- *Corresponding Author:
- Qian Zhang
Department of Respiratory and Critical Care Medicine, The First Central Hospital Affiliated to Nankai University, Nankai, Tianjin 300052, China
E-mail: jenna9898@163.com
| This article was originally published in a special issue,“Exploring the Role of Biomedicine in Pharmaceutical Sciences” |
| Indian J Pharm Sci 2024:86(1) Spl Issue “287-293” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Idiopathic pulmonary fibrosis is a fatal and irreversible lung disease with limited therapeutic options. It has always been a difficult problem that we cannot overcome and there is a need for new therapies in clinical treatment. More and more experimental data suggests that the histone deacetylase family of transcriptional corepressors has emerged as crucial mediators of idiopathic pulmonary fibrosis pathogenesis. In order to clarify the role of histone deacetylase inhibitors in pulmonary fibrosis, we searched the database for relevant literatures on histone deacetylase inhibitors and pulmonary fibrosis, and summarized the latest literatures. Epigenetic regulation, such as histone modification and deoxyribonucleic acid methylation, plays an increasingly important role in idiopathic pulmonary fibrosis. Through the regulation of histone modification, we can affect the occurrence and development of idiopathic pulmonary fibrosis. Histone deacetylases not only participates in the development of tumor, but also they are proved to be related to the progress of organ fibrosis. Histone deacetylase inhibitors affect the development of protein function by changing the acetylation level of histone or non-histones. At present, histone deacetylase inhibitors and gene transcription are mostly used in the treatment of skin lymphoma. Many studies have shown that there is a common signal pathway in the occurrence of tumor and lung fiber. By summarizing the literature, upregulated histone deacetylase activities are observed in fibrotic diseases involving the heart, liver, kidneys and lungs. We suggest the significant imbalance of histone deacetylase activity in the onset and progression of idiopathic pulmonary fibrosis lungs. Therefore, the role of histone deacetylase inhibitors in pulmonary fibrosis needs a new understanding and we have also supported histone deacetylase inhibitors in targeting class I histone deacetylase activity in fibroblasts as a therapeutic option and a promising field for idiopathic pulmonary fibrosis patients.
Keywords
Idiopathic pulmonary fibrosis, histone deacetylase inhibitors, fibroblasts, myofibroblasts
Idiopathic Pulmonary Fibrosis (IPF) is a progressive interstitial lung disease that is characterized with airway epithelial cell injury, fibroblast and myofibroblast aggregation, inflammatory cell infiltration, and extracellular matrix deposition[1]. The incidence rate of IPF is 50/10 000, with an average survival time of 3-5 y[2]. It also has a high mortality rate. At present, IPF is commonly managed with therapeutics like antioxidants (acetylcysteine), glucocorticoids (methylprednisolone), immunosuppressants (cyclophosphamide, azathioprine, methotrexate), new anti-pulmonary fibrosis drugs (pirfenidone, nintedanib) and so on. However, whether used alone or in combination, the outcome of these therapies is poor. In most cases, the pulmonary fibrosis progression does not cease and the patient ultimately dies. The potential multifactorial etiology of IPF is still under investigation[3]. In emerging studies, epigenetic regulation like histone modification (acetylation/deacetylation) and Deoxyribonucleic Acid (DNA) Methylation (DM) were reported to contribute to IPF development and progression. Hence, IPF occurrence and advancement may be modulated through histone modification. This study reviews the research progress of Histone Deacetylases (HDACs) and Histone Deacetylase inhibitors (HDACi) in general and in IPF conditions.
Functional Characteristics and Classification of HDACs
HDACs are enzymes that work in consent with Histone Acetylase (HAT) to regulate gene transcription. Gene expression is often modulated by acetylation, phosphorylation and methylation, among which the activities of HATs and HDACs are the most prominent[4]. These enzymes function in a state of dynamic balance, by regulating the number of charged histones, via acetylation and deacetylation of histone lysine residues, in order to alter the histone structure, local state of protein octamer and DNA spatial conformation to affect the binding efficiency of transcription factors that regulate gene transcription[5,6].
Based on the structural and functional homology between HDACs and yeast, HDACs can be classified into four categories. Group I HDACs are similar to Reduced Potassium Dependency 3 (RPD3) and consist of HDAC1, HDAC2, HDAC3 and HDAC8, whereas group II HDACs are similar to Histone Deacetylase 1 (HDA1), and consist of HDAC4, HDAC5, HDAC6, HDAC7, HDAC9 and HDAC10. Among the group II HDACs, there exists further classification. Group IIA has an active catalytic domain similar to HDAC4, HDAC5, HDAC7 and HDAC9, whereas group IIB has two active catalytic domains, similar to HDAC6 and HDAC10. Group III HDACs include silent information regulator 2 related enzymes and Nicotinamide Adenine Dinucleotide (NAD+) dependent HDAC. Lastly, group IV HDACs include HDAC11 and behave differently from group I and II HDACs[7,8]. Each HDAC member offers a unique regulation of gene transcription and is also involved in the modulation of inflammatory responses, cell proliferation, cell differentiation and cell apoptosis[9].
The Relationship Between HDACs and Tumor
The HDACs mediates to loose or compact chromatin structures and regulate multiple cellular functions. Humans possess four categories of HDACs. Type I is found in the nucleus. Type II can translocate from the nucleus to the cytoplasm and vice versa and is expressed in a cell-specific fashion. Type III can be found in small organelles like the mitochondria and exists in the form of Sirtuin (SIRT) 3, SIRT4 and SIRT5. The primary function of HDAC is to serve as a messenger connecting genome to the extracellular environment. Hence, it utilizes numerous downstream target proteins such as Suppressor of Mothers against Decapentaplegic (SMAD) protein family, tumor suppressor protein p53, Heat Shock Proteins (HSPs) and so on, to regulate inflammation, tumor and other diseases via activation of appropriate signaling pathways[10].
The Relationship Between HDACs and Fibrosis
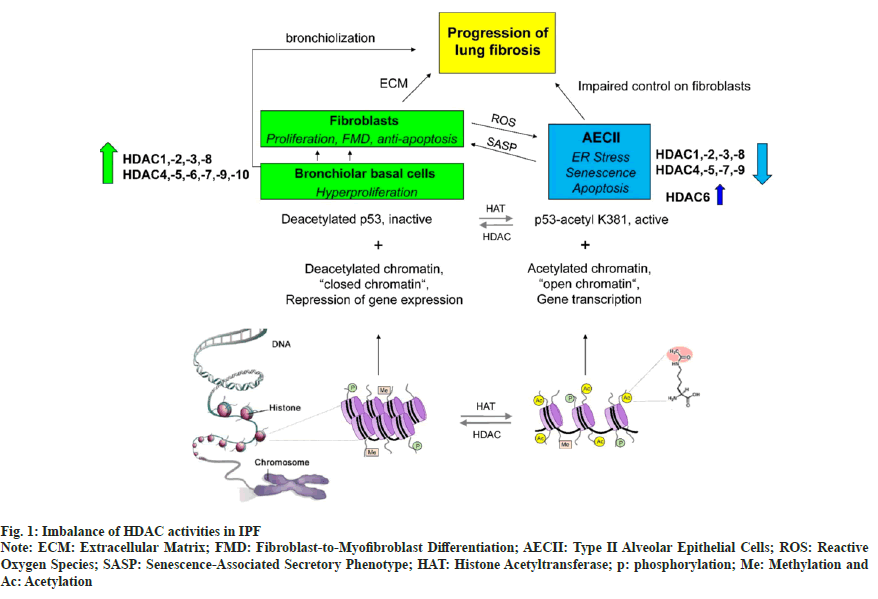
Emerging reports suggest that HDACs not only contribute to tumor development and advancement, but are also related to certain fibrotic diseases[11,12] like IPF, myocardial fibrosis, chronic kidney disease, Systemic Sclerosis (SSc), and pulmonary cystic fibrosis. IPF is characterized by a significant imbalance of HDAC activities, with an abnormal increase of HDAC expression in fibroblasts/ myofibroblasts and bronchiolar basal cells, but lack of HDAC expression in type II Alveolar Epithelial Cells (AECII) is due to Endoplasmic Reticulum (ER stress), senescence and ptosis. This imbalance contributes and perpetuates the fibrotic process as shown in fig. 1[13]. Transforming Growth Factor-beta 1 (TGFβ1) is a powerful fibrogenic agent, which can mediate the transformation of fibroblasts into myofibroblasts. However, it is only partly responsible for the pathogenesis of fibrosis. In patients with IPF, the expression of HDAC1, HDAC2, HDAC3, HDAC8 and SIRT1 increase significantly, particularly in the fibrotic area, along with markedly upregulated nuclear localization of HDAC2 and HDAC3[14]. Moreover, it is reported that HDAC4 can regulate Protein Kinase B (AKT) phosphorylation, stimulate fibroblast differentiation and Epithelial-Mesenchymal Transition (EMT), and inhibit expression of alpha-Smooth Muscle Actin (α-SMA) and collagen[15,16]. Phosphorylation of HDAC4 and AKT is also essential in transforming healthy human lung fibroblasts into myofibroblasts[17]. Phosphorylation and dephosphorylation processes are generally maintained by protein kinase and Protein Phosphatase (PP) and are keys to numerous pathophysiological conditions. PP1 and PP2A are part of the serine/threonine phosphatase family. In vitro, PP2A can dephosphorylate AKT, while PP1 modulates HDAC1 and HDAC6. Moreover, PP activities can be inhibited by small interfering Ribonucleic Acid (siRNA), which increases the interaction between PP1 and AKT. In addition, the expression of TGF-β1-induced α-SMA requires AKT phosphorylation. Several reports also suggest that AKT activation is essential in the differentiation of TGF-β1-regulated lung fibroblasts. Furthermore, this effect can be blocked by HDACi or siRNA. Hence, PP1 and PP2A can reduce TGF-β1-modulated expression of α-SMA, thus inhibiting fibrosis.
Fig. 1: Imbalance of HDAC activities in IPF
Note: ECM: Extracellular Matrix; FMD: Fibroblast-to-Myofibroblast Differentiation; AECII: Type II Alveolar Epithelial Cells; ROS: Reactive Oxygen Species; SASP: Senescence-Associated Secretory Phenotype; HAT: Histone Acetyltransferase; p: phosphorylation; Me: Methylation and
Ac: Acetylation
Functions and Clinical Applications of HDACi
HDACi suppresses HDACs function by concealing the catalytically active fragment of HDACs. Furthermore, it alters histone and non-histone acetylation status to further prevent gene transcription and bring about cell cycle arrest, apoptosis, cell differentiation, autophagy, anti-angiogenesis, and so on. Several studies have established that HDACs can severely reduce levels of Tumor Suppressor Genes (TSGs). Alternately, HDACi can upregulate TSGs levels, delay tumor growth and induce tumor cell death in vitro. Similar to non-pathological conditions, HDACi can inhibit the growth and cytotoxicity of tumor cells via acetylation and modification of target proteins. In fact, scarcely acetylated histone proteins are correlated with development of numerous hematological tumors and malignant solid tumors[18]. At present, HDACi has entered the clinical trial stage as a new class of anti-tumor drugs. It exerts significant inhibitory action on tumor proliferation and angiogenesis, and induces programmed cell death and cell differentiation of a variety of tumor cells, while having almost no effect on healthy cells. Its anti-tumor activity is especially promising due to its high efficiency and low toxicity [19].
Clinical Applications of HDACs
HDACs can be classified into four categories, based on their chemical structures. The first category includes short chain fatty acids like benzoic and isovaleric acid. The second category includes hydroxamic acids like Trichostatin A (TSA), vorinostat (Suberoylanilide Hydroxamic Acid (SAHA)) and Belinostat (PXD101). Interestingly, vorinostat is the first HDACi to be approved by the United States Food and Drug Administration (US FDA) and it treats cutaneous T-cell lymphoma[20]. PXD101, on the other hand, was FDA-approved in 2014 for the management of peripheral T-cell lymphoma[21]. The third category includes cyclic tetrapeptide compounds like Trapoxin (TPX) and Romidepsin (FK228), which can effectively inhibit HDAC1 and HDAC4. Among the third category of HDACi, FK228 was FDA-approved in 2009 and 2011 to treat cutaneous T-cell lymphoma and peripheral T-cell lymphoma, respectively[22,23]. Lastly, the fourth category includes benzamide compounds like Entinostat (MS-275).
New Drug Development of HDACs
Most HDACi can inhibit a variety of HDACs. Unfortunately, due to its lack of specificity, therapies with HDACi may produce rampant toxic side effects. Therefore, it is crucial for doctors to choose selective HDACs for treatment, which can maximize therapeutic effect while minimizing side effects. Recently, a group of specific HDACs have gained much attention. MS-275, for instance, is a specific inhibitor of HDAC1, HDAC2 and HDAC3, and can effectively inhibit renal interstitial fibrosis and vascular remodeling in hypertensive nephropathy. Similarly, tasquinimod is a HDAC4-specific inhibitor, which has been shown to inhibit Vascular Endothelial Growth Factor (VEGF) expression and angiogenesis. Likewise, Tubulin acetylation inducer (Tubacin), a HDAC6-specific inhibitor, regulates the acetylation status of various substrate proteins involved in multiple pathological processes and it further suppress angiogenesis, remodeling, organ fibrosis and extracellular collagen deposition[24-27]. In addition, PCI-34051 is a specific inhibitor of HDAC8, which can regulate the acetylation status of H3K9 and H3K27 sites on histones and contributes to the modulation of T cell differentiation, autoimmune response and smooth muscle cell differentiation[28-31]. Korfei et al.[13] summarizes the broad therapeutic effects of various pan-HDACi on preclinical models of lung fibrosis or IPF in recent years. HDACi for treatment of pulmonary fibrosis/IPF are shown in Table 1[32-47].
| Study | Lung fibrosis model | HDAC inhibition | Effect/Involved molecules |
|---|---|---|---|
| Coward et al.[32] | TGF-β-treated IPF fibroblasts | Panobinostat (LBH589), pan-HDAC | H3 and H4 acetylation at COX-2 promoter, derepression of COX-2 expression |
| Huang et al.[33] | Lung fibroblasts of bleomycin mice, primary IPF fibroblasts | TSA, vorinostat (SAHA), pan-HDAC | H3 acetylation at the Fas/Fas promoter, derepression of Fas/Fas expression |
| Sanders et al.[34] | Primary IPF fibroblasts, bleomycin mouse model | Vorinostat (SAHA), pan-HDAC | In vitro: Proliferation, H3 and H4 acetylation, H3K9Ac↑, BAK↑, BID↑, BCL2L1↓. In vivo: Ameliorated lung fibrosis H3K9Ac↓ and BAK+BCL2L1↓ |
| Korfei et al.[14] | Primary IPF fibroblasts | Panobinostat (LBH589), pan-HDAC | Tubulin acetylation↑, H3K27 acetylation, CIP1/p21↑, CHOP↑, proliferation (CCND1)↓, FMD (ACTA2, COL1A1, COL1A3, FN)↓, surviving↓, BCL-XL↓ |
| Zhang et al.[35] | Primary IPF fibroblasts, bleomycin mouse model | Vorinostat (SAHA), pan-HDAC | In vitro: H3 and H4 acetylation, COL3A1 (mRNA and protein)↓, In vivo: Ameliorated lung fibrosis, collagen-Ⅲ↓ |
| Ota et al.[36] | TGF-β-stimulated A549 cells, bleomycin mouse model | TSA | In vitro: EMT↓ restoration of CDH1 expression. In vivo: Partial attenuation of fibrosis, restoration of AECII-Sftpc expression |
| Kim et al.[37] | Bleomycin mouse model, PHMG induced lung fibrosis | CG-745, Class I-HDAC+HDAC6 | Abrogation of bleomycin-fibrosis, H3 acetylation, Pai-1↓, α-SMA↓, collagen-I↓, BALF:TNF-α↓, II-6↓ and attenuation of PHMG-fibrosis |
| Jones et al.[38] | TGF-β-treated primary IPF fibroblasts | Pracinostat, pan-HDAC except HDAC6 | H3 acetylation at PGC1A promoter, derepression of PGC1A expression, HDAC7 signalling↓, ACTA2↓, TNC↓, IL6↓, PDGFA↓ and inhibition of FMD |
| Coward et al.[39] | TGF-β-treated primary IPF fibroblasts | Panobinostat (LBH589), pan-HDAC | H3 and H4 acetylation at CXCL10 promoter, reduction of repressive H3K9Me3 at CXCL10 promoter, derepression of CXCL10 expression |
| Sanders et al.[40] | Fibrotic rat Thy1 (-) lung fibroblasts | TSA | H3 and H4 acetylation, derepression of Thy1 (CD90) expression |
| Korfei et al.[41] | Primary IPF fibroblasts | Panobinostat (LBH589), pan-HDAC | Tubulin acetylation↑, H3K27Ac↑, STAT3-pTyr705↓, proliferation, FMD↓, ECM (pro-collagen-I)↓, HDAC1↓, HDAC2↓, HDAC7 (mRNA and protein)↓ |
| Guo et al.[15] | TGF-β-treated human normal lung fibroblasts | TSA, pan-HDAC | HDAC4 signalling↓, ACTA2↓, COL1A1↓, CTGF↑, inhibition of FMD, α-SMA, AKT phosphorylation↓ |
| Ye et al.[42] | Bleomycin rat model | TSA, pan-HDAC | Reduction of lung fibrosis, HDAC2 (mRNA and protein)↓ |
| Rao et al.[43] | TGF-β-treated normal Human, Lung Fibroblasts (HFL1), paraquat-induced lung fibrosis in rats | Vorinostat (SAHA), pan-HDAC | In vitro and in vivo: SMAD7 acetylation and stabilization, SMAD3 dephosphorylation, FMD↓, attenuation of lung fibrosis |
| Glenisson et al.[44] | TGF-β-treated primary normal skin fibroblasts (human) | TSA, pan-HDAC | HDAC4 signalling↓, ACTA2/α-SMA↓, inhibition of FMD |
| Kabel et al.[45] | Bleomycin rat model | 4-Phenylbutyric Acid (4-PBA), class I and class IIA-HDAC | Attenuation of lung fibrosis, oxidative stress↓, BALF: IL6↓,TGF-β↓,TNF-α↓ |
| Jiang et al.[46] | A549 cells overexpressing mutant SP-AG231V, or SP-A F198S | 4-PBA, class I and class II A-HDAC | GRP78↑, suppressed protein aggregation, improved secretion |
| Zhao et al.[47] | Bleomycin mouse model | 4-PBA, class I and class II A-HDAC | ER stress↓, EMT↓, NF-κB (p65)↓, cytokines↓, α-SMA↓, COL1A1↓, COL1A2↓, alleviation of lung fibrosis |
Note: IPF: Idiopathic Pulmonary Fibrosis; EMT: Epithelial-Mesenchymal Transition; ECM: Extracellular Matrix; FMD: Fibroblast-To-Myofibroblast Differentiation; H3/H4: Histone H3/H4; Ac: Acetylation; BALF: Bronchoalveolar Lavage Fluid; PHMG: Polyhexamethylene Guanidine; ↑: Upregulation; ↓: Downregulation; BAK: Bcl-2 Homologous Antagonist/Killer; BID: BH3 Interacting Domain Death Agonist; BCL2L1: Bcl-2-Like Protein 1; CIP1/p21: Cyclin-Dependent Kinase Inhibitor p21; CHOP: C/EBP Homologous Protein; CCND1: Cyclin D1; ACTA2: Actin Alpha 2; COL1A1: Collagen type I Alpha 1; COL1A3: Collagen type I Alpha 1; BCL-XL: B-Cell Lymphoma-Extra Large; mRNA: messenger RNA; CDH1: Cadherin 1; TNF-α: Tumor Necrosis Factor alpha; PGC1A; Peroxisome Proliferator-Activated Receptor-Gamma Coactivator 1 Alpha; TNC: Tenascin-C; IL6: Interleukin 6; PDGFA: Platelet-Derived Growth Factor Alpha; H3K9me3: Histone 3 lysine 9 trimethylation; CXCL10: C-X-C motif Chemokine Ligand 10; Thy-1: Thymocyte differentiation antigen 1; CD90: Cluster of Differentiation 90; H3K27Ac: H3 lysine 27 Acetylation; STAT3-pTyr705: Signal transducer and activator of transcription 3-phosphorylated Tyr705; CTGF: Connective Tissue Growth Factor; GRP78: Glucose-Regulated Protein 78 and NF-κB: Nuclear Factor kappa B
Table 1: HDACi for Treatment of Pulmonary Fibrosis/Ipf
TSA and Pulmonary Fibrosis
TSA is the most common HDACi used in clinical settings and it inhibits group I and II HDACs. It works by suppressing TGF-β1-stimulated collagen I via reducing the levels of conversion factors. In mice, HDACi can prevent cardiac interstitial fibrosis induced by abnormal expression of HDAC2 related nucleoprotein. Other studies have discovered that deacetylation of the Fas cell surface death receptor (Fas) protein gene promoter leads to the downregulation of Fas proteins in the fibroblasts of IPF patients, whereas TSA up-regulates Fas gene expression and promotes cell apoptosis, induced by the Fas signaling pathway. Other reports have suggested that TSA enhances Thymocyte differentiation antigen 1 (Thy-1) expression in pulmonary myofibroblasts and suppresses fibroblasts proliferation, thereby serving an anti-fibrotic role[33,40]. Recent studies[32] discovered that excessive deacetylation of the Cyclooxygenase (COX) gene promoter can reduce the expression of COX gene and aggravate pulmonary fibrosis, while vorinostat, an inhibitor of HDAC, can reduce the deacetylation state of COX, thus increasing expression of COX and producing prostaglandin E2 that serve as an antifibrotic role.
Conclusion
IPF is associated with a progressive deterioration of lung function and poor prognosis. Approved antifibrotic drugs, nintedanib and pirfenidone, only can delay the progression of the disease, but IPF remains incurable and there is an urgent need for new therapies. The majority of the evidence generated to date indicates that the overexpression of Class I and Class II HDACs is associated with fibroblast proliferation and Fibroblast-to- Myofibroblast Differentiation (FMD), as well as accounts for the apoptosis resistant, invasive phenotype of fibroblast/myofibroblast and bronchiolar basal cells in IPF. Therefore, they hold great potential as therapeutics for pulmonary fibrosis. However, its mechanism remains elusive. Hence, future investigations on the different types of HDACs and the mechanisms of HDACi in IPF have not yet been addressed and should be elucidated in future studies.
Author’s contributions:
Qian Zhang is the major contributor in writing the manuscript and Ping Jiang is mainly responsible for the editing the manuscript.
Acknowledgements:
We thank professor, Jie Cao, a professional in interstitial lung disease who critically reviewed and language-edited the manuscript prior to submission. Furthermore, we thank Shuo Li for administrative and funding support.
Conflict of interests:
The authors declared no conflict of interest.
References
- Wolters PJ, Collard HR, Jones KD. Pathogenesis of idiopathic pulmonary fibrosis. Annu Rev Pathol 2014;9:157-79.
[Crossref] [Google scholar] [PubMed]
- Loomis-King H, Flaherty KR, Moore BB. Pathogenesis, current treatments and future directions for idiopathic pulmonary fibrosis. Curr Opin Pharmacol 2013;13(3):377-85.
[Crossref] [Google scholar] [PubMed]
- Scelfo C, Caminati A, Harari S. Recent advances in managing idiopathic pulmonary fibrosis. F1000Res 2017;6:2052.
[Crossref] [Google scholar] [PubMed]
- Pang M, Zhuang S. Histone deacetylase: A potential therapeutic target for fibrotic disorders. J Pharmacol Exp Ther 2010;335(2):266-72.
[Crossref] [Google scholar] [PubMed]
- Allfrey VG, Faulkner R, Mirsky A. Acetylation and methylation of histones and their possible role in the regulation of RNA synthesis. Proc Natl Acad Sci USA 1964;51(5):786-94.
[Crossref] [Google scholar] [PubMed]
- McKinsey TA, Zhang CL, Olson EN. Control of muscle development by dueling HATs and HDACs. Curr Opin Genet Dev 2001;11(5):497-504.
[Crossref] [Google scholar] [PubMed]
- de Ruijter AJM, van Gennip AH, Caron HN, Kemp S, van Kuilenburg ABP. Histone deacetylases (HDACs): Characterization of the classical HDAC family. Biochem J 2003;370(3):737-49.
[Crossref] [Google scholar] [PubMed]
- Benedetti R, Conte M, Altucci L. Targeting histone deacetylases in diseases: Where are we? Antioxid Redox Signal 2015;23(1):99-126.
[Crossref] [Google scholar] [PubMed]
- Shahbazian MD, Grunstein M. Functions of site-specific histone acetylation and deacetylation. Annu Rev Biochem 2007;76:75-100.
[Crossref] [Google scholar] [PubMed]
- Minucci S, Pelicci PG. Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat Rev Cancer 2006;6(1):38-51.
[Crossref] [Google scholar] [PubMed]
- KING Jr TE, Schwarz MI, Brown K, Tooze JA, Colby TV, Waldron Jr JA, et al. Idiopathic pulmonary fibrosis: Relationship between histopathologic features and mortality. Am J Respir Crit Care Med 2001;164(6):1025-32.
[Crossref] [Google scholar] [PubMed]
- Selman M, Pardo A, Richeldi L, Cerri S. Emerging drugs for idiopathic pulmonary fibrosis. Expert Opin Emerg Drugs 2011;16(2):341-62.
[Crossref] [Google scholar] [PubMed]
- Korfei M, Mahavadi P, Guenther A. Targeting histone deacetylases in idiopathic pulmonary fibrosis: A future therapeutic option. Cells 2022;11(10):1626.
[Crossref] [Google scholar] [PubMed]
- Korfei M, Skwarna S, Henneke I, MacKenzie B, Klymenko O, Saito S, et al. Aberrant expression and activity of histone deacetylases in sporadic idiopathic pulmonary fibrosis. Thorax 2015;70(11):1022-32.
[Crossref] [Google scholar] [PubMed]
- Guo W, Shan B, Klingsberg RC, Qin X, Lasky JA. Abrogation of TGF-β1-induced fibroblast-myofibroblast differentiation by histone deacetylase inhibition. Am J Physiol Lung Cell Mol Physiol 2009;297(5):864-70.
[Crossref] [Google scholar] [PubMed]
- Davies ER, Haitchi HM, Thatcher TH, Sime PJ, Kottmann RM, Ganesan A, et al. Spiruchostatin A inhibits proliferation and differentiation of fibroblasts from patients with pulmonary fibrosis. Am J Respir Cell Mol Biol 2012;46(5):687-94.
[Crossref] [Google scholar] [PubMed]
- Khalil W, Xia H, Bodempudi V, Kahm J, Hergert P, Smith K, et al. Pathologic regulation of collagen I by an aberrant protein phosphatase 2A/histone deacetylase C4/microRNA-29 signal axis in idiopathic pulmonary fibrosis fibroblasts. Am J Respir Cell Mol Biol 2015;53(3):391-9.
[Crossref] [Google scholar] [PubMed]
- Nagathihalli NS, Massion PP, Gonzalez AL, Lu P, Datta PK. Smoking induces epithelial-to-mesenchymal transition in non–small cell lung cancer through HDAC-mediated downregulation of E-cadherin. Mol Cancer Ther 2012;11(11):2362-72.
[Crossref] [Google scholar] [PubMed]
- Zhang J, Zhong Q. Histone deacetylase inhibitors and cell death. Cell Mol Life Sci 2014;71:3885-901.
[Crossref] [Google scholar] [PubMed]
- Mann BS, Johnson JR, Cohen MH, Justice R, Pazdur R. FDA approval summary: Vorinostat for treatment of advanced primary cutaneous T-cell lymphoma. Oncologist 2007;12(10):1247-52.
[Crossref] [Google scholar] [PubMed]
- McDermott J, Jimeno A. Belinostat for the treatment of peripheral T-cell lymphomas. Drugs Today 2014;50(5):337-45.
[Crossref] [Google scholar] [PubMed]
- Whittaker SJ, Demierre MF, Kim EJ, Rook AH, Lerner A, Duvic M, et al. Final results from a multicenter, international, pivotal study of romidepsin in refractory cutaneous T-cell lymphoma. J Clin Oncol 2010;28(29):4485-91.
[Crossref] [Google scholar] [PubMed]
- Coiffier B, Pro B, Prince HM, Foss F, Sokol L, Greenwood M, et al. Results from a pivotal, open-label, phase II study of romidepsin in relapsed or refractory peripheral T-cell lymphoma after prior systemic therapy. J Clin Oncol 2012;30(6):631-6.
[Crossref] [Google scholar] [PubMed]
- Leucker TM, Nomura Y, Kim JH, Bhatta A, Wang V, Wecker A, et al. Cystathionine γ-lyase protects vascular endothelium: A role for inhibition of histone deacetylase 6. Am J Physiol Heart Circ Physiol 2017;312(4):711-20.
[Crossref] [Google scholar] [PubMed]
- Meng Z, Jia LF, Gan YH. PTEN activation through K163 acetylation by inhibiting HDAC6 contributes to tumour inhibition. Oncogene 2016;35(18):2333-44.
[Crossref] [Google scholar] [PubMed]
- Chang Z, Li Y, He W, Liu B, Duan X, Halaweish I, et al. Inhibition of histone deacetylase 6 restores intestinal tight junction in hemorrhagic shock. J Trauma Acute Care Surg 2016;81(3):512-9.
[Crossref] [Google scholar] [PubMed]
- Li ZY, Zhang C, Zhang Y, Chen L, Chen BD, Li QZ, et al. A novel HDAC6 inhibitor Tubastatin A: Controls HDAC6-p97/VCP-mediated ubiquitination-autophagy turnover and reverses temozolomide-induced ER stress-tolerance in GBM cells. Cancer Lett 2017;391:89-99.
[Crossref] [Google scholar] [PubMed]
- Ha SD, Reid C, Meshkibaf S, Kim SO. Inhibition of Interleukin 1β (IL-1β) expression by anthrax Lethal Toxin (LeTx) is reversed by Histone Deacetylase 8 (HDAC8) inhibition in murine macrophages. J Biol Chem 2016;291(16):8745-55.
[Crossref] [Google scholar] [PubMed]
- Rettig I, Koeneke E, Trippel F, Mueller WC, Burhenne J, Kopp-Schneider A, et al. Selective inhibition of HDAC8 decreases neuroblastoma growth in vitro and in vivo and enhances retinoic acid-mediated differentiation. Cell Death Dis 2015;6(2):e1657.
[Crossref] [Google scholar] [PubMed]
- di Liddo R, Valente S, Taurone S, Zwergel C, Marrocco B, Turchetta R, et al. Histone deacetylase inhibitors restore IL-10 expression in lipopolysaccharide-induced cell inflammation and reduce IL-1β and IL-6 production in breast silicone implant in C57BL/6J wild-type murine model. Autoimmunity 2016:1-11.
[Crossref] [Google scholar] [PubMed]
- Li J, Chen S, Cleary RA, Wang R, Gannon OJ, Seto E, et al. Histone deacetylase 8 regulates cortactin deacetylation and contraction in smooth muscle tissues. Am J Physiol Cell Physiol 2014;307(3):C288-95.
[Crossref] [Google scholar] [PubMed]
- Coward WR, Watts K, Feghali-Bostwick CA, Knox A, Pang L. Defective histone acetylation is responsible for the diminished expression of cyclooxygenase 2 in idiopathic pulmonary fibrosis. Mol Cell Biol 2009;29(15):4325-39.
[Crossref] [Google scholar] [PubMed]
- Huang SK, Scruggs AM, Donaghy J, Horowitz JC, Zaslona Z, Przybranowski S, et al. Histone modifications are responsible for decreased Fas expression and apoptosis resistance in fibrotic lung fibroblasts. Cell Death Dis 2013;4(5):e621.
[Crossref] [Google scholar] [PubMed]
- Sanders YY, Hagood JS, Liu H, Zhang W, Ambalavanan N, Thannickal VJ. Histone deacetylase inhibition promotes fibroblast apoptosis and ameliorates pulmonary fibrosis in mice. Eur Respir J 2014;43(5):1448-58.
[Crossref] [Google scholar] [PubMed]
- Zhang X, Liu H, Hock T, Thannickal VJ, Sanders YY. Histone deacetylase inhibition downregulates collagen 3A1 in fibrotic lung fibroblasts. Int J Mol Sci 2013;14(10):19605-17.
[Crossref] [Google scholar] [PubMed]
- Ota C, Yamada M, Fujino N, Motohashi H, Tando Y, Takei Y, et al. Histone deacetylase inhibitor restores surfactant protein-C expression in alveolar-epithelial type II cells and attenuates bleomycin-induced pulmonary fibrosis in vivo. Exp Lung Res 2015;41(8):422-34.
[Crossref] [Google scholar] [PubMed]
- Kim YS, Cha H, Kim HJ, Cho JM, Kim HR. The anti-fibrotic effects of CG-745, an HDAC inhibitor, in bleomycin and PHMG-induced mouse models. Molecules 2019;24(15):2792.
[Crossref] [Google scholar] [PubMed]
- Jones DL, Haak AJ, Caporarello N, Choi KM, Ye Z, Yan H, et al. TGFβ-induced fibroblast activation requires persistent and targeted HDAC-mediated gene repression. J Cell Sci 2019;132(20):233486.
[Crossref] [Google scholar] [PubMed]
- Coward WR, Watts K, Feghali-Bostwick CA, Jenkins G, Pang L. Repression of IP-10 by interactions between histone deacetylation and hypermethylation in idiopathic pulmonary fibrosis. Mol Cell Biol 2010;30(12):2874-86.
[Crossref] [Google scholar] [PubMed]
- Sanders YY, Tollefsbol TO, Varisco BM, Hagood JS. Epigenetic regulation of thy-1 by histone deacetylase inhibitor in rat lung fibroblasts. Am J Respir Cell Mol Biol 2011;45(1):16-23.
[Crossref] [Google scholar] [PubMed]
- Korfei M, Stelmaszek D, MacKenzie B, Skwarna S, Chillappagari S, Bach AC, et al. Comparison of the antifibrotic effects of the pan-histone deacetylase-inhibitor panobinostat versus the IPF-drug pirfenidone in fibroblasts from patients with idiopathic pulmonary fibrosis. PLoS One 2018;13(11):e0207915.
[Crossref] [Google scholar] [PubMed]
- Ye Q, Li Y, Jiang H, Xiong J, Xu J, Qin H, et al. Prevention of pulmonary fibrosis via Trichostatin A (TSA) in bleomycin induced rats. Sarcoidosis Vasc Diffuse Lung Dis 2014;31(3):219-26.
[Google scholar] [PubMed]
- Rao SS, Zhang XY, Shi MJ, Xiao Y, Zhang YY, Wang YY, et al. Suberoylanilide hydroxamic acid attenuates paraquat-induced pulmonary fibrosis by preventing Smad7 from deacetylation in rats. J Thorac Dis 2016;8(9):2485-94.
[Crossref] [Google scholar] [PubMed]
- Glenisson W, Castronovo V, Waltregny D. Histone deacetylase 4 is required for TGFβ1-induced myofibroblastic differentiation. Biochim Biophys Acta Mol Cell Res 2007;1773(10):1572-82.
[Crossref] [Google scholar] [PubMed]
- Kabel AM, Omar MS, Abd Elmaaboud MA. Amelioration of bleomycin-induced lung fibrosis in rats by valproic acid and butyrate: Role of nuclear factor kappa-B, proinflammatory cytokines and oxidative stress. Int Immunopharmacol 2016;39:335-42.
[Crossref] [Google scholar] [PubMed]
- Jiang X, Fang G, Dong L, Jin P, Ding L, Zhang H, et al. Chemical chaperone 4-phenylbutyric acid alleviates the aggregation of human familial pulmonary fibrosis-related mutant SP-A2 protein in part through effects on GRP78. Biochim Biophys Acta Mol Basis Dis 2018;1864(10):3546-57.
[Crossref] [Google scholar] [PubMed]
- Zhao H, Qin HY, Cao LF, Chen YH, Tan ZX, Zhang C, et al. Phenylbutyric acid inhibits epithelial-mesenchymal transition during bleomycin-induced lung fibrosis. Toxicol Lett 2015;232(1):213-20.
[Crossref] [Google scholar] [PubMed]