- *Corresponding Author:
- Lianhua Chen
Department of Anesthesiology, Shanghai General Hospital of Nanjing Medical University, Hongkou, Shanghai 200080, China
E-mail: emily19941010@126.com
| Date of Received | 26 December 2022 |
| Date of Revision | 21 May 2023 |
| Date of Accepted | 10 November 2023 |
| Indian J Pharm Sci 2023;85(6):1669-1676 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To compare the anesthesia effect of compound ketamine and pentobarbital sodium in orthopedic surgery, 30 specific pathogen free C57BL/6J male mice were included and randomly enrolled into group A (compound ketamine group) and group B (pentobarbital sodium group). The changes of respiratory frequency, heart rate, body temperature, blood oxygen saturation, anesthesia duration, and anesthesia depth among mice in the two groups were compared. It was demonstrated that respiratory frequency declined in the two groups. Heart rate showed no apparent changes as anesthesia was continued. Heart rate was high in group A at 50 min while it was low in group B at 90 min. Body temperature among mice in the two groups generally declined. After 90 min, it dramatically declined in group A. Body temperature was the lowest in group B at 90 min. Blood oxygen saturation generally raised in the two groups. As to anesthesia duration, induction and anesthesia periods were relatively longer in group B. The score for anesthesia depth of group B remained above 3 points 20 min after rectification disappeared and anesthesia duration of group B was notably longer. The research findings revealed that compound ketamine and pentobarbital sodium showed excellent anesthesia effects and physiological indicators. The latter one was characterized by long anesthesia duration, low safety, and high fatality.
Keywords
Ketamine, pentobarbital sodium, orthopedic surgery, anesthesia
The observation period of cartilage defect and repair experiment is long. Medium and small animals rather than high-cost large animals are widely applied in clinical orthopedic treatment and scientific research. Mice are commonly used in laboratory study and the main anesthetics for mice include chloral hydrate and Rompun®[1,2]. Post-anesthesia surgeries mainly include incision of trachea and enter ostomy, which are featured with short duration and mild pain. Chloral hydrate and Rompun® have significant anesthesia effects. However, orthopedic surgery leads to severe pain and lasts for a long time so that the above anesthesia methods are not effective. Anesthesia is an essential procedure for mammal experiment. Inappropriate anesthesia may result in near-death state among mammals, which affects experimental results[3-5]. Therefore, safer anesthesia methods should be adopted to promote anesthesia techniques and reduce experimental costs.
Ketamine injection is a compound preparation consisting of ketamine, xylazine, and phenethylpiperate. Ketamine is a nonbarbiturate analgesic that can effectively relieve pain and treat various diseases. It can quickly penetrates blood brain barrier to have an analgesic effect on central and peripheral nerves[6]. In addition, it has few inhibition effects on respiratory system. After intravenous administration, the drug is quickly distributed to all tissues and organs of body. Consequently, the effectiveness lasts for a short time. Metabolic pathways in liver include demethylation and hydroxylation. Under the influence of enzyme, the transformation rate of phenyl cyclohexanone reaches 30 % and it completely recovers after 30 min[7]. Ketamine possesses multiple action mechanisms. It can simultaneously have an analgesic effect on cardiac and peripheral nerve network systems. The effect is widely applied in animal experiment. Moreover, ketamine can quickly penetrate blood brain barrier to play several roles in clinical treatment. The recent study shows that ketamine has a remarkable anti-inflammatory effect and can effectively inhibit hyperalgesia, psychiatric symptom, and depression. In addition, it can balance acute inflammatory reflex and dysimmunology because it initiates congenital immune response mechanism[8-10]. Amobarbital sodium is an injection drug and its main component is amobarbital sodium, which appears as white particles or powder[11]. Low-dose amobarbital sodium has sedation and hypnosis effects. In contrast, high-dose amobarbital sodium has anti-spasm and anesthesia effects and anesthesia duration becomes longer. Nonetheless, the induction period becomes longer[12,13]. Amobarbital sodium is highly lipid-soluble and its content is high in brain, liver, and kidney. In most cases, the metabolites of liver function are inactive hydroxyl radical derivatives and a few of them are excreted in the original form with urine through kidney. Nonetheless, conventional pentobarbital sodium anesthesia method possesses many shortcomings, such as long induction period, high requirement of abdominal anesthesia and blood puncture anesthesia, and uncontrollable administration rate and dose. According to related research, pentobarbital sodium can effectively improve anesthesia effects, which last for a long time[14-16].
This research was conducted to compare the anesthesia effects of compound ketamine and pentobarbital sodium in orthopedic surgery to provide valuable references for clinical anesthesia. Based on the comparison of the two anesthetics, the action mechanisms in different situations can be better understood.
Materials and Methods
Laboratory animals:
30 Specific Pathogen Free (SPF) C57BL/6J male mice were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. They were aged between (9-12) w with an average weight of 26 g. Laboratory mice were fed in independent ventilation cages of animal laboratory equipped with SPF barriers. They were offered free access to food and water and the feeding temperature and humidity were kept at 25° and about 55 % with an alternation of day and night every 12 h. 30 mice were randomly enrolled into group A (compound ketamine group) and group B (pentobarbital sodium group).
Experimental drugs:
The experimental drugs included 2 ml compound ketamine injection (veterinary drug approval number (2015): 060022095, Shenyang Veterinary Pharmaceutical Factory), pentobarbital sodium powder (Beijing Propbs Biotechnology Co., Ltd.), and 0.9 % sodium chloride injection (N21082201B, Kelun Pharmaceutical Co., Ltd.). The concentrations of all the above drugs were made or diluted to the required levels using sterile sodium chloride injection.
Experimental methods:
Abdominal injection was performed on the mice in group A at a dose of 90 mg/kg. Before anesthesia, fasting was carried out for 1 d and water deprivation was implemented for 12 h. Then, weighing was performed. Next, indwelling venous catheter was inserted into left forelimb vein for intravenous anesthesia and first aid pathway. After the mice stayed quiet, blood oxygen saturation monitor was installed and all pre-anesthesia physiological indicators were recorded.
Before anesthesia, the mice in group B underwent fasting for 12 h and water deprivation for 4 h. Next, 3 ml/kg 2 % pentobarbital sodium was prepared and then 2 g pentobarbital sodium powder was weighed using an electronic scale. After that, it was mixed with 100 ml distilled water. Then, the solution was filtered with cell sieve before the injecting into mice muscle. Finally, the drugs took effect.
Indicator monitoring:
Induction time recording: The starting time of administration was recorded. After the administration, mice were allowed to roam freely. The occurrence of the 1st righting reflex suggested that mice generally entered anesthesia stage. In this case, forepaw toes of mice were clamped with a hemostatic force. If mice made no response, they formally entered anesthesia stage. After that, the duration from the beginning of administration to no reaction was recorded as the induction period.
Body position fixation: After mice completely entered anesthesia stage, mice tongue was pulled out using a tweezer and it was fixed on one side to prevent glossoptosis, airway blockage, asphyxia, and even death. Then, a large sheet was spread on the operating table and mice were moved onto the operating table with the assistants. Next, mice were immobilized in supine position with their heads tilted back and the airway open.
Surgery after sheet disinfection: The surgical area was disinfected with iodophor and the sheet was spread. Next, the surgery was performed (femoral trochlea cartilage defect model).
Intraoperative monitoring: The multifunctional vital sign monitor was connected to detect Heart Rate (HR) and blood oxygen saturation. Then, the researchers worked with the assistants to record HR, blood oxygen saturation, respiratory frequency, and body temperature every 10 min. During the surgery, they should always pay attention to mice tongue to prevent airway blockage caused by glossoptosis.
Postoperative treatment:
Resuscitation: After the surgery, the duration from toe-pinch reflex to free activity was recorded as recovery period for each mouse.
Postoperative biochemical detection: Ear venous blood was extracted 24 h after the surgery for postoperative chemical analysis and testing. Then, mice ears were disinfected with iodophor. Next, ear vein was punctured using a 5 ml syringe and about 0.5 ml venous blood was extracted. After the needle was removed, the extracted venous blood was injected into 1.5 ml centrifuge tube containing anticoagulant and they were thoroughly mixed upside down. In addition, mice numbers were labeled on the centrifuge tubes with a marker. Next, 100 μl postoperative mice venous blood was extracted using 200 μl pipette and instilled on a health 16-item quantitative detection reagent tray. After that, it was placed into a fully automatic biochemical analyzer. The parameters were set as mice mode and named. Next, the fully automatic biochemical analyzer was started. After chemical analysis indicators were analyzed, laboratory test report was printed out and data were collected.
Observation indicators: The laboratory mice were placed in a quiet and clean room, and allowed to roam freely. After they were stabilized, pre-anesthesia body temperature, blood oxygen saturation, HR, and respiratory frequency were detected. After that, 0.5 ml ear venous blood was extracted for biochemical testing. Then, anesthesia was carried out. Mice reflex and all physiological indicators were monitored 10 min, 20 min and 30 min, (every 10 min) after the anesthesia. What’s more, ear venous blood was extracted from mice that were awake for 24 h for biochemical testing.
Monitoring: Induction, anesthesia, and recovery periods were recorded. The induction period stared from the beginning of administration to first righting reflex. The anesthesia period included the entire processes of toe-pinch reflex and surgery. The recovery period started from toe-pinch reflex to free activity.
Statistical methods:
IBM Statistical Package for Social Sciences (SPSS) Statistics 26 was employed to analyze experimental data. The test level was set as Alpha (α)=0.05. p<0.05 suggested that differences revealed statistical significance. Measurement indicators were denoted by mean±standard deviation (x±s) and data distribution was performed with test of normality and homogeneity test for variance. Pre and post-interventional target angles of all groups were compared using matched samples t-test and the differences between two groups were compared using independent sample t-test. Data among multiple groups were compared using one-factor analysis of variance. Postoperative comparison was carried by Least Significant Difference (LSD) method. Different therapeutic plans were compared based on analysis of variance of factorial design to determine the main, individual, and interaction effects of different therapeutic parameters. The correlation was analyzed based on Spearman correlation analysis method. If data showed skewed distribution, Wilcoxon rank-sum test of non-parametric test was conducted.
Results and Discussion
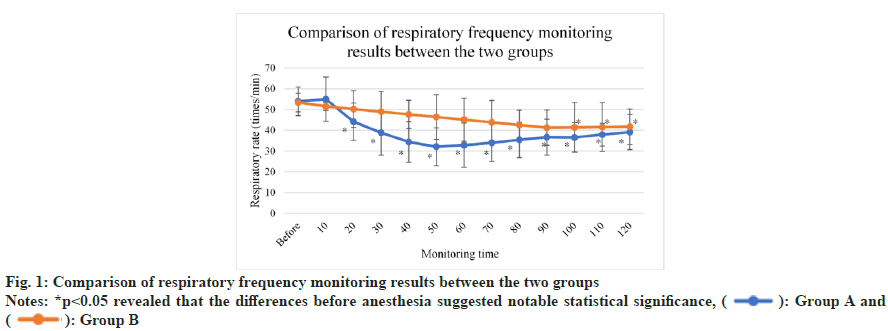
As illustrated in fig. 1, respiratory depression was remarkable among mice in group A and group B.
As anesthesia progressed, respiratory frequency declined in the two groups. The differences before and after anesthesia indicated notable statistical significance (p<0.05). After anesthesia, respiratory frequency declined (p>0.05).
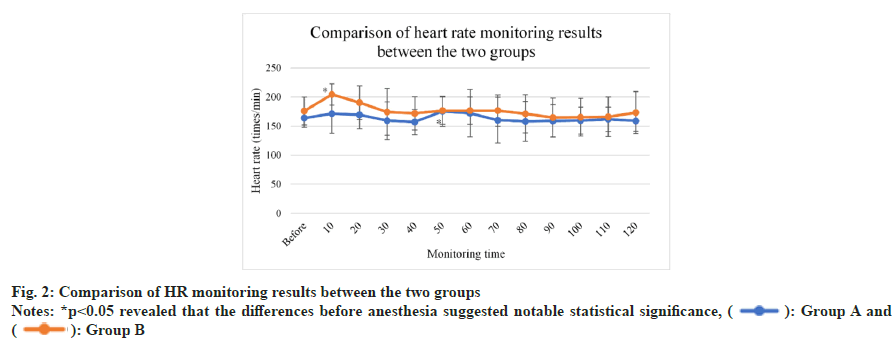
As presented in fig. 2, no notable changes were detected in HR among mice in group A and group B. HR was high among mice in group A at 50 min (p<0.05). In contrast, it was low among those in group B at 90 min (p<0.05). No statistical significance was detected in the difference in HR between the two groups at other time points before and after anesthesia (p>0.05) and so was the case with the difference in HR (p>0.05).
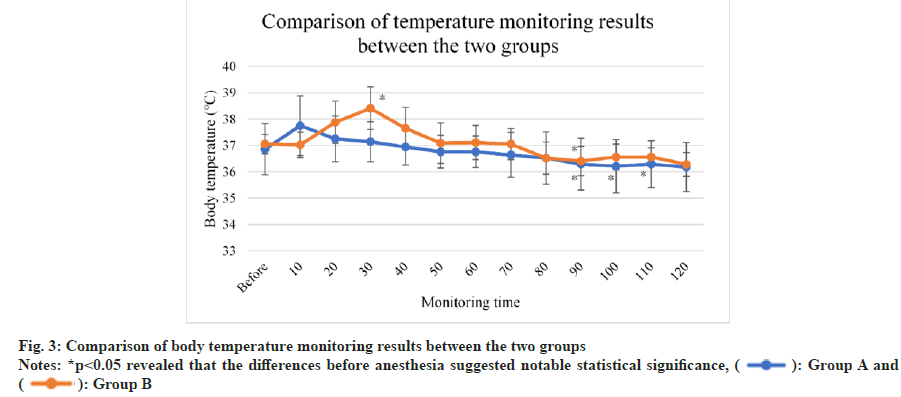
As displayed in fig. 3, body temperature generally declined among mice in group A and group B, and it dramatically dropped down among mice in group A after 90 min (p<0.05) and reached the lowest among mice in group B at 90 min (p<0.05). In group B, body temperature slightly rose after 90 min. No statistical significance was detected in the difference between the two groups (p>0.05).
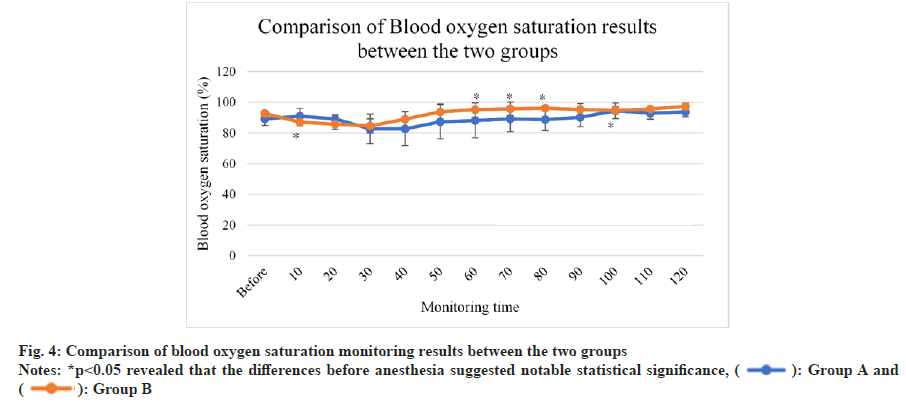
As illustrated in fig. 4, blood oxygen saturation generally raised among mice in group A and group B. It apparently increased among mice in group A after 50 min and the difference between that at 100 min and before anesthesia suggested statistical significance (p<0.05). In contrast, it notably raise among mice in group B after 40 min, while slightly declined after 70 min.
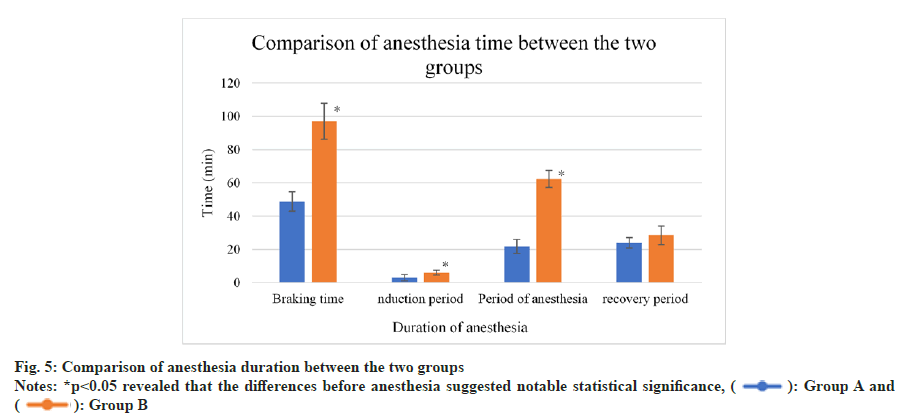
As displayed in fig. 5, braking time was relatively shorter in group A, while induction and anesthesia periods were longer in group B (p<0.05).
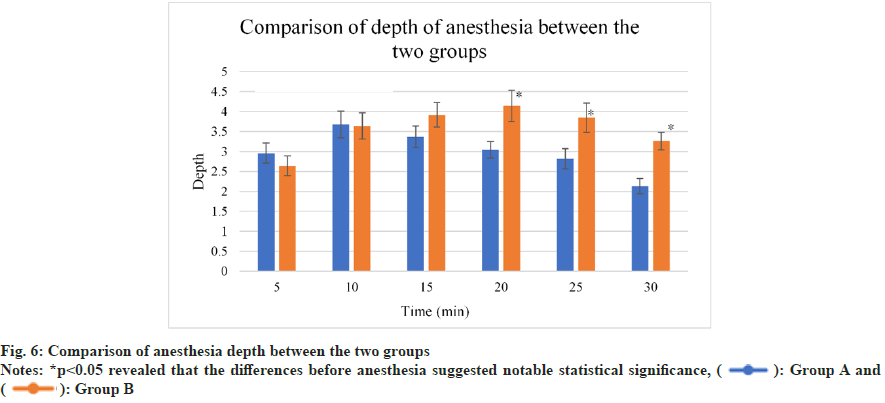
The scores for anesthesia depth of the two groups were displayed in fig. 6. The scores for group A were >3 points 5 min and longer after righting reflex disappeared; while those for group B were <3 points at the same periods (the requirement of surgical anesthesia was not met). The scores for group A were <3 points 20 min after righting reflex disappeared. In this case, mice entered recovery period. In contrast, the scores for group B remained above 3 points and anesthesia duration was relatively longer (p<0.05).
Ketamine has a strong excitation effect and can rapidly induce anesthesia. Pentobarbital sodium has significant long-term sedation and hypnosis effects. Hence, it is usually applied in longterm anesthesia. However, induction is timeconsuming[17]. Low-dose compound ketamine (less than half of the original lowest dose) has the same anesthesia effect with routine dose without excessive respiratory depression and bradycardia. The research findings demonstrated that ketamine could effectively alleviate cardiac function depression caused by xylazine hydrochloride injection and has few adverse effects on respiratory system[18,19]. What’s more, no remarkable changes were detected in respiratory system monitoring after the anesthesia by compound ketamine. Some research demonstrates that there seems to be a slight difference between the dose of pentobarbital sodium for surgical anesthesia and that leading to respiratory arrest[20,21]. Because pentobarbital sodium only has limited analgesic effect, higher dose of pentobarbital sodium is required when more stimulus were applied, such as pinching ears. Consequently, the incidence of respiratory arrest becomes higher. In addition, it is verified that pentobarbital sodium causes no impacts on ventilation, respiration, and circulation[22].
According to the research findings, xylazine hydrochloride injection has remarkable effects on respiratory depression[23,24]. In the experiment, the change of HR during compound ketamine anesthesia was consistent with previously reported result. HR declined among the subjects in the two groups, suggesting that compound ketamine anesthesia possessed remarkable therapeutic effect on cardiovascular system. During the early stage of anesthesia, pentobarbital sodium might result in slight rise of HR. As anesthesia progressed, it dramatically declined. In the whole process of anesthesia, HR of compound ketamine group ranged from 50 to 60 times/min. The stability of HR in compound ketamine group was related to ketamine in compound ketamine[25]. In general, ketamine could effectively alleviate bradycardia, but its effect can’t be completely eliminated. Therefore, it is safer and more reliable to apply compound ketamine in anesthesia for cardiovascular system.
Anesthetics may inhibit central nervous system and affect thermoregulation center in hypothalamus. Consequently, the ability to regulate body temperature may be attenuated during anesthesia. In addition, anaesthetics reduce basal metabolic rate of animals. Heat loss and environmental temperature in operating room reduce body temperature[26]. Xylazine hydrochloride is a α2 adrenoreceptor agonist that has apparent soothing and sedative effects on central muscle relaxation. In contrast, benzoate hydrochloride enhances therapeutic effect and the excitability of parasympathetic nerve. Xylazine hydrochloride injection can effectively prevent heat production in animal bodies. Before anesthesia, laboratory dogs should under fasting and water deprivation for 8 h. Because the basal metabolic rate is low and they get little heat energy, body temperature is unstable. Hence, thermal insulation should be carried out and the duration of nonsensical fasting should be minimized during clinical surgery.
Changing body temperature may indicate physical health condition[27,28]. Hyperthermia usually results from infectious diseases or other causes. During anesthesia, hypothermia may occur in laboratory animals, which may be caused by low environmental temperature, surgical conditions, anesthesia methods and patient condition. It was revealed that the decline in body temperature might lead to a variety of complications, especially the change of the circulation in cardiovascular system. The above complications are detrimental to animals with serious illness or myocardial lesions[29]. Moreover, more oxygen is consumed by tissues, liver and kidney functions are impaired, and immune system is affected. In the experiment, no remarkable difference was detected in body temperature between the two groups. Body temperature dramatically declined and remained at about 36° in the two groups.
With the development of modern clinical treatment and medicine, animal experiment plays more and more significant role in biomedical experiments and study, and new technologies and drug screening are widely applied in clinical treatment. Mammal experimental study is one of the steps of the test and mammal mode is the key factor for test quality, process and outcome. They can be applied for orthopedic surgery, systemic diseases and spontaneous mammal mode. Anesthesia quality directly affects experimental outcome[30,31]. In particular, experimental animal models are widely applied in orthopedic surgery in surgical research. The anesthesia requirement for experimental animal models is the highest.
Reasonable orthopedic anesthesia effect should possess the following characteristics, short induction period and long anesthesia duration; strong soothing and analgesic effects without dysphoria and other adverse reactions; significant effect on muscle relaxation and short recovery period, few postoperative adverse effects, few toxic effects and low mortality.
In this research, it was found that pentobarbital sodium possessed remarkable sedation and hypnosis effects and could be used for long-term anesthesia. Nevertheless, it led to long induction period. Simple pentobarbital sodium could hardly anesthetize mice and some mice jittered. Besides, there seemed to be a slight difference between the dose for surgical anesthesia and that resulting in respiratory arrest, which was consistent with previously reported result[32]. What’s more, pentobarbital sodium has apparent ventilator and respiration depression effects and no impacts on circulation. Compound ketamine could induce the disappearance of righting reflex within 10 s and pain reflex disappeared within 1 min. Nonetheless, nystagmus and head shaking might occur during anesthesia. The induction time of xylazine hydrochloride injection was limited to 10 min. After mice entered anesthesia stage, it would take some time to completely eliminate pain reflex. However, xylazine hydrochloride injection was more effective in muscle relaxation during anesthesia.
According to the experimental results, it was found that compound ketamine and pentobarbital sodium were both excellent in terms of anesthesia effects and physiological indicators. Compound ketamine was safer with few side effects. Hence, it was widely applied in clinical practice. By contrast, pentobarbital sodium was characterized by long anesthesia period, low safety, and high fatality.
Conflict of interests:
The authors declared no conflict of interests.
References
- Carbone L, Austin J. Pain and laboratory animals: Publication practices for better data reproducibility and better animal welfare. PLoS One 2016;11(5):e0155001.
[Google Scholar] [PubMed] [Crossref]
- Erickson RL, Blevins CE, Souza Dyer C, Marx JO. Alfaxalone-xylazine anesthesia in laboratory mice (Mus musculus). J Am Assoc Lab Anim Sci 2019;58(1):30-9.
[Google Scholar] [PubMed] [Crossref]
- Lebaschi A, Deng XH, Coleman NW, Camp CL, Zong J, Carbone A, et al. Restriction of postoperative joint loading in a murine model of anterior cruciate ligament reconstruction: Botulinum toxin paralysis and external fixation. J Knee Surg 2017;30(7):687-93.
[Google Scholar] [PubMed] [Crossref]
- Miller-Rhodes P, Kong C, Baht GS, Saminathan P, Rodriguiz RM, Wetsel WC, et al. The broad spectrum mixed-lineage kinase 3 inhibitor URMC-099 prevents acute microgliosis and cognitive decline in a mouse model of perioperative neurocognitive disorders. J Neuroinflammation 2019;16(1):1-15.
[Google Scholar] [PubMed] [Crossref]
- Adıyaman D, Kuyucu M. Difference between near-field and far-field of middle cerebral artery doppler pulsatility index and peak systolic velocity: A prospective study. Clin Exp Obstet Gynecol 2021;48(1):66-72.
- Cakir L, Aktas G, Enginyurt OZ, Cakir SA. Mean platelet volume increases in type 2 diabetes mellitus independent of HbA1c level. Acta Medica Mediterranea 2014;30(2):425-8.
- Sun L, Dong R, Xu X, Yang X, Peng M. Activation of cannabinoid receptor type 2 attenuates surgery-induced cognitive impairment in mice through anti-inflammatory activity. J Neuroinflammation 2017;14(1):138.
[Google Scholar] [PubMed] [Crossref]
- Wang P, Velagapudi R, Kong C, Rodriguiz RM, Wetsel WC, Yang T, et al. Neurovascular and immune mechanisms that regulate postoperative delirium superimposed on dementia. Alzheimers Dement 2020;16(5):734-49.
[Google Scholar] [PubMed] [Crossref]
- Nunes IK, de Souza ET, Cardozo SV, Carvalho VF, Romeiro NC, Silva PM, et al. Synthesis, pharmacological profile and docking studies of new sulfonamides designed as phosphodiesterase-4 inhibitors. PLoS One 2016;11(10):e0162895.
[Google Scholar] [PubMed] [Crossref]
- Siriarchavatana P, Ayers JD, Kendall LV. Anesthetic activity of alfaxalone compared with ketamine in mice. J Am Assoc Lab Anim Sci 2016;55(4):426-30.
[Google Scholar] [PubMed]
- Verdoodt D, Eens S, van Dam D, de Deyn PP, Vanderveken OM, Szewczyk K, et al. Effect of oral allylnitrile administration on cochlear functioning in mice following comparison of different anesthetics for hearing assessment. Front Toxicol 2021;3:641569.
[Google Scholar] [PubMed] [Crossref]
- Villetti G, Carnini C, Battipaglia L, Preynat L, Bolzoni PT, Bassani F, et al. CHF6001 II: A novel phosphodiesterase 4 inhibitor, suitable for topical pulmonary administration-in vivo preclinical pharmacology profile defines a potent anti-inflammatory compound with a wide therapeutic window. J Pharmacol Exp Ther 2015;352(3):568-78.
[Google Scholar] [PubMed] [Crossref]
- Xia C, He JP, Feng KW, Liu L, Zheng L, Wang HT, et al. Discovery of novel 3-Amino-4-alkoxyphenylketones as PDE4 inhibitors with improved oral bioavailability and safety against spatial memory impairments. ACS Chem Neurosci 2022;13(3):390-405.
[Google Scholar] [PubMed] [Crossref]
- Zheng Z, Kyzer JL, Worob A, Wenthur CJ. Family of structurally related bioconjugates yields antibodies with differential selectivity against ketamine and 6-hydroxynorketamine. ACS Chem Neurosci 2021;12(21):4113-22.
[Google Scholar] [PubMed] [Crossref]
- Deciga-Campos M, Navarrete-Vazquez G, López-Muñoz FJ, Librowski T, Sanchez-Recillas A, Yanez-Pérez V, et al. Pharmacological profile of N-(2,6-dichlorophenyl)-2-(4-methyl-1-piperidinyl) acetamide, a novel analogue of lidocaine. Life Sci 2016;155:48-55.
[Google Scholar] [PubMed] [Crossref]
- Feridooni HA, MacDonald JK, Ghimire A, Pyle WG, Howlett SE. Acute exposure to progesterone attenuates cardiac contraction by modifying myofilament calcium sensitivity in the female mouse heart. Am J Physiol Heart Circ Physiol 2017;312(1):H46-59.
[Google Scholar] [PubMed] [Crossref]
- Gomes MM, Wang L, Jiang H, Kahl CA, Brigande JV. A rapid, cost-effective method to prepare recombinant adeno-associated virus for efficient gene transfer to the developing mouse inner ear. Methods Mol Biol 2016;1427:43-57.
[Google Scholar] [PubMed] [Crossref]
- Gui MX, Huang B, Peng J, Chen X, Muthu R, Gao Y, et al. Babao dan alleviates 5-fluorouracil-induced intestinal damage via wnt/β-catenin pathway. Chin J Integr Med 2022;28(11):1000-6.
[Google Scholar] [PubMed] [Crossref]
- Kageyama A, Tohei A, Ushijima H, Okada K. Anesthetic effects of a combination of medetomidine, midazolam and butorphanol on the production of offspring in Japanese field vole, Microtus montebelli. J Vet Med Sci 2016;78(8):1283-91.
[Google Scholar] [PubMed] [Crossref]
- Laferriere CA, Leung VS, Pang DS. Evaluating intrahepatic and intraperitoneal sodium pentobarbital or ethanol for mouse euthanasia. J Am Assoc Lab Anim Sci 2020;59(3):264-8.
- Li C, Dai J, Wu F, Zhang H. Impacts of different anesthetic agents on left ventricular systolic function in mice assessed by echocardiography. Physiol Res 2019;68(3):365-74.
[Google Scholar] [PubMed] [Crossref]
- Li Z, Cheng JM, Peng HX, Jiang XY, Gong J, Xiao M. Sini decoction inhibits TLR4/NF-κB signaling pathway to improve airway remodeling of allergic asthmatic mice. Zhongguo Zhong Yao Za Zhi 2022;47(22):6191-8.
[Google Scholar] [PubMed] [Crossref]
- Ma D, Yoon SI, Yang CH, Marcy G, Zhao N, Leong WY, et al. Rescue of methyl-cpg binding protein 2 dysfunction-induced defects in newborn neurons by pentobarbital. Neurotherapeutics 2015;12(2):477-90.
[Google Scholar] [PubMed] [Crossref]
- Gonzalez-Diaz A, Medina-Polo J, Abad-Lopez P, Gonzalez-Padilla DA, Garcia-Rojo E, Santos-Perez de la Blanca R, et al. The challenge of Pseudomonas aeruginosa related infections in a urology ward: Epidemiology, risk factors and patterns of antibiotic resistance. Arch Esp Urol 2020;73(4):299-306.
[Google Scholar] [PubMed]
- Miwa Y, Tsubota K, Kurihara T. Effect of midazolam, medetomidine and butorphanol tartrate combination anesthetic on electroretinograms of mice. Mol Vis 2019;25:645-53.
[Google Scholar] [PubMed]
- Yang J, Zheng X, Zhang S, Wang H. Epidemiology-based analysis of characteristics of dual infection of tuberculosis/Acquired Immune Deficiency Syndrome (AIDS) and drug resistance mechanism of related genes. Cell Mol Biol 2022;68(2):109-18.
[Google Scholar] [PubMed] [Crossref]
- Niles DJ, Gordon JW, Fain SB. Effect of anesthesia on renal R2* measured by blood oxygen level-dependent MRI. NMR Biomed 2015;28(7):811-7.
[Google Scholar] [PubMed] [Crossref]
- Tsukamoto A, Serizawa K, Sato R, Yamazaki J, Inomata T. Vital signs monitoring during injectable and inhalant anesthesia in mice. Exp Anim 2015;64(1):57-64.
[Google Scholar] [PubMed] [Crossref]
- Wang X, Guo J, Deng X, Huang Y, Ye C, Liang H, et al. Comparative safety assessments of the biosimilar APZ001 and Erbitux in pre-clinical animal models. Acta Cir Bras 2018;33(8):690-702.
[Google Scholar] [PubMed] [Crossref]
- Carramolino-Cuéllar E, Lauritano D, Carinci F, Silvestre-Rangil J, Bañuls-Morant C, Silvestre FJ, et al. Salivary glucose as a metabolic control marker in patients with type 2 diabetes. J Biol Regul Homeost Agents 2017;31(2):181-7.
[Google Scholar] [PubMed] [Crossref]
- Xiong C, Liu J, Lin D, Zhang J, Terrando N, Wu A. Complement activation contributes to perioperative neurocognitive disorders in mice. J Neuroinflammation 2018;15(1):254.
[Google Scholar] [PubMed] [Crossref]
- Zhang C, Chen D, Gu Y, Wang T, Wang C. Effects of lncRNA GAS5/miR-137 general anesthesia on cognitive function by TCF4 inflammatory bodies in patients undergoing lumbar spinal canal decompression. Medicine 2022;101(49):e31880.
[Google Scholar] [PubMed] [Crossref]
 ): Group A and
(
): Group A and
( ): Group B
): Group B