- *Corresponding Author:
- R. kumaravelrajan
Department of Pharmaceutics,
CL Baid Metha College of Pharmacy,
Tamil Nadu 600047,
Chennai,
India
E-mail: rkumaravelrajan@gmail.com
| Date of Received | 18 September 2020 |
| Date of Revision | 10 August 2021 |
| Date of Acceptance | 30 March 2022 |
| Indian J Pharm Sci 2021;84(2):390-399 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The purpose of this study was to prepare diltiazem timed-release pellets using non pareil seeds. An important step in the preparation of timed-release multiparticulate dosage forms was to mix a pilot mixture composed of various polymers or waxes with different concentration. By using the three-factor and twolevel factorial design for the pilot mixing process, the mixing can be distinguished technically than the trial and error method. Core, non pareil seeds prepared by using a sugar solution. A fluidized bed processor was used to coat diltiazem on to the non pareil seeds. Hydroxypropyl methylcellulose E15 was used as coatings for non pareil seeds, with various percentage (10 %, 20 % and 30 %) in two levels (16.66 % and 33.33 %). According to the design matrix, pilot mixing was carried out for in vitro dissolution studies and optimized batches were prepared for masterblend and mixture based on the results. The Pareto chart supported the analysis of variance results for the model simplification and eliminating non-significant terms (p>0.05). The optimal process variable selected for optimizing the diltiazem timed-release pellets formulation was set to be pilot mix I=24.9 %, pilot mix II=16.6 % and pilot mix III=16.6 %. The optimized formula showed that half-life period was 2.361±0.243 h and shelf-life period was 6.875 h.
Keywords
Quality by design, diltiazem, palletization, factorial design, timed-release
Modified-release preparations can be administered orally in single or multiple-unit dosage forms. Singleunit formulations contain the active ingredient within the single tablet or capsule, where as multiple-unit dosage forms comprise of number of discrete particles that are combined into one dosage unit. They may exist as pellets, granules, sugar seeds (non pareil) and minitablets [1]. Multi-Unit Pellet System (MUPS) is one of the most popular Multi-Unit Dosage Form (MUDF) because of its unique advantages in physical properties (e.g., spherical shape) and mechanical properties (e.g., elastic and deformation ability and tensile strength) [2,3]. Pellet formulations were better at producing sustained release effects and reducing variability in blood levels, it was claimed that a pellet formulation had shorter gastric emptying times and longer total intestinal residence times than tablets [4,5]. Non Pareil Seeds (NPS) can be prepared by conventional and fluid-bed process [6,7]. Quality by Design (QbD) is formally defined as “a systematic approach for product development that begins with predefined objectives and emphasizes product and process understanding and process control, based on sound science and quality risk management” [8]. QbD is now seen as a key enabler for achieving the desired performance of developed formulation. Screening designs (full-factorial, Plackett–Burman designs and mixture designs) are used to indicate the most important of all factors, potentially influencing the formulation, product or process. Response surface designs (central-composite design, Box–Behnken design and Doehlert design) are again applied to find the optimal factor settings and mixture designs to optimize, for instance, the excipients composition in formulations [9,10]. Conventional pharmaceutical development procedures are based on quality by testing and have become out of use. In these methods, product quality was assured by controlling raw materials (i.e. drugs and excipients) and manufacturing techniques [11].
The challenges in the production of a timed-release dosage form to the manufacturing industry are pilot mixing of different level of the coated pellets by trial and error until satisfactory results obtained. This can be overcome by QbD approach [12]. The identification of Critical Process Parameters (CPPs) becomes a part of production process parameters and finally the risk assessment of the drug substance attributes to the development of the product [13,14]. Taking antihypertensive drugs at night can significantly reduce Morning Blood Pressure Fluctuations (MBPS), thereby improving blood pressure variability [15]. Diltiazem Hydrochloride (HCl) has a short biological half-life and high dosing frequency (usually three to four times a day), making it a potential candidate for the development of Sustained-Release (SR) formulations [16]. Diltiazem pellets prepared by Fluid-bed coating process reported to be ideal for the preparation of controlled-release formulations [17]. The purpose of this study was to prepare diltiazem timed-release pellets using NPS. An important step in the preparation of timed-release multiparticulate dosage forms is to mix a pilot mixture composed of various polymers or waxes with different concentration. By using the 23 factorial design for the pilot mixing process, the mixing can be distinguished technically than the trial and error method. Core, NPS prepared by using sugar solution. A fluidized bed processor was used to coat diltiazem to the NPS. Hydroxypropyl methylcellulose (HPMC) E15 used as NPS coating polymer. According to the design matrix, pilot mixing was carried out for in vitro dissolution studies and optimized batches were prepared for master blend and mixture based on the results. The threefactor and two-level (23) factorial design was used to optimize the Diltiazem NPS (DNPS) pilot mix. Hence, the amount of hydrophilic polymers coated with DNPS namely, amount of HPMC K15-10 %, HPMC K15- 20 % and HPMC K15-30 % as the prime selected independent variables (factors), which were varied at two levels (low 16.66 %) and 33.33 % high) for pilot mixing operation. 50 % drug released (t50) and 90 % drug release (t90) were chosen as a dependent variable to test the release property of DNPS. Various studies have been conducted on the optimized mixture, such as Entrapment Efficiency (EE) and surface morphology.
Materials and Methods
Materials:
Diltiazem HCl was purchased from Divi’s Laboratories Limited, Hyderabad. Hydroxypropyl Cellulose (HPCDiltiazem HCl was purchased from Divi’s Laboratories Limited, Hyderabad. Hydroxypropyl Cellulose (HPCM- FP-300) was procured from Aualon, Gujarat. HPMC 15 cps E15-LV (12-15) obtained from Colorcon Asia Pvt. Ltd., Singapore. Polyvinyl pyrrolidone (PVP K30, 5.5-8.5) was purchased from Nanchang Industrial Holding Group Co. Ltd. and Feicheng Rui Tai Fine Chemicals, China. Triethyl citrate (C12-35.2) was purchased from Zhonglan Industry Co.Ltd, China. All other chemicals purchased for manufacturing and quality control as an analytical grade.
Methods:
Thermal analysis: Thermograms of unplasticized and plasticized polymeric films were obtained by using a Differential Scanning Calorimetry (DSC) (Mettler Toledo DSC2A-00837, Mumbai, India) and STAR® software (Mettler Toledo, Giessen, Germany) to determine the melting point or the glass Transition (Tg) temperature (n=2–3). The temperature calibration was completed with the melting transition of indium. The sample (7–10 mg, stored in a vacuum desiccator prior to analysis) was sealed in an aluminium tank and placed in the NETZSCH DSC. The scanning rate was 10°/min over a temperature range of 50°-300°. All tests were run under a nitrogen atmosphere.
Formulation development: Diltiazem 120 mg timed release pellets were prepared. At first, preliminary experiments were conducted to prepare NPS with a syrup base, but after a long fluidized bed dryer coating process, particles with the desired shape were formed. Hence, combining 3 % PVP with the syrup solution resulted in a satisfactory particle size. Another method of developing DNPS is to use a traditional coating pan, but the drug loading effect is poor, so the coating pan method is abandoned. Diltiazem and HPC (7 % w/v) polymers are separately dissolved in water and mixed. Water was used as the granulating fluid, sufficient to prepare a wet blend that is suitable for extrusion. The prepared aqueous suspension was sprayed on to the NPS. The third stage of development is HPMC polymer coating, which carried out through a conventional coating pan to form pellets of sufficient thickness. For coating, the most common cellulose-based polymer used for sustained release dosage forms is a HPMC because, HPMC is known as a pellets-hardening agent and also reported as a pore-forming agent in pellets manufacturing. Plasticizers are other components incorporated into polymer coatings to enhance filmforming properties, because most polymers become brittle at room temperature. The commonly used plasticizer for particle coating is 10 % w/w triethyl citrate.
Preparation of NPS: The pellets were prepared in a fluid-bed granulator (WSG-Pro, Pam Glatt, Thane, India) using the wet granulation technique (Table 1A). 1 kg of pharma grade sugar and 5 % starch passed to a 60 mesh sieve, was used at first [18]. This powder mixture was loaded into the product chamber of the machine. After a fluidization for 3 min, a pre-weighed amount of syrup approximately 300 ml was sprayed through an atomizing nozzle (1.2 mm diameter) into the powder at a rate of 30 ml/min with the aid of a peristaltic pump. The process continued for an hour until pellets developed a 0.4 mm thickness in size. Later pellets passed through 30 mesh, but retained in the 50 mesh used further coating process. The inlet air temperature was kept constant at 45°±1° and the process airflow at 0.3-0.5 bar. Once all the syrup was sprayed, the pellets were dried for 15 min at 450°.
| Preparation of NPS: Ingredients | (g) | |
|---|---|---|
| Pharma grade sugar* | 1000 | |
| Starch | 50.0 | |
| Povidone | 30.0 | |
| Sugar syrup | qs | |
| Purified water | qs | |
| Prepartion of DNPS** | ||
| Diltiazem HCl | 1000 | |
| Hydroxy propyl cellulose | 28.0 | |
| Purified water | qs | |
| Formulation of polymer coating with DNPS | ||
| HPMC | 1.5-2.5 | |
| Triethyl citrate | 1.0 | |
| Talc | 0.0125 | |
| Isopropyl Alcohol | 50.0 | |
| Methylene Chloride | 50.0 | |
Note: *formula for 1 kg of NPS and **formula for 1 kg of diltiazem pellets
Table 1: Composition of NPS, Preparation and Coating of Diltiazem Pellets
Preparation of DNPS: The core consists of diltiazem, which was coated on the NPS using HPC as a binder (Table 1B). HPC was dissolved in purified water at a ratio of 1:0.0028 (drug-polymer) [19]. Using the conditions previously described, the drug was suspended in the binder solution and sprayed onto the sugar spheres (NPS) in a fluidized bed processor. Binder weight divided by drug weight was 0.028. Core composition was calculated as 1 kg of the drug in 1000 g of NPS (1:1) basis and drug content was expressed as fractional content (Fc, the weight of the drug in the cores/total weight of the cores×100 %=250×1245×100 =20.08 %). Spray efficiency of the drug layering process was reported as the actual weight gain divided by the theoretical weight gain expressed as percentage. Cores were sieved to remove undersized material and agglomerates before coating.
Coating of polymer with DNPS: The polymer membrane consisted of a HPMC (Table 1C) were prepared as three variables of the coating solution HPMC 10 %, 20 % and 30 %. The triethyl citrate (10 % w/v) was used as plasticizer and talc as antistatic material. Coating polymers and plasticizer were dissolved in isopropyl alcohol and methylene chloride at 1:1 ratio. Talc was suspended in the coating solution at 10 % of the weight of dissolved solids as an anti-tack agent. The suspension was sprayed onto the DNPS of traditional coating pan (316 stainless steel size 18"×12", RPM 0-28, temperature 30°-65°).
Pilot mixing of pellets:
Experimental design: A 23 factorial design was used to optimize the diltiazem HCl pellets pilot mix operation. Before performing the dissolution study of diltiazem pellets in vitro, a factorial design of 23 was applied to all trail batches (F1-F8) of pilot mixtures, as shown in Table 2. The pilot mix was prepared by mixing of diltiazem coated pellets of three different polymer coat 10 %, 20 % and 30 % HPMC E15, at higher (33.33 %) and lower (16.66 %) level with or without dummy pellets to make 100 % according to design matrix. Hence, every batch consisted of either a lower or higher level of HPMC coated diltiazem core. These pellets (DNPS) were mixed after the determination of their drug content. The t90 and t50 are studied as dependent variables (responses). Design-Expert 11.0.6.1 software (Stat-Ease Inc., USA) was used for generation and evaluation of the statistical experimental design.
| Batch | A: Pilot mix Ia (HPMC-10) % | B: Pilot mix IIb (HPMC-20) % | C: Pilot mix IIIb (HPMC-30) % | Dummy pellets (%) | Total pilot mix (%) | t50 (h)* | t90 (h)** |
|---|---|---|---|---|---|---|---|
| F1 | 16.66 (-1) | 16.66 (-1) | 16.66 (-1) | 50.02 | 100 | 0.756 | 4.629 |
| F2 | 33.33 (+1) | 16.66 (-1) | 16.66 (-1) | 33.35 | 100 | 1.2089 | 5.31 |
| F3 | 16.66 (-1) | 33.36 (+1) | 16.66 (-1) | 33.35 | 100 | 1.2476 | 5.999 |
| F4 | 33.33 (+1) | 33.33 (+1) | 16.66 (-1) | 16.68 | 100 | 1.973 | 6.26 |
| F5 | 16.66 (-1) | 16.66 (-1) | 33.33 (+1) | 33.35 | 100 | 2.222 | 6.48 |
| F6 | 33.33 (+1) | 16.66 (-1) | 33.33 (+1) | 16.68 | 100 | 2.789 | 6.98 |
| F7 | 16.66 (-1) | 33.3 (+1) | 33.33 (+1) | 16.68 | 100 | 3.062 | 7.30 |
| F8 | 33.33 (+1) | 33.33 (+1) | 33.33 (+1) | 0.01 | 100 | 4.197 | 7.50 |
Note: A, B and C represent the main effects (factors); (+1): Higher level (33.3 %) and (−1): Lower level (16.6 %), *t50 (h) (%): The time required to release 50 % of the drug. Response variable I, value of mean±standard deviation, n=3, **t90 (h) (%): The time required to release 90 % of the drug. Response variable II, value of mean±standard deviation, n=3, acontent of pilot mix I, both level used to mix 100 % pellets and bcontent of pilot mix II and pilot mix III, was higher level (+1) used to mix 100 % pellets
Table 2: Design Matrix for Pilot mix with Responses
Y=b0+b1A+ b2B+b3C+b4AB+b5AC+b6BC Eqn (1)
Where Y is the dependent variable and b0 is the intercept; b1, b2, b3, b4, b5 and b6 are regression coefficients; A, B and C are independent variables and AB, AC and BC are the interactions between variables. One-way Analysis of Variance (ANOVA) was applied to estimate the significance of the model (p<0.05) and individual response parameters.
Evaluation of diltiazem pellets:
Pellet size analysis: Using a combination of vertical movements (sieve numbers 10, 14, 16 and 18), diltiazem HCl pellets were analyzed through a series of successively decreasing sieve vibrations [20]. The pellets in motion finally oriented to the screen (sieve 16) with its smallest two sizes, opens and passes through the screen with the next smaller nominal opening (sieve 18).Upon completion of the sieving process, the weight of the sieves was measured and compared to the weight of the sieves before the addition of the sample. Through the addition of the mass fraction on each sieve, from the smallest to the largest sieve, the cumulative mass distribution of the test sample was calculated.
EE: The prepared pellets were evaluated for the percentage of drug loading and drug EE [21]. The pellets were crushed well and a powder equivalent to 100 mg dissolved in 100 ml of methanol and filtered through a 0.45 μm cellulose acetate membrane filter, 5 ml of aliquot was withdrawn. The drug loading was determined by Ultraviolet (UV) detection at 236 nm and the percentage (%) drug release and EE was calculated using the following equations:
EE % =Drug added-Free unentrapped drug/drug added×100
Theoretical Polymer Coat Weight (TPCW): TPCW is defined as the weight of dissolved solids’ applied (including plasticizer) divided by the sum of core and applied dissolved solids weights. During this process, the sample was removed from the coating pan to ensure that the TPCW passes improved, through the sample port without interrupting the spraying process. Drug content was reported as fractional content (Fc, weight of drug in total pellet weight) or potency (Fc×100 %). Samples were sieved to remove undersize material and agglomerates before image analysis.
In vitro dissolution study: Drug release tests were carried out using a dissolution tester. The dissolution tester was calibrated using salicylic acid by United States Pharmacopeia (USP) dissolution calibrator. Test samples containing 120 mg of diltiazem HCl pellets was placed in a USP dissolution apparatus II containing 900 ml of distilled water at 37° (paddle method at 100 rpm). Diltiazem HCl belongs to the BCS class I drug (high solubility and high permeability). Drug release and absorption do not depend on the pH value of gastrointestinal tract. Therefore, distilled water is used as the dissolution medium [22]. By comparing the UV scanning (200-400 nm) of different products with the UV scanning of pure drugs and was found that the detection method was not interfered by excipients at 236 nm. The USP XXIII rotating paddle method (VanKel 700, VK 650a, 37°, 900 ml, 0.1 M HCl, 0.1 M pH 7.4 phosphate buffer, 100 RPM, n=3) was used to study the release of drug from coated pellets. The samples (5.0 ml) were collected at 1, 2, 3, 4, 5, 6, 8 and 9 h intervals and analysed or stored in the refrigerator until analysis (within 24 h). Diltiazem content and release were detected, spectrophotometrically at 236 nm [23] (Electrolab, TDT-0P, India).
Fitting kinetic model: In order to analyze the drug release mechanism of these diltiazem timed release pellets, the in vitro dissolution data were fitted to various mathematical models, such as zero-order, firstorder, Higuchi and Korsmeyer-Peppas models [24].
Statistical analysis:
Statistical optimization was performed using Design- Expert 8.0.6.1 software (Stat-Ease Inc., USA). All other data were analysed with simple statistics.
Scanning Electron Microscopy (SEM) study:
The external and surface morphology of diltiazem pellets were analyzed by SEM [25]. The pellets were fixed on supports with carbon-glue and coated with gold-palladium under an argon atmosphere using a gold sputter module in a high-vacuum evaporator. Samples were then observed with an FEI, Quanta 200 scanning electron microscope (FEI, Quanta 200 SEM, USA) at 20 kv.
Results and Discussion
In order to confirm the physical state of diltiazem HCl in the pellets, the DSC of Diltiazem HCl and the physical mixture of diltiazem HCl and polymer were measured. The DSC trace of diltiazem HCl showed a sharp endothermic peak at 186.23°, its melting point. The physical mixture of diltiazem HCl and polymers showed the same thermal behavior 171.31° as the individual component, indicating that there was no interaction between the diltiazem HCl and the polymer in the solid state. These suggest that the diltiazem HCl existed in an amorphous or disordered crystalline phase as a molecular dispersion in polymeric matrix. The size of the prepared diltiazem HCl pellets was measured using sieve diameter method and found to be between 0.761 and 0.882 mm. It was found that the trapping efficiency of experiment 1 was 77 %. The encapsulation efficiency was increased by 85 % in another trial batch by optimizing coating condition. This serves to calculate the equivalent value of diltiazem HCl in the pellets. The angle of repose, flow through an orifice, moisture content, bulk/tapped volume and density, compressibility index, and Hausner ratio were evaluated to determine the flow characteristics. The calculated angle of repose for the powder was 22.67°±0.4° to 27.24°±0.2° indicating that powder exhibits a fair flow. The inter-particle interaction that affects the performance of the powder body affects the powder flow. A comparison of the tapped and bulk densities is useful to give a measure of the relative importance of these interactions in powder. Such a comparison is often said as an index (the compressibility index or the Hausner ratio) of the ability of the powder to flow. Estimated compressibility index and Hausner ratio for powder mixture were 1.99 %±06 % to 9.39 %±1.06 % and 1.022 %±0.03 % to 1.13 %±0.04 %, respectively. Friability values were 0.079 % to 0.096 %.
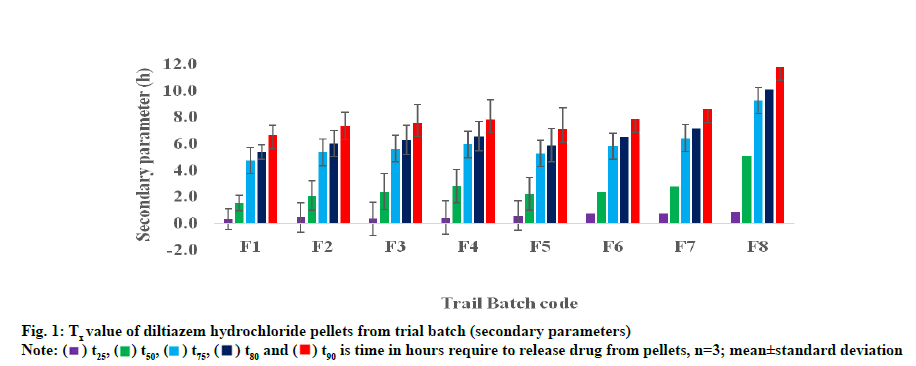
Table 2 lists the time required for all formulations to release 50 % and 90 % (t50 and t90 of the drug. The drug release from pilot-mix according to design matrix was largely depends on the coating level. Firstly, t50 of the drug was found to be 0.75 and 4.19 h for F1 (all three variable low level) and F8 (all high level) as level of pilot mix in coating increased, proportionately the drug was released from pellets irrespective of polymer concentration. Similarly, when comparing with high level coating for F4, F6 and F7, the t50 was found to be 1.97, 2.78 and 3.06 h. Although the coating thickness was increased to the greatest extent, when the HPMC concentration higher (F6, 2.78 h), the release of the polymer better due to the swelling and solubility of the polymer. On the other hand, when comparing t50 of F2, F3 and F5, t50 was 1.20, 1.24 and 2.22 h respectively. However, t50 for F3 slightly higher than other two formulations. This fact could be due to level and more ethyl cellulose present in coating membrane.
The time required for all formulations to release 90 % of the shown in fig. 1. In pilot mix F1 and F8, lower and higher t90 values are given for 4.6 and 7.5 h. Formulations 4, 6 and 7 composed of pilot mix II and pilot mix III largely, thickness showed a higher t90 of 6.2 h, 6.9 h and 7.3 h. Similarly, F2, F3 and F5 are released for 5.3 h, 5.9 h and 6.4 h, respectively. Because of small differences in the pilot mix I concentration, no significant change in the pattern of release between F3 and F5.
Through 23 factorial designs, a total of 8 pilot mixtures of diltiazem HCl pellets were proposed for 3 independent variables HPMC 10 %, 20 %, 30 % coat as pilot mix I, II and III respectively. In the current study, the effects of these independent variables, t50 (h) and t90 (h) were studied as the optimal response parameters. The results of the ANOVA indicated that these models were significant for all response parameters as shown in Table 3. After fitting these data, Design-Expert 10.0.6.1 software provides suitable polynomial model equations including each main factor and interaction factor. The model simplified by eliminating the unimportant terms (p>0.05) in the modeling equation obtained by the multiple regression analysis. The model equation with t50 (h) as the response becomes,
| Source | Sum of squares | D.f | Mean square | F value | p value | |
|---|---|---|---|---|---|---|
| Model–t50 | 7.81 | 2 | 3.9 | 15.09 | 0.0076 | S |
| B-Pilot mix II (HPMC-20) | 1.53 | 1 | 1.53 | 5.93 | 0.059 | |
| C-Pilot mix III (HPMC-30) | 6.27 | 1 | 6.27 | 24.25 | 0.0044 | |
| Model-t90 | 6.6 | 3 | 2.2 | 45.4 | 0.0015 | S |
| A-Pilot mix I (HPMC-10) | 0.337 | 1 | 0.337 | 6.95 | 0.0578 | |
| B-Pilot mix II (HPMC-20) | 1.67 | 1 | 1.67 | 34.53 | 0.0042 | |
| C-Pilot mix III (HPMC-30) | 4.59 | 1 | 4.59 | 94.73 | 0.0006 |
Note: D.f: Degree of freedom; A, B and C represent the main effects (factors) and S: Model Significant
Table 3: Paraphrase of Anova for Response Parameters
t50=+2.1819375+0.3600375×A+0.4379625×B+0.88 55625×C+6.2×10−3AB+9.4×10−2AC+7.1.2×10−3BC, (R2=0.9991; F value=15.09; p<0.0076) Eqn. (2)
The model equation relating with t90 (h) as the response becomes
t90=+6.30725+0.20525×A+0.4575×B+0.75775×C+5. 50×10−3AB−6.12×10−3AC−5.02×10−3BC, (R2=0.9994; F value=45.40; p<0.0015) Eqn. (3)
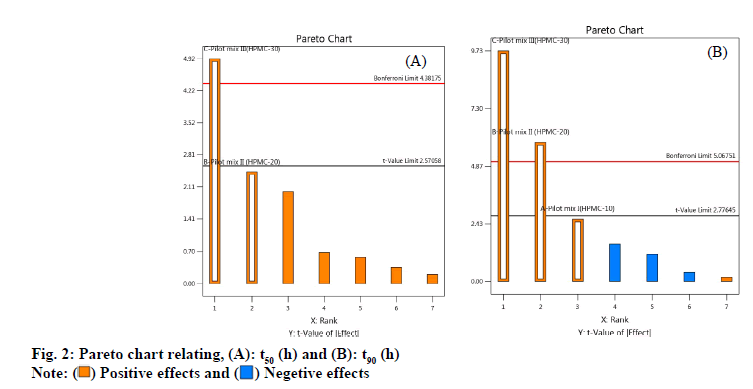
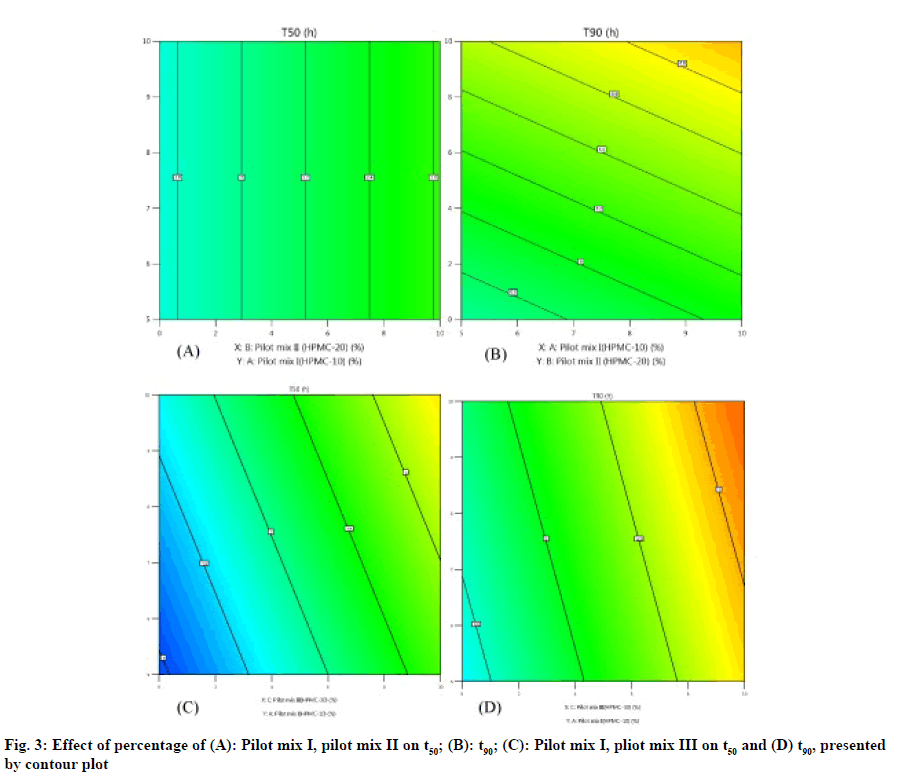
As shown in fig. 2 A-fig. 2B, the statistical significance of each response coefficient was studied through the Pareto chart. These graphs describe the statistical significance of each response coefficient. Coefficients with t values of effects above the Bonferroni line are designated as significant coefficientswith t values of effects between Bonferroniline and t limit line are termed as coefficients likely to be significant. Coefficients with t values of effects below the t limit line are statistically insignificant coefficients. Therefore, these Pareto charts supported also the ANOVA results for the model simplification by eliminating non-significant terms (p>0.05) in both the model equations [26]. A numerical optimization technique based on the desired method was adopted to realize a new optimized formula with the required response. The ideal range of these responses is limited to 2 h≤t50≤50 % and 7 h≤t90≤90 %, while the factor range is limited to 20 %≤A≤25 %, 15 %≤B≤20 %, 15 %≤C≤20 %. In order to evaluate the optimization capabilities of these models generated from the results of 23 factorial design, the optimal process variable selected for optimizing the diltiazem timed-release pellets formulation was set to be pilot mix I=24.9 %, pilot mix II=16.6 % and pilot mix III=16.6 %. Numerical analysis was performed to obtain the best value based on the expected response. Table 4 depicts the results (actual values) of predicted values obtained from the mathematical model and actually observed. The optimized formula shows that t50 is 2.361±0.243 h, t90 is 6.875 h and the error range is small (<10 %), indicating that the mathematical model obtained through 23 factorial designs is very suitable.
| Factor | Name | Level (%) | Response | Predicted mean (h) | Observed* (h) |
|---|---|---|---|---|---|
| A | Pilot mix I (HPMC-10) | 24.9 | t50 | 2.18194 | 2.361 |
| B | Pilot mix II (HPMC-20) | 16.6 | t90 | 6.30725 | 6.875 |
| C | Pilot mix III (HPMC-30) | 16.6 | % error** | 8.203 | 9.005 |
Note: *Observed values=mean±standard deviation, n=3; **Percent error=(Actual value−predicted value)/predicted value×100 and A, B and C represent the main effects (factors)
Table 4: Design Space, Analyses for Optimization
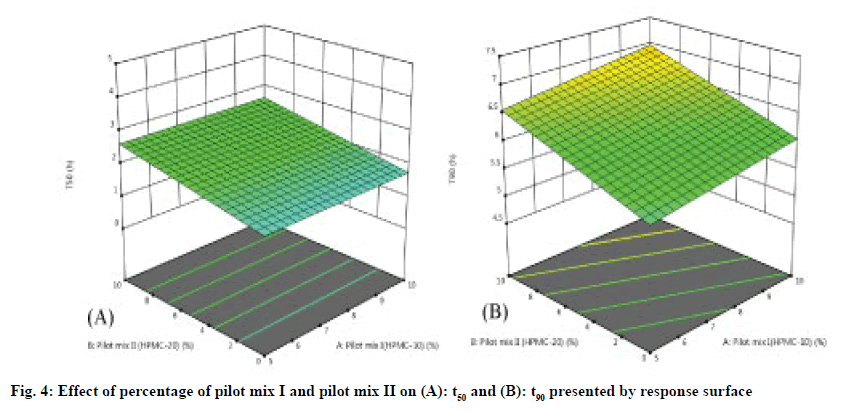
The three-dimensional response surface plot is very useful in learning about the main and interaction. The influence of independent variables (factors) and the two-dimensional contour map (fig. 3A-fig. 3D) is a visual representation of the response value. The threedimensional response surface map and corresponding contour map related to t50 (h) and t90 (h) show that with the increase of HPMC concentration (20 % pilot II and 30% pilot mix III), the dead value of t50 and t90 (fig. 4A-fig. 4B). The model found to be significant (p≤0.005) indicating that the selected model described the relationship between the independent variable and the dependent variable well.
Dissolution data of the optimized formulation was fitted to various mathematical models (zero-order, first-order, Higuchi and Korsemeyar-Peppas) in order to describe the kinetics of drug release as shown in Table 5. Regression coefficient and slope (rate) were compared in all the formulations to study their effect on drug release. In addition, the optimized formula is also equipped with selective zero-order and Peppas models to calculate the residual sum of squares, Akaike Information Criteria (AIC) and the value of the best fit test (R2).
| Parameters | Report |
|---|---|
| Average pellet sizea (mm, n=20) | 0.827 |
| EEa (%) | 94.06 |
| TPCWa ( %, n=5) | 14.66 |
| Angle of reposea | 22.31±0.03 |
| Bulk densitya (g/cm3) | 0.561 |
| Tapped densitya (g/cm3) | 0.583 |
| Carr’s indexa (% ) | 3.99 |
| Hausner’s ratioa | 1.042±0.03 |
| Friabiltya | 0.09% |
| Content uniformityb (%, n=5) | 119.67±2.66 |
| Disintegration timeb (min) | 10.02 |
| Weight variationb (%, n=20) | 0.61 |
| Zero-orderb | 0.9714 |
| Sum of square | 33.59 |
| AIC | 32.11 |
Note: aProperties of the pellets and bproperties of the optimized batch
Table 5: Evaluation Parameters of the Optimized Formulation
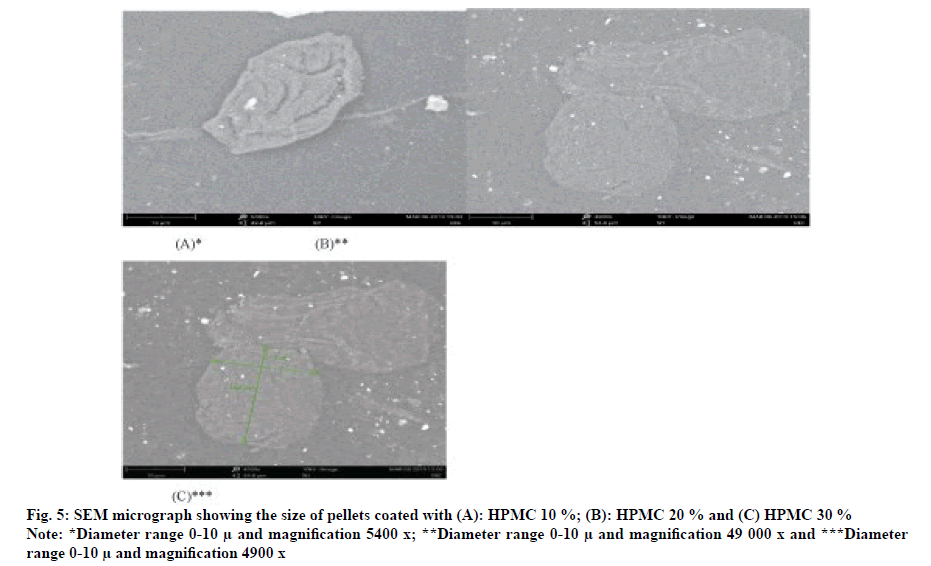
The surface of optimized formulation F9 pellets (HPMC-10 %) is continuous (fig. 4A), but compared with smooth and uniform particles (fig. 5B, fig. 5C), it was coated with 20 % and 30 % HPMC for pilot mixing. The surface of the pill is dense, continuous and uniform. Therefore, at a higher coating level in the pilot mixture, the diffusion length of the dissolution medium in the drug layer and the diffusion of the dissolved drug will increase, which will result in a decrease in the release rate of formulation F9.These optimized timed-release pellets can release drugs in vitro for up to 7 h, which may be better than conventional dosage form, thereby reducing the frequency of dosing and improving patient compliance.
A 23 full- factorial design used for pre-mixing (pilotmix) operations to obtain the desired release (t90) up to 7 h, as it was found to be one of the CPPs for timedrelease pellets. The core, NPS prepared by using a sugar solution. A fluidized bed processor was used to coat diltiazem to the NPS. HPMC E15 used as a coating for NPS, with different percentages (10 % A, 20 % B, and 30 % C), divided into two levels (16.66 % and 33. 33 %). According to the design matrix, pilot mixing was carried out for the in vitro dissolution studies and optimized batch was prepared for master blend and mixture based on the results. The Pareto charts supported the ANOVA results for the model simplification and eliminating non-significant terms (p>0.05). The optimal process variable (independent variable) selected to optimize the formulation of diltiazem timed-release pellets was set as the pilot mix I=24.9 %, pilot mix II=16.6 % and pilot mix III=16.6 %. Therefore, the pilot blend composed of 58.1 % of A, B and C coated pellets, and the remaining 41.9 % is uncoated NPS. The optimized pilot blend showed desired release pattern of t50 was 2.361±0.243 h, t90 is 6.875 h.
Acknowledgements
The authors thank FOURTTS (INDIA) PVT LTD, for permitted us to carry out the entire project work. The authors also thank T.K computers for assisting in drawing pictures and tables.
Conflict of interests
The authors declared no conflict of interest.
References
- Abdul S, Chandewar AV, Jaiswal SB. A flexible technology for modified-release drugs: Multiple-unit pellet system (MUPS). J Control Release 2010;147(1):2-16.
[Crossref] [Google Scholar] [PubMed]
- Chen T, Li J, Chen T, Sun CC, Zheng Y. Tablets of multi-unit pellet system for controlled drug delivery. J Control Release 2017;262:222-31.
[Crossref] [Google Scholar] [PubMed]
- Al-Hashimi N, Begg N, Alany RG, Hassanin H, Elshaer A. Oral modified release multiple-unit particulate systems: Compressed pellets, microparticles and nanoparticles. Pharmaceutics 2018;10(4):176-82.
[Crossref] [Google Scholar] [PubMed]
- Zakowiecki D, Frankiewicz M, Hess T, Cal K, Gajda M, Dabrowska J, et al. Development of a biphasic-release multiple-unit pellet system with diclofenac sodium using novel calcium phosphate-based starter pellets. Pharmaceutics 2021;13(6):805-11.
[Crossref] [Google Scholar] [PubMed]
- Newton JM. Gastric emptying of multi-particulate dosage forms. Int J Pharm 2010;395(1-2):2-8.
[Crossref] [Google Scholar] [PubMed]
- Lakio S, Heinämäki J, Yliruusi J. Colorful drying. AAPS PharmSciTech 2010;11(1):46-53.
[Crossref] [Google Scholar] [PubMed]
- Elsergany RN, Chan LW, Heng PW. Influence of the porosity of cushioning excipients on the compaction of coated multi-particulates. Eur J Pharm Biopharm 2020;152:218-28.
- International Conference on Harmonisation (ICH) of Technical Requirements for Registration of Pharmaceuticals for Human Use, ICH Harmonised Tripartite Guideline: Q8(R2) Pharmaceutical Development, 2009.
- Dejaegher B, Vander Heyden Y. Experimental designs and their recent advances in set-up, data interpretation and analytical applications. J Pharm Biomed Anal 2011;56(2):141-58.
[Crossref] [Google Scholar] [PubMed]
- Grangeia HB, Silva C, Simões SP, Reis MS. Quality by design in pharmaceutical manufacturing: A systematic review of current status, challenges and future perspectives. Eur J Pharm Biopharm 2020;147:19-37.
- Cunha S, Costa CP, Moreira JN, Lobo JM, Silva AC. Using the quality by design (QbD) approach to optimize formulations of lipid nanoparticles and nanoemulsions: A review. Nanomedicine 2020;28:102206.
[Crossref] [Google Scholar] [PubMed]
- Soni G, Kale K, Shetty S, Gupta MK, Yadav KS. Quality by design (QbD) approach in processing polymeric nanoparticles loading anticancer drugs by high pressure homogenizer. Heliyon 2020;6(4):e03846.
[Crossref] [Google Scholar] [PubMed]
- Albadry MA, Khan IA. Roadmap for quality by design implementation for dietary supplements. J AOAC Int 2020;103(1):103-16.
[Crossref] [Google Scholar] [PubMed]
- Santos B, Carmo F, Schlindwein W, Muirhead G, Rodrigues C, Cabral L, et al. Pharmaceutical excipients properties and screw feeder performance in continuous processing lines: A Quality by Design (QbD) approach. Drug Develop Ind Pharm 2018;44(12):2089-97.
[Crossref] [Google Scholar] [PubMed]
- Xie Z, Zhang J, Wang C, Yan X. Chronotherapy for morning blood pressure surge in hypertensive patients: A systematic review and meta-analysis. BMC Cardiovasc Disord 2021;21(1):1-1.
[Crossref] [Google Scholar] [PubMed]
- Emara LH, El-Ashmawy AA, Taha NF. Stability and bioavailability of diltiazem/polyethylene oxide matrix tablets. Pharm Dev Technol 2018;23(10):1057-66.
[Crossref] [Google Scholar] [PubMed]
- Prasad MB, Vidyadhara S, Sasidhar RL, Balakrishna T, Trilochani P. Development and evaluation of diltiazem hydrochloride controlled-release pellets by fluid bed coating process. J Adv Pharm Technol Res 2013;4(2):101-7.
[Crossref] [Google Scholar] [PubMed]
- Shin TH, Im SH, Goh MS, Lee ES, Ho MJ, Kim CH, et al. Novel extended-release multiple-unit system of imidafenacin prepared by fluid-bed coating technique. AAPS PharmSciTech 2018;19(6):2639-45.
[Crossref] [Google Scholar] [PubMed]
- Shin TH, Ho MJ, Kim SR, Im SH, Kim CH, Lee S, et al. Formulation and in vivo pharmacokinetic evaluation of ethyl cellulose-coated sustained release multiple-unit system of tacrolimus. Int J Biol Macromol 2018;109:544-50.
[Crossref] [Google Scholar] [PubMed]
- Killivalavan P, Kumaravelrajan R, Gopi M, Suba V. Development of nifedipine timed-release spansule dosage form by extrusion-spheronization technology. Asian J Pharm 2017;11(3):192-204.
- Brar V, Kaur G. Thiolated okra chitosan nanoparticles: Preparation and optimisation as intranasal drug delivery agents. J Microencapsul 2020;37(8):624-39.
[Crossref] [Google Scholar] [PubMed]
- Varma MV, Gardner I, Steyn SJ, Nkansah P, Rotter CJ, Whitney-Pickett C, et al. pH-Dependent solubility and permeability criteria for provisional biopharmaceutics classification (BCS and BDDCS) in early drug discovery. Mol Pharm 2012;9(5):1199-212.
[Crossref] [Google Scholar] [PubMed]
- Mircioiu C, Anuta V, Mircioiu I, Nicolescu A, Fotaki N. In vitro–in vivo correlations based on in vitro dissolution of parent drug diltiazem and pharmacokinetics of its metabolite. Pharmaceutics 2019;11(7):344-55.
[Crossref] [Google Scholar] [PubMed]
- Siepmann J, Karrout Y, Gehrke M, Penz FK, Siepmann F. Predicting drug release from HPMC/lactose tablets. Int J Pharm 2013;441(1-2):826-34.
[Crossref] [Google Scholar] [PubMed]
- Elfiky M, Salahuddin N, Matsuda A. Green fabrication of 3D hierarchical blossom-like hybrid of peeled montmorillonite-ZnO for in vitro electrochemical sensing of diltiazem hydrochloride drug. Mater Sci Eng C Mater Biol Appl 2020;111:110773.
[Crossref] [Google Scholar] [PubMed]
- Malakar J, Nayak AK, Goswami S. Use of response surface methodology in the formulation and optimization of bisoprolol fumarate matrix tablets for sustained drug release. ISRN Pharm 2012;2012.
[Crossref] [Google Scholar] [PubMed]