of 103 patients considered for this study, who were enrolled between July 2017 to August 2021. This study analyzed the treatment with aripiprazole along with the standard of care based treatment procedure. The patient data reviewed in this study during the screening process moved closer to an interactive voice response system after obtaining informed consent. In the aripiprazole treatment group (n=49), the results were significantly better and effective in comparison to the standard of care treatment group (n=54; p<0.001; w 26, last observation carried forward) as reflected by the interactive voice response system total score. Similar results were noted in patients who duly completed the study design where aripiprazole was significantly associated with effectiveness at all phases in comparison to the standard of care group. The patients treated with aripiprazole also showed a significantly higher increase in quality of life and lesser adverse effects at w 26 (p<0.001). A high percentage of patients who received aripiprazole as a study medication was termed as “much better’’ on the preference scale of the medication questionnaire in comparison to the standard of care. The schizophrenia trial of aripiprazole provides a detailed overview of the real-world rendering and the enhanced benefits of aripiprazole compared to other major atypical antipsychotics advised in the hospital.
Keywords
Schizophrenia, aripiprazole, schizophrenia trial of aripiprazole study, antipsychotic standard of care, antipsychotic medication
Mental disorders include depression and anxiety. There are multiple factors that can result in this disease. Psychosocial factors including stress and disease awareness as well as biological factors may influence the course of a disease[1]. Benzodiazepines are useful for anxiety[2]. However, it has a trend for dependency. Chinese medical treatment (Shakannzoutou, Keishikaryuukotsuboreitou and Hangekoubokutou) is also used in treating Basedow psychosis, but the effect was not so clear. Schizophrenia is a severe mental disorder with lifelong effects in around 1 % of the world’s population[3]. There is rising proof, where most atypical antipsychotic treatment is proposing benefits over the first-generation antipsychotics, comprising of considerable development with negative symptoms, functional dimensions, cognitive potential as well as the quality of lifestyle for patients suffering from schizophrenia[4]. Additionally to this established efficiency, the second-generation anti-psychotics transmit a diminished unpredictability of critical Extrapyramidal Symptoms (EPS) as well as the Tardive Dyskinesia (TD) in maximum of the researches which are differentiated with the first- generation antipsychotics[5].
The Schizophrenia Trial of Aripiprazole (STAR) research is naturally an efficient study performed with the primary objective of yielding crucial information over the association between the state, type of medication as well as the patients in their routine care setting[6]. The United Kingdom (UK) National Institute for Health and Care Excellence (NICE) followed an initial analysis of atypical antipsychotics in the year 2002 and discovered the absence of the “real-world” data like the patient-reported results, patient priorities as well as the treatment regimen depended on the expert’s decision[7].
Eventually, NICE was referred to acknowledge the subsequent reviews during the randomized clinical trials of the limited appropriateness towards the common patient population because of their bounded timeline as well as the count of variables calculated and the naturalistic researches like the STAR permit the analysis of the treatment efficacy utilizing few patient criteria along with a broad spectrum of efficacy variables like the tolerance, security, symptom relief of worsening, patient’s priority for medication, quality of lifestyle as well as the cost-effectiveness.
The purpose of the research was to analyze the efficacy of a 26 w based treatment of aripiprazole compared to the Standard of Care (SOC) among the patients undergoing treatment for schizophrenia within the hospital-based outpatient setting[8]. This study will provide the basic theory and treatment for patients who survived chronic schizophrenia.
Materials and Methods
This study was performed at hospital. There were a total of 103 patients considered for this study, who were enrolled on July 2017 to August 2021.
Inclusion and exclusion criteria:
In this study, the age range of men and women was taken as 14 to 90 y with recognition of schizophrenia and were treated in the hospital-based outpatient care centre, possessing symptoms which need to be treated with antipsychotic medicines and basic treatment, and needed a modification of antipsychotic medication which was not well-structured by the clinicians. Those patients were excluded from the study, if they were still going through some critical psychotic symptoms which needed hospitalization or were at the risk of executing suicide or had a detection of schizoaffective disarray, depression along with psychotic symptoms or bipolar disorder. There should be more patients enrolled in the schizoaffective treatment group than the Reactive Oxygen Species (ROS) group in order to ensure the number of patients in the whole study.
Those patients who were found to be resistant against the treatment procedure and possess a significant psychoactive substance use dysfunction in 3 mo before the screening process or with any background or neuroleptic malignancy based syndrome or seizures, any critical head injury, epilepsy, stroke or abnormal electroencephalogram. Furthermore, the exclusion criteria comprised of any significant background of intolerance against multiple antipsychotic treating procedures, identified as hypersensitivity towards aripiprazole other dihydrocarbostyrils, earlier randomness among the aripiprazole clinical analysis with the investigation agency in the past month or the receiving of long-term antipsychotics in 3 w of the screening process.
Designing study:
This study analyzed the treatment with aripiprazole along with the SOC based treatment procedure. The patient data considered in this study during the screening process was within the Interactive Voice Response (IVR) approach, after informed consent was obtained.
Randomized patients in the SOC category were given one of the following atypical antipsychotics that were available at the time of the search, i.e. quetiapine, olanzapine or risperidone. This randomization study implemented and prohibited any classification within the SOC group. Furthermore, the patients cannot accept the medication which they have been advised before the study or any medication treatment which they had not undergone in their past.
The cross-titration of the earlier and the treatment- stage antipsychotics were permitted for 2 w and the follow-up investigation existed at the following weeks: 2nd w, 4th w, 8th w, 12th w, 18th w and the 27th w to analyze the efficacy of the treatment.
Medication utilization:
The patients were randomized to the aripiprazole category, obtained around 10-30 mg per day, managed orally at least once every day in portions of 10 mg or 15 mg tablets with a prescribed initial dosage of 15 mg per day. The patients were randomized to the SOC category, obtained the olanzapine with 5 to 20 mg per day with an advised initial dosage of 10 mg per day, quetiapine with 100-800 mg per day advised dosage titration (50 mg on the 1st d followed by 200 mg on the 2nd d, 200 mg on 3rd d and 300 mg on the 4th d), after the 4th d the doses can be titrated as per the reaction based results, the risperidone in proportions of 2-16 mg per day, advised dose titration, where 2 mg of it on the 1st d followed by 4 mg on the 2nd d and the added dosage expanded as per the need. All the doses of this research medication were to regulate within the permitted and verified local medication label.
The use of benzodiazepines as well as anticholinergics, were permitted at any timeline, if needed. The use of other psychotropic medication like the antidepressant as well as the mood stabilizer were permitted if a suffering patient was accepting these medicated treatments at the study centre, wherein only small modifications in dosing were allowed while conducting the study and there was no novel psychotropic medication treatment which could have been included while the treatment phase. There were no other antipsychotics permitted during the study phase except while the process of cross-titration till the 15th d of this medication period.
Assessment of efficacy:
The efficacy of the treatment process was analyzed with the total score obtained using the Investigator Assessment Questionnaire (IAQ), which was comprehended as the total of 10 from the given 12 elements i.e. somnolence, signs and symptoms of the prolactin rise, EPS, energy, akathisia, mood, cognition, positive symptoms, weight gain and negative symptoms, each of them on a 5-point Likert scale with the range as 1: Much better, 2: Slightly better, 3: About the same, 4: Slightly worse and 5: Much worse.
The protocol revision while the trial eliminated the inclusion of the two elements from the total of the IAQ score because of the higher reaction rate of the “not applicable” while the performance of validation study. The factorial study of the 10 IAQ elements means they were all equivalent in weight and were loaded on only one factor. The IAQ also showed a good reliability score (Cronbach’s alpha (α)=0.87). To analyze the overall effectiveness of antipsychotic therapy in patients, it is found that IAQ is the most trusted and verified tool.
The use of the Preference of Medication Questionnaire (POM), which is a two-item based questionnaire to analyze the preference for the present antipsychotics in comparison to the latest pre-study antipsychotic was also concluded. From the POM, one element is communicated to the patient and the other to the care provider associated with the patient. The POM was regulated at different weeks: 8th w, 18th w and the end of the 26th w, thus scored over the following scaling parameters, 1: Much better and I prefer this medication, 2: Slightly better, 3: About the same, 4: Slightly worse, 5: Much worse and I would prefer my previous medication.
Statistical analysis:
The statistical analysis implemented varied for the efficacy and the result of research-based specimens. We used Statistical Package for the Social Sciences (SPSS) 22, a statistical tool to perform the statistical investigation over the gathered outcomes.
Results and Discussion
Patient demographics and disposition was explained here. A total of 103 patients participated in this trial. Here 49 patients were enrolled in the aripiprazole study group, in which 53.06 % were male, 46.93 % female, with a mean age of 64.5 y in the group, while in the SOC group, 53.70 % were males and 38.88 % were females with a mean age of 57.18 y in the group. The mean weight of the aripiprazole study group was 135.50 kg while in the SOC group is 104.07 kg (Table 1).
| S. No. | Characteristics | Aripiprazole (n=49) | All SOC (n=54) | Total (n=103) |
|---|---|---|---|---|
| 1 | Age | |||
| Mean | 64.5 | 57.18 | 60.85 | |
| Median | 58 | 59.5 | 60.84 | |
| 2 | Gender (n, %) | |||
| Male | 26, 53.06 % | 29, 53.70 % | 55, 53.39 % | |
| Female | 23, 46.93 % | 21, 38.88 % | 44, 42.72 % | |
| 3 | Race (n, %) | |||
| White | 5, 10.20 | 8, 14.81 | 13, 12.61 | |
| Asian | 39, 79.59 | 41, 75.92 | 80, 77.66 | |
| Black | 2, 4.08 | 3, 5.55 | 5, 4.85 | |
| Other | 3, 6.12 | 2, 3.07 | 5, 4.85 | |
| 4 | Schizophrenia type (n, %) | |||
| Paranoid | 25, 51.02 | 28, 51.85 | 53, 51.46 | |
| Catatonic | 4, 8.16 | 5, 9.26 | 9, 8.73 | |
| Residual | 9, 18.36 | 9, 16.66 | 18, 17.47 | |
| Disorganized | 6, 12.24 | 7, 12.90 | 13, 12.62 | |
| Undifferentiated | 5, 10.20 | 5, 9.26 | 10, 9.71 | |
| 5 | Weight, kg | |||
| Weight (Mean) | 135.5 | 104.07 | 119.785 | |
| Weight (Median) | 104 | 106 | 105 |
Table 1: Characteristics of Patients
A total of 103 patients enrolled for the study complied with the inclusion criteria with patients randomized either to aripiprazole or SOC. The 54 atypical antipsychotic SOC group was randomized to drugs like olanzapine (27 patients), risperidone (15 patients) and quetiapine (12 patients). In the aripiprazole group of 49 randomized patients, 4 patients were randomized but did not receive medication because of non-compliance with the criteria.
Similarly, in the SOC group of 54 randomized patients, 4 patients were randomized but received no medication because of non-compliance and violation of the criteria. The baseline of the demographics of patients is shown in Table 1 and Table 2.
| S. No. | Status of patients included in the study | Aripiprazole (n=49) | All SOC (n=54) | Total (n=103) |
|---|---|---|---|---|
| 1 | Randomized | 49 | 54 | 103 |
| 2 | Randomized but no medication received | 4 | 4 | 8 |
| 3 | Discontinued from treatment phase | 23 (46.93) | 24 (44.44) | 47(45.63) |
| Lack of efficacy | 1 (2.04) | 5 (9.25) | 6 (5.82) | |
| Adverse event | 4 (8.16) | 7 (12.96) | 11 (10.67) | |
| Withdrew consent | 4 (8.16) | 2 (3.07) | 6 (5.82) | |
| Death | 2 (4.08) | 1 (1.85) | 3 (2.09) | |
| Lost to follow-up | 4 (8.16) | 2 (3.70) | 6 (5.82) | |
| Poor or non-compliance | 4 (8.16) | 2 (3.70) | 6 (5.82) | |
| Pregnancy | 2 (4.08) | 1 (1.85) | 3 (2.09) | |
| No longer meets study criteria | 1 (2.04) | 2 (3.07) | 3 (2.09) | |
| Other | 1 (2.04) | 2 (3.07) | 3 (2.09) | |
| 4 | Completed treatment phase | 26 | 30 | 56 |
Table 2: Patient Disposition of Aripiprazole and SOC Group
A 26 w treatment period was completed by the patients of the aripiprazole and SOC group. The major reason for the discontinuation was an adverse effect (8.16 %), withdrew consent (8.16 %), lost to follow-up (8.16 %), poor or non-compliance (8.16 %) in the aripiprazole group and adverse effect (12.96 %) as well as lack of efficacy (9.25 %) in SOC groups (Table 2).
The major positive symptoms (aripiprazole: 46.98 and SOC: 53.70) and negative symptoms (aripiprazole: 53.70 and SOC: 27.77) were the major prime reasons for the changes in medication also followed by the gain in weight (aripiprazole: 24.49 % and SOC: 27.77 %). All the reasons for changing the medication were analyzed and the positive, negative control was most common amongst them for the change of the antipsychotic medication. The mean dose in the case of the study medication was 10.5 mg olanzapine, 15.7 mg aripiprazole, 378.3 mg quetiapine and 3.9 mg risperidone. The anxiolytic and anticholinergic drugs used in the study group, who underwent treatment was 19.8 % and 39.6 % respectively. The treatment group of SOC was 13.8 % and 29.3 % respectively. New drugs were introduced for 2.8 % of patients who received aripiprazole and 3.9 % of patients in the SOC arm. A new category of anxiolytic medications was started in 20.1 % of the patients who received aripiprazole and 7.9 % of the SOC group patients. The primary reason for drug switching at the time of listing is presented in Table 3.
| S. No. | The main reason for switching drugs | Aripiprazole (n=49) | All SOC (n=54) | Total (n=103) |
|---|---|---|---|---|
| 1 | Lack of optimum control, n (%) | |||
| Positive symptoms | 23, 46.98 | 29, 53.70 | 52, 50.48 | |
| Negative symptoms | 20, 40.82 | 15, 27.77 | 35,33.98 | |
| Other symptoms | 6, 12.24 | 10, 18.52 | 16, 15.53 | |
| 2 | Tolerability, n (%) | |||
| Somnolence | 4, 8.16 | 5, 9.26 | 9, 8.74 | |
| Weight gain | 12, 24.49 | 15, 27.77 | 27, 26.21 | |
| Prolactin elevation | 6, 12.24 | 1, 1.85 | 7, 6.80 | |
| Akathisia | 1, 2.04 | 2, 3.70 | 3, 2.91 | |
| EPS other than akathisia | 9, 18.37 | 6, 11.11 | 15, 14.56 | |
| Other safety/tolerability issue | 1, 2.04 | 2, 3.70 | 3, 2.91 | |
| 3 | Lack of optimizing, n (%) | |||
| Cognition | 8, 16.32 | 9, 16.66 | 17, 16.50 | |
| Mood | 7, 14.28 | 5, 9.26 | 12, 11.65 | |
| Energy | 1, 2.04 | 2, 3.70 | 3, 2.91 |
Table 3: Reasons for Switching Medication
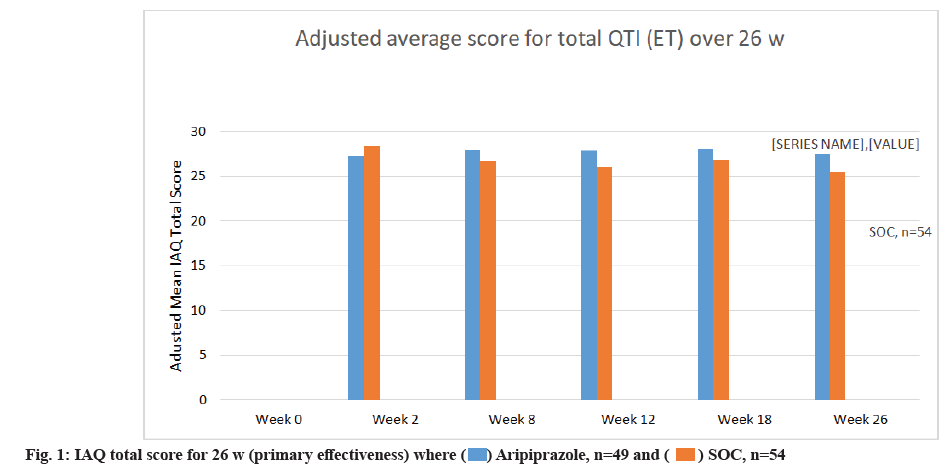
Primary and secondary effectiveness outcomes were explained in this study. Aripiprazole was linked to significantly lower IAQ mean scores when compared with the SOC group at w 26 where p<0.001 and aripiprazole were found to be significantly better than the SOC group for assessment of the medication when compared with prior studied antipsychotics. Mean IAQ scores were found to be lower significantly in the aripiprazole treated group at beginning of w 4 which was sustained till w 26 as shown in fig. 1.
IAQ scores for 26 w are shown in fig. 1 which reflects the rating by investigator of the currently used antipsychotic drugs when compared with the anti-psychotic drugs prescribed before the randomization process. IAQ total score for 26 w where ratings for the currently used antipsychotic drug is compared against medicine used before randomization. The study was performed for 26 w (primary effectiveness).
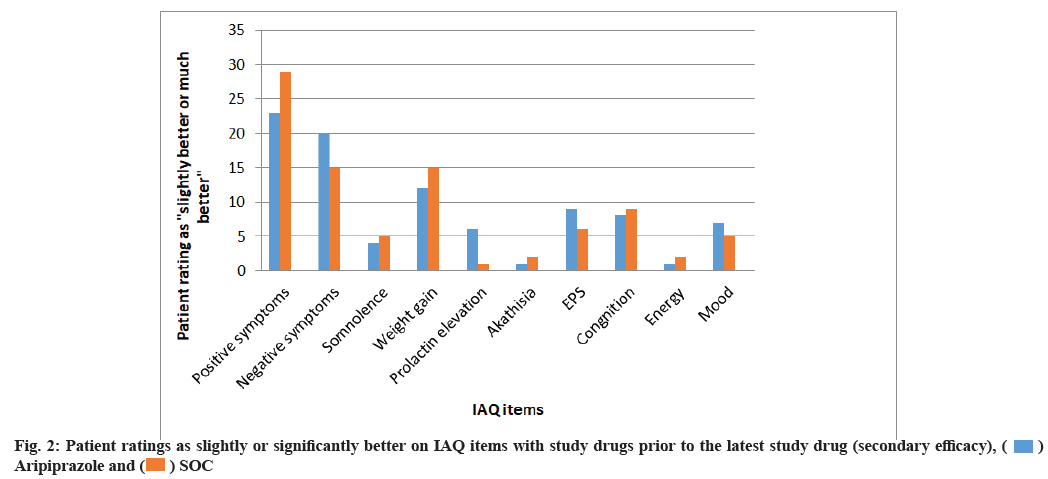
The measures for secondary effectiveness for every IAQ item in proportion to all the patients who were rated as slightly better’’ or ‘‘much better’’ in the study before medication were bigger for the aripiprazole group in comparison to the SOC group at 26th w of study. The ratio of patients who were rated as slightly or much better for the study were around 10 % larger for aripiprazole than the SOC in aspects of IAQ items: Negative symptoms (20 % vs. 15 %), somnolence (4 % vs. 5 %), weight gain (12 % vs. 15 %), prolactin elevation (6 % vs. 1 %), akathisia (1 %, 2 %), EPS (9 %, 6 %), cognition (8 %, 9 %), energy (1 %, 2 %) and mood (7 %, 5 %) respectively as shown in fig. 2.
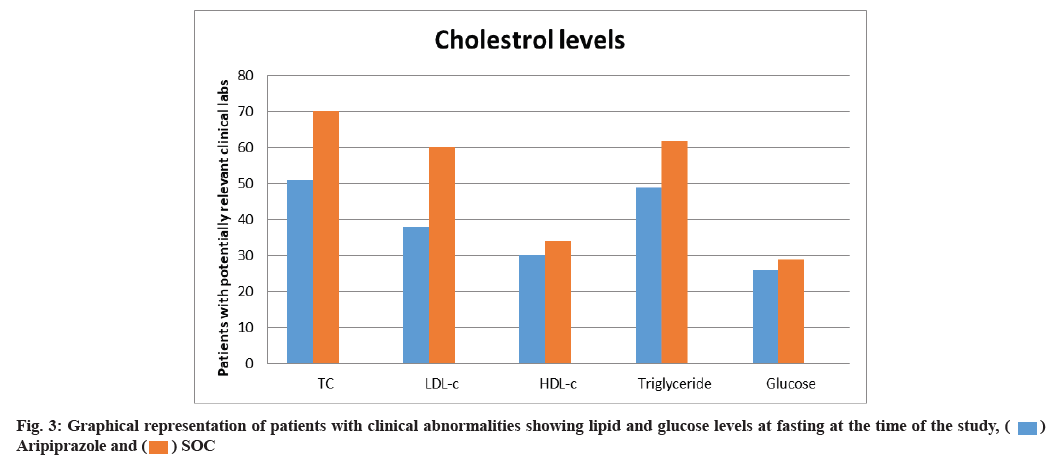
Cholesterol levels and weight changes have significant impact on the choice of medication, thereby both were studied for the 26 w course. Aripiprazole was associated with a lesser proportion of patients who were linked with clinically relevant lipid levels (fasting) total cholesterol (51 % aripiprazole vs. 70 % SOC), Low-Density Lipoprotein Cholesterol (LDL-C) (38 % aripiprazole vs. 60 % SOC), High-Density Lipoprotein Cholesterol (HDL-C) (30 % aripiprazole vs. 34 % SOC), triglycerides (49 % aripiprazole vs. 62 % SOC) thereby controlling baseline levels. No significant difference was noted in the ratio of patients with fasting glucose (26 % aripiprazole vs. 29 % SOC) as shown in fig. 3.
The ratio of patients with significant abnormalities in the case of fasting lipid and glucose levels at the time of the study for controlling the baseline values was considered as SOC.
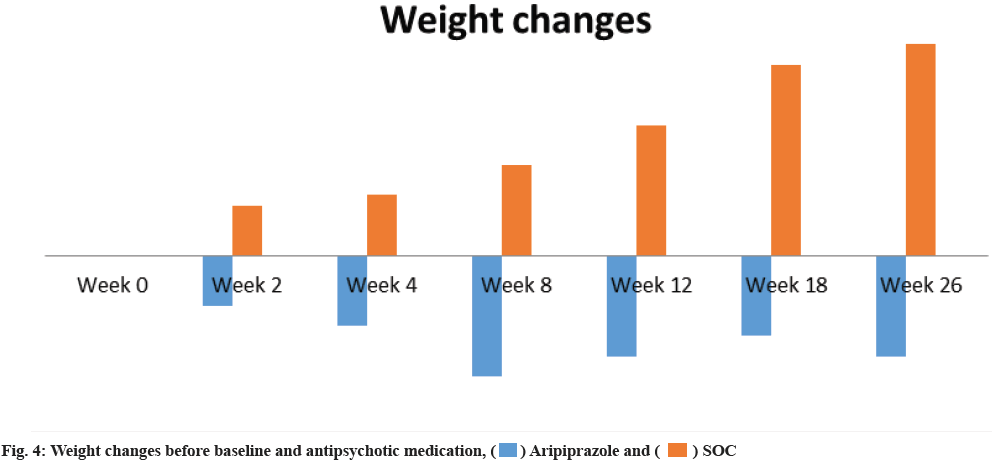
The weight changes were similar in the cases of treatment groups. At 26th w the loss in weight for treated group with aripiprazole noted a decrease of 0.5 % and an increase of 0.5 % in the SOC group. On 26th w aripiprazole group had a 1 % decrease whereas the SOC group had a 2.1 % increase from the baseline. The larger proportion of patients gained a clinically significant weight increase when compared against the aripiprazole group at 26th w as shown in fig. 4.
The patients who received medication were analyzed for the safety of the drug for all 103 patients. The cases of adverse effects in the treatment groups, aripiprazole and SOC were high. The cases of serious adverse effect led to the discontinuation of the therapy. Reported adverse effects were nausea 44.89 % in aripiprazole group and 5.55 % in SOC group; fatigue 6.12 % in aripiprazole group vs. 14.81 % in SOC group; psychotic disorder 4.08 % in aripiprazole group vs. 5.55 % in SOC group; insomnia 14.28 % in aripiprazole group vs. 9.26 % in SOC group and schizophrenia 4.08 % in aripiprazole group vs. 5.55 % in SOC group etc. Most commonly experienced adverse effect in the treated group with SOC was headache 11.11 %, somnolence 9.26 %, insomnia 9.26 % and weight increase 18.51 % as shown in Table 4.
| S. No. | Adverse effects | Aripiprazole (n=49), % | All SOC (n=54), (%) |
|---|---|---|---|
| 1 | Nausea | 22 (44.89) | 3 (5.55) |
| 2 | Fatigue | 3 (6.12) | 8 (14.81) |
| 3 | Weight increase | 1 (2.04) | 10 (18.51) |
| 4 | Akathisia | 2 (4.08) | 2 (3.70) |
| 5 | Headache | 5 (10.20) | 6 (11.11) |
| 6 | Somnolence | 2 (4.08) | 5 (9.26) |
| 7 | Agitation | 2 (4.08) | 2 (3.70) |
| 8 | Anxiety | 1 (2.04) | 4 (7.41) |
| 9 | Insomnia | 7 (14.28) | 5 (9.26) |
| 10 | Psychotic disorder | 2 (4.08) | 3 (5.55) |
| 11 | Schizophrenia | 2 (4.08) | 3 (5.55) |
Table 4: Adverse Effects in Treatment Groups
The most commonly reported adverse effects in the aripiprazole group were insomnia 14.28 %, headache 10.20 %, fatigue 6.12 % and nausea 44.89 %. Here the highest percentage was noted for nausea as shown in Table 4.
Untreated schizophrenia might lead to significant functional impairment. With the development of chlorpromazine in the 1950s, medication treatment became available and antipsychotic medication development continues until this day. The rating parameters of the severity of symptoms have been critically expressed for net showcasing, the well- being of patients and the efficacy among the natural clinical practices. The Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study is utilized for the overall termination rates as the chief element of measure for the effectiveness, assurance of restrictions to the rating parameters. In this research, there was no difference in termination rates between the groups of aripiprazole and SOC patients.
However, the group variation came when the effectiveness was investigated based on key elements which contributed towards the effectiveness of antipsychotics. The main results are measured in the current study, i.e. STAR was the IAQ overall score, which was a verified measure of efficacy that assessed both the effectiveness and the side effects of the present antipsychotic medication treatment when compared with the patient’s background antipsychotic[9-11].
In this naturalistic analysis, the aripiprazole treatment procedure was associated with significantly better efficacy when compared with the SOC treatment. However, the physicians select one of these three atypical agents as the favourable treatment for the independent patient, based on the overall IAQ score, a verified measure of the antipsychotic effectiveness at all the time-values. For each IAQ element, the aripiprazole was also associated with the higher proportions of the patients which were rated as “slightly better” or “much better” in comparison to the prior medication than that of the SOC group. Digitally we found that there appears to be excessive use of anxiolytic, in the category treated with aripiprazole[12].
Although, it is estimated that most of the patients approaching this research were first prescribed sedative drugs, in addition, the data prioritized by the patient suggest that the subjects will prefer such an arrangement rather than the induced sedative and thus weight gain including effects on the initial drug. The side effect outcomes from the antipsychotic treatment are other elements that can adversely influence a patient’s attitude towards a future process of treatment, the medication attachment, quality of lifestyle and the relapse as well as the hospitalization numbers.
The advantage of this study was to provide a higher degree of efficacy of aripiprazole in the treatment of the outpatients, i.e., treatment patients with schizophrenia, specifically in the areas of symptom enhancement, patient medication priority, clinical reactions and the quality of lifestyle. The STAR provides a detailed overview of the actual rendering and increased benefits of aripiprazole compared to other major atypical antipsychotics recommended in hospital.
However, there are also limitations of this study. The patients suffering from schizophrenia possess a 5-8 times greater cardiovascular mortality rate as compared to the common population. However, the aripiprazole used to treat patients might not have the ability to treat cardiovascular disease. Also, this study only compared the effectiveness of aripiprazole with SOC and it is not clear whether aripiprazole have the superiority of other atypical antipsychotic drugs. The STAR provides a detailed overview of the real-world rendering and the enhanced benefits of aripiprazole compared to other major atypical antipsychotics advised in the hospital.
Conflict of interests:
The authors declared no conflict of interest.
References
- Fukao A, Takamatsu J, Arishima T, Tanaka M, Kawai T, Okamoto Y, et al. Graves disease and mental disorders. J Clin Transl Endocrinol 2020;19:100207.
[Crossref] [Google Scholar] [PubMed]
- Vita R, Lapa D, Vita G, Trimarchi F, Benvenga S. A patient with stress-related onset and exacerbations of graves disease. Nat Clin Pract Endocrinol Metab 2009;5(1):55-61.
[Crossref] [Google Scholar] [PubMed]
- Nucifora Jr FC, Woznica E, Lee BJ, Cascella N, Sawa A. Treatment resistant schizophrenia: Clinical, biological, and therapeutic perspectives. Neurobiol Dis 2019;131:104257.
[Crossref] [Google Scholar] [PubMed]
- Hasan A, Falkai P, Wobrock T, Lieberman J, Glenthoj B, Gattaz WF, et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 2: Update 2012 on the long-term treatment of schizophrenia and management of antipsychotic-induced side effects. World J Biol Psychiatry 2013;14(1):2-44.
[Crossref] [Google Scholar] [PubMed]
- Chan RC, Gottesman II. Neurological soft signs as candidate endophenotypes for schizophrenia: A shooting star or a northern star? Neurosci Biobehav Rev 2008;32(5):957-71.
[Crossref] [Google Scholar] [PubMed]
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: A STAR*D report. Am J Psychiatry 2006;163(11):1905-17.
[Crossref] [Google Scholar] [PubMed]
- Breton F, Plante A, Legauffre C, Morel N, Adès J, Gorwood P, et al. The executive control of attention differentiates patients with schizophrenia, their first-degree relatives and healthy controls. Neuropsychologia 2011;49(2):203-8.
[Crossref] [Google Scholar] [PubMed]
- Sullivan G, Bienroth M, Jones M, Millar H, Ratna L, Taylor D. Practical prescribing with aripiprazole in schizophrenia: Consensus recommendations of a UK multidisciplinary panel. Curr Med Res Opin 2007;23(7):1733-44.
[Crossref] [Google Scholar] [PubMed]
- Liu Z, Palaniyappan L, Wu X, Zhang K, Du J, Zhao Q, et al. Resolving heterogeneity in schizophrenia through a novel systems approach to brain structure: Individualized structural covariance network analysis. Mol Psychiatry 2021;26(12):7719-31.
[Crossref] [Google Scholar] [PubMed]
- Perlis RH, Laje G, Smoller JW, Fava M, Rush AJ, McMahon FJ. Genetic and clinical predictors of sexual dysfunction in citalopram-treated depressed patients. Neuropsychopharmacology 2009;34(7):1819-28.
[Crossref] [Google Scholar] [PubMed]
- Sobis J, Rykaczewska-Czerwińska M, Swiętochowska E, Gorczyca P. Therapeutic effect of aripiprazole in chronic schizophrenia is accompanied by anti-inflammatory activity. Pharmacol Rep 2015;67(2):353-9.
[Crossref] [Google Scholar] [PubMed]
- Kerwin R, Millet B, Herman E, Banki CM, Lublin H, Pans M, et al. A multicentre, randomized, naturalistic, open-label study between aripiprazole and standard of care in the management of community-treated schizophrenic patients Schizophrenia Trial of Aripiprazole: (STAR) study. Eur Psychiatry 2007;22(7):433-43.
[Crossref] [Google Scholar] [PubMed]
 Aripiprazole, n=49 and
Aripiprazole, n=49 and  SOC, n=54
SOC, n=54