- *Corresponding Author:
- A. G. Ji
Marine College, Shandong University, Weihai-264209, China
E-mail: jiaiguo@sdu.edu.cn
| Date of Submission | 08 August 2016 |
| Date of Revision | 01 January 2017 |
| Date of Acceptance | 08 April 2017 |
| Indian J Pharm Sci 2017;79(3):361-368 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Earthworm fibrinolytic enzyme from Chinese earthworm Eisenia foetida was isolated to investigate its antitumor activity in breast cancer cells. The protein isolated was characterised as earthworm fibrinolytic enzyme using the fibrin plate method. The molecular weight of earthworm fibrinolytic enzyme component was determined to be 25 kDa by sodium dodecyl sulfate polyacrylamide gel electrophoresis assay using a standard protein ladder. Earthworm fibrinolytic enzyme can markedly inhibit the growth and migration of MCF-7 cells in a dose and time dependent manner. In addition, MCF-7 cells treated with earthworm fibrinolytic enzyme (40 μg/ml) began to undergo apoptosis after 24 h. Expression of focal adhesion kinase and CD44v6 measured using reverse transcription polymerase chain reaction and Western blot was down-regulated in a concentration dependent manner (20-80 μg/ml), resulting in the suppression of MCF-7 cells adhesion. The obtained earthworm fibrinolytic enzyme displayed an antitumor effect on MCF-7 cells in vitro, revealing the therapeutic potential of E. foetida.
Keywords
Earthworm fibrinolytic enzyme component A, MCF-7 cells, migration, antitumor activity
Breast cancer is the most common malignancy among women presenting an emerging major health problem around the world and the number of cases of has increased dramatically in Asia over the past few decades [1-4]. The development drug resistance in breast cancer is a growing challenge in the field of cancer therapy. Thus, there is an urgent need to discover new therapeutic agents.
Earthworm is widely used in Chinese traditional medicine. Previous studies on earthworm extracts have demonstrated its ability to improve haemostatic disorders [5] and stimulate wound healing [6]. In addition, higher concentrations of earthworm extracts are nontoxic and have excellent antiinflammatory and antipyretic properties [7]. Further researches have revealed the potential for using earthworm extracts as antibacterial [8], antifungal [9], antiviral [10] and antitumor [8,9] agents. In this study, earthworms were studied to determine their active components used in the traditional prescriptions as starting materials.
Earthworm fibrinolytic enzyme (EFE, also known as lumbrokinase, a group of six serine proteases, was first isolated by Mihara and coworkers from Lumbricus rubellus who divided them into four groups: F-I-0, F-I-1/2, F-II and F-III-1/2 [11,12]. To date, many other species of earthworm including Eisenia foetida [13], Neanthes japonica [14] and Pheretima posthuma [15] have been explored for several fibrinolytic enzymes. EFE has been applied as an oral thrombolytic agents for the prevention of cardiac and cerebrovascular diseases due to its strong fibrinolytic activity [16]. In recent times, the anticarcinogenic effect of EFE has drawn amounts of global research attention. A previous study has shown that EFE isolated from E. foetida has strong antitumor activity in human hepatoma cells in vitro and in vivo [17]. A novel acidic serine protease purified from N. japonica could inhibit the proliferation of cells, induce apoptosis and enhance chemo-susceptibility of acute promyelocytic leukemia cells NB4 [14]. A recent study suggested that a serine protease from P. posthuma had potent cytotoxic activity against Vero cells and satisfactory antitumor activity in MCF-7 cells [3]. EFE component A (EFEA, EC#: 3.4.21), a protein, which is typical for an elastase-like enzyme and functions both as a direct fibrinolytic enzyme and a plasminogen activator, was purified from the earthworm E. fetida [18]. The crystal structure of the EFEa is the first reported for an EFE component and belongs to the trypsin-like serine protease family [19]. However, whether it has antitumor activity against mammary cancer is not yet clear.
In this study, EFEA was isolated and purified using size and charge based chromatographic separation techniques from E. foetida. MCF-7 is a type of cell line representing the breast cancer adenoma cells that can be expanded and differentiated in culture. Using different techniques, EFEA was determined that it could act as antiproliferative and antimetastatic factors, inhibiting the viability and migration of MCF-7 cells.
Materials and Methods
The MCF-7, a human breast adenocarcinoma cell line, was obtained from Chinese academy sciences (Shanghai, China). Earthworms, E. foetida were provided by Huaneng Pharmaceutical Co. Ltd (Shandong, China). Columns of diethylaminoethanol (DEAE)-Sepharose FF, Sephacryl S-200 HR and Sephadex G-10 were purchased from GE (USA). Primers for glyceraldehyde 3-phosphate dehydrogenase (GAPDH), focal adhesion kinase (FAK) and CD44v6 were purchased from GenScript (Jiangsu, China); antibodies for GAPDH and FAK were purchased from Santa Cruz (USA). Dulbecco's modified Eagle medium (DMEM), foetal bovine serum (FBS) and trypsin were all purchased from Gibco (USA); CCK-8 kit Beyotime (Shanghai, China); human fibrinogen and thrombin were purchased from Sigma (USA). Other reagents and chemicals used were of analytical grade and commercially available.
Isolation and purification of EFFA
The preparation of EFEA was carried out as described earlier [19] with a slight change. Crude protein was isolated from 500 g homogenate of frozen E. fetida by ammonium sulphate precipitation. Afterwards, the precipitated protein was resuspended in 50 mM Tris-HCl buffer (pH 8.0) and subjected to successive DEAE-cellulose ion-exchange chromatography and Sephacryl S-200 HR gel filtration after dialysis. The eluates were loaded onto a Sephadex-G10 column for desalination. Finally, the active fractions were pooled, freeze-dried and stored at –20° for further study.
Enzyme assay on fibrin plate
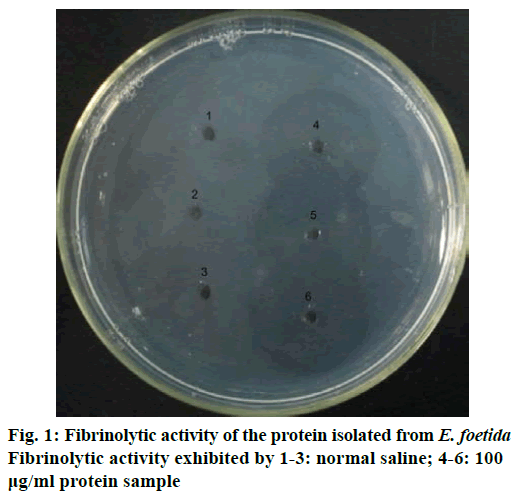
Enzyme activity was evaluated using the fibrin plate method [20] with some minor adjustments. Briefly, the solution composed of 6 mg/ml human fibrinogen in 50 mM Tris-HCl buffer (pH 7.4) containing 0.15 M NaCl, 0.8% agarose and 100 IU/ml thrombin was poured into a sterile petri dish. The solution in the plate was left still for 1 h to form fibrin clot and then 2 mm diameter wells were made in the plate for sample application. To observe the fibrinolytic activity, 10 μl of sample solution was carefully dropped into each well and incubated at 37° for 18 h.
Determination of molecular weight
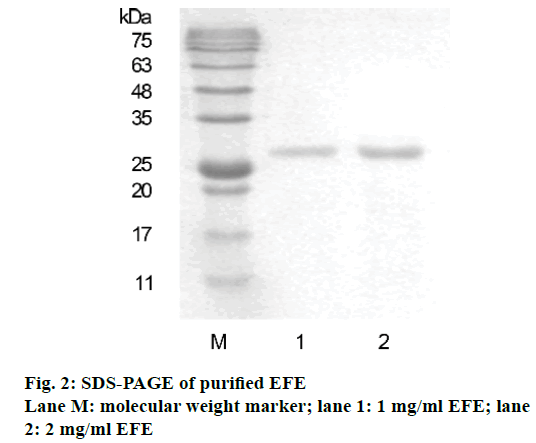
Sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) was performed according to Laemmli [21]. A 12% acrylamide matrix was prepared and protein samples (10 μl) were loaded with protein marker. After completion of electrophoresis, gels were stained with Coomassie brilliant blue G250 and destained with a destaining solution. The average molecular weight was determined with a standard protein ladder.
Liquid chromatography-mass spectrometry (LC-MS) analysis
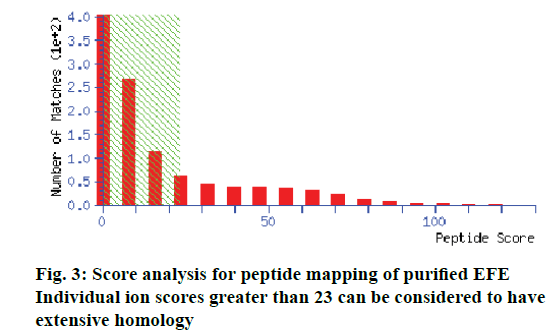
After SDS-PAGE, gel bands were stained with Coomassie brilliant blue G250 and destained with a destaining solution, and subsequently sent to Beijing Protein Innovation Co. Ltd for LC-MS analysis.
Cell culture
Human breast adenocarcinoma cell line MCF-7 was cultured in DMEM, supplemented with 10% FBS, 100 U/ml penicillin and 100 μg/ml streptomycin, and incubated at 37° in a 5% CO2 atmosphere. The medium was routinely changed every two days and at confluence, cells were subcultured by 0.25% trypsinisation. In all experiments, cells were used between P4 and P5 passage cultures.
Cell viability assay and morphology assay
Cell viability was examined using a Cell Counting Kit-8 (CCK-8) [22]. The MCF-7 cells were seeded in 96-well plates (8×103 cells/well) and cultured at 37° in 5% CO2 to adhere. After 24 h incubation, the supernatant was discarded and the cells were treated with a growth medium containing EFEa of varying concentrations. Cells treated with paclitaxel (PTX) were adopted as positive controls. Cell morphology was observed under the invert microscope [23] after being treated for 12, 24 and 48 h. After incubation for 48 h, cell viability was tested by CCK-8.
Scratch-wound assay
MCF-7 cells in logarithmic growth phase were plated in 24-well plates (5×105 cells/well) and grew until confluence. Thereafter, the procedure described [24] was followed. A straight scratch was made with a new 200 μl pipette tip in a monolayer of the cells to simulate a wound. The wound was exposed to EFEa or PTX and the cells without treatment were used as controls. After wounding for 4, 24 and 48 h, respectively, the scratches were observed under the invert microscope.
Reverse transcription polymerase chain reaction (RT-PCR)
The mRNA expression of related genes was investigated as previously described [22] with some modifications. Total RNA was isolated from cultured MCF-7 cells using TRIzol reagent according to the manufacturer’s instructions. Isolated RNAs were reverse transcribed using Revert AidTM First Strand cDNA synthesis kit. Amplification of the reverse transcription products by PCR with GAPDH serving as an internal control was carried out using Dream TaqTM PCR Master Mix (2×). The corresponding cDNA fragments were subjected to 35 cycles of annealing. After electrophoresis of the PCR products on agarose gel, which was stained with DuRed and visualised using Bio-Rad Gel Doc EQ, the results were analysed by Image J 1.47. The sequences of the PCR primers were: GAPDH (forward: 5'-GACACATATTGCTTCAATGCTTCAGC- 3', reverse: 5'-GTCCACCACCCTGTTGCTGTAG-3'); FAK (forward: 5'-GGCAGCATCTATCCAGGTCAGG-3', reverse:5'-CAGGGCGAGGCGGTTTCTTT-3'); CD44v6 (forward: 5'-GACACATATTGCTTC AATGCTTCAGC- 3', reverse: 5'-TACTAGGAGTTGCCTGGATGGTAG -3').
Western blot analysis
The protein expression of related genes was carried out as previously described [25]. Cells were homogenised in RIPA lysis buffer containing protease inhibitor phenylmethane sulfonyl fluoride (PMSF). Protein samples were loaded on an acrylamide/bisacrylamide SDS-PAGE gel, electrophoresed and transferred to polyvinylidene difluoride membranes. The polyvinylidene difluoride blots were blocked with 5% non-fat dry milk in tris-buffered saline tween 20 (TBST) buffer and then incubated with primary antibodies. The blots were then incubated with second antibody for 2 h. After three washes with TBST buffer, the resulting immunocomplex bands were developed with Immobilon Western Chemiluminescent HRP Substrate.
Statistical analysis
Data were expressed as the means±standard deviation (SD) and paired t tests were used to evaluate statistical significance by the GraphPad Prism software package for Windows (v. 5.0 GraphPad Prism Software Inc, San Diego, CA). Values with 95% confidence (P<0.05) were considered significant.
Results and Discussion
The protein isolated displayed strong fibrinolytic activity during testing by the fibrin plate method (Figure 1). Its molecular weight was 25 kDa, as determined by SDS-PAGE (Figure 2). The protein sequence was 67% identical to EFEA (Q8MX72) as determined by LCMS (Figure 3) from Beijing Protein Innovation Co. Ltd. Therefore, it could be speculated that this protein was EFEA.
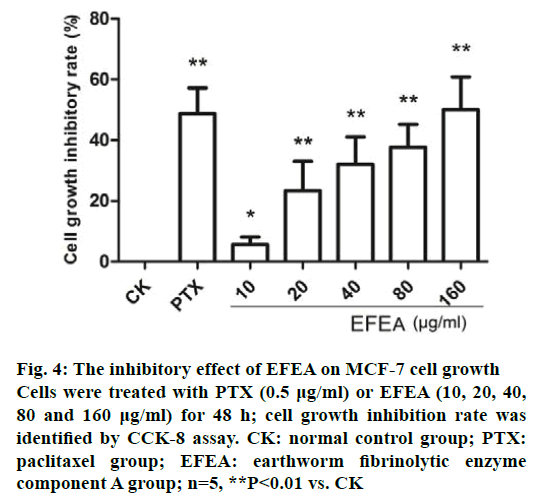
After incubation with PTX for 48 h, the cell growth was approximately 50% of the controls (Figure 4). EFEA caused a proliferation inhibition of MCF-7 cells in a dose-dependent manner, with significant reuction, which was comparable to PTX group at the highest tested dose (160 μg/ml). The IC50 value was 130.26 μg/ml. These data suggested that EFEA could effectively inhibit the proliferation of MCF-7 cells.
Figure 4: The inhibitory effect of EFEA on MCF-7 cell growth
Cells were treated with PTX (0.5 μg/ml) or EFEA (10, 20, 40,
80 and 160 μg/ml) for 48 h; cell growth inhibition rate was
identified by CCK-8 assay. CK: normal control group; PTX:
paclitaxel group; EFEA: earthworm fibrinolytic enzyme
component A group; n=5, **P<0.01 vs. CK
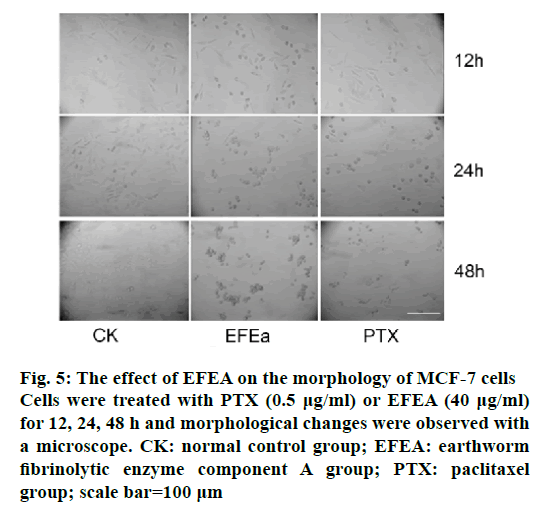
The majority of MCF-7 cells changed their morphology initially when they were exposed to 40 μg/ml of EFEA. As shown in Figure 5, after exposure to EFEA for 12 h, spindle cells gradually became swollen compared to control group. Round cells gathered and some predisposed to undergo apoptosis after 24 h. These changes were more pronounced with the prolonged treatment time 48 h, which was consistent with the PTX (0.5 μg/ml) treatment group.
Figure 5: The effect of EFEA on the morphology of MCF-7 cells
Cells were treated with PTX (0.5 μg/ml) or EFEA (40 μg/ml)
for 12, 24, 48 h and morphological changes were observed with
a microscope. CK: normal control group; EFEA: earthworm
fibrinolytic enzyme component A group; PTX: paclitaxel
group; scale bar=100 μm
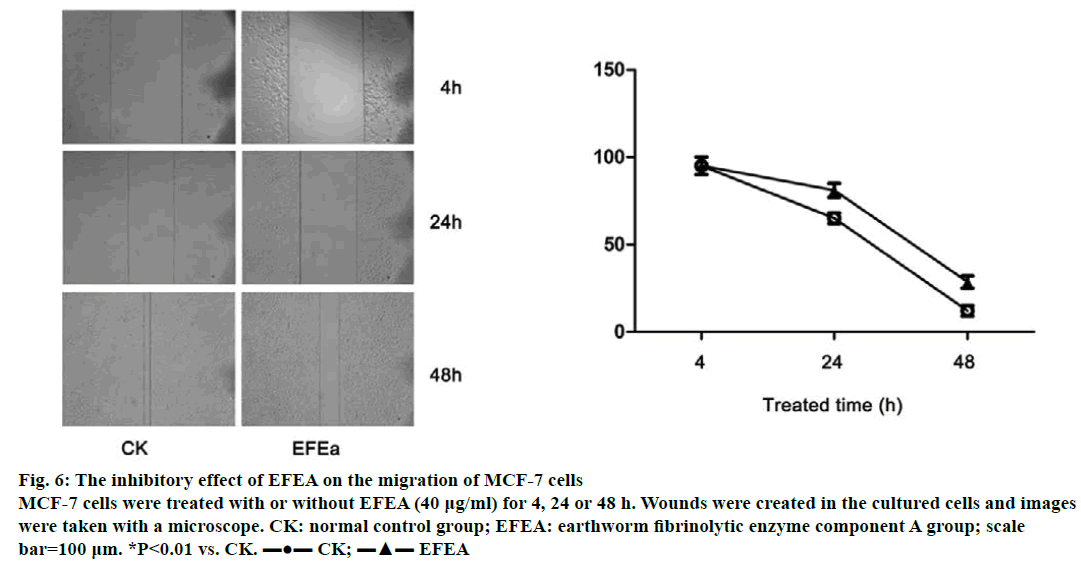
The ability of MCF-7 cells to migrate was examined at three time points (4, 24 and 48 h; Figure 6). The untreated control cells grew and migrated more rapidly compared to the EFEA treated group (40 μg/ml). The scratch band healed almost completely without EFEA treatment. Conversely, the migration of MCF-7 treated with EFEA were predominantly inhibited. These results showed that EFEA could suppress the migration of MCF-7 cells.
Figure 6: The inhibitory effect of EFEA on the migration of MCF-7 cells
MCF-7 cells were treated with or without EFEA (40 μg/ml) for 4, 24 or 48 h. Wounds were created in the cultured cells and images
were taken with a microscope. CK: normal control group; EFEA: earthworm fibrinolytic enzyme component A group; scale
bar=100 μm. *P<0.01 vs. CK. ▬●▬ CK; ▬▲▬ EFEA
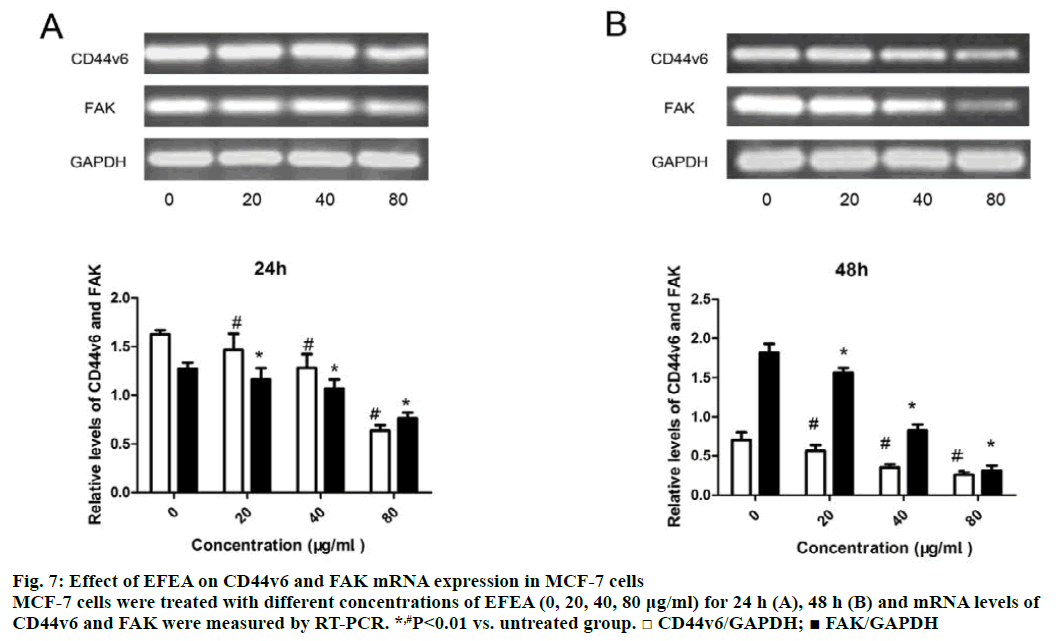
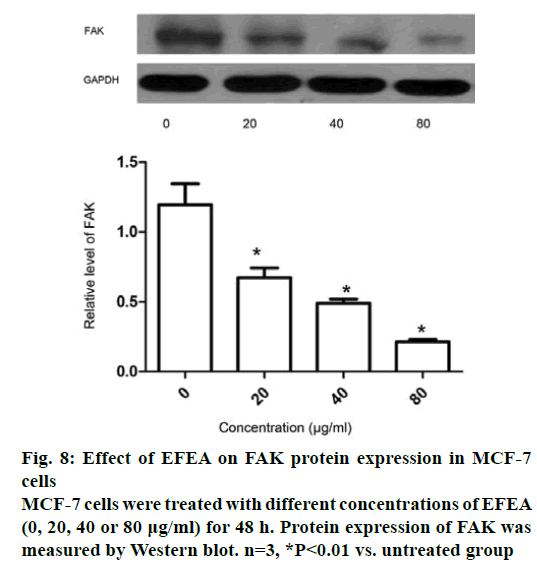
The mRNA expression of CD44v6 and FAK in MCF- 7 cells was detected using RT-PCR assay at two time points (24 and 48 h). Exposure of MCF-7 cells to various concentrations of EFEA for 24 h (Figure 7A) decreased the expression of CD44v6 and FAK in mRNA level, with the greatest reduction at 80 μg/ml, the highest concentration ever measured. After 48 h (Figure 7B), the mRNA expression of CD44v6 and FAK was decreased further. It has been demonstrated that EFEA could resist cell adhesion by reducing the mRNA expression of CD44v6 and FAK in a concentration-dependent manner. The protein expression of FAK in MCF-7 cells treated with EFEA for 48 h was effectively attenuated in a concentration-dependent manner (Figure 8). The result, in accordance with Figure 7, showed that EFEA could inhibit cell adhesion by reducing the protein expression of FAK.
In recent years, among the diverse pharmceutical effects of EFE, its antitumor activity has attracted interest [3,14,17,26,27]. Using the CCK-8 assay, it could be inferred that EFEA could inhibit the proliferation of MCF-7 cells. The IC50 value was 130.26 μg/ml, which was lower than the protease purified from the Indian earthworm P. posthuma by Verma [3]. The antitumor machanisms of earthworm extract are complex. Some studies verified it could enhance the swallowing ability of abdominal macrophage in tumor bearing mice, increase the spleen index and thymus index and activate the catalase (CAT), superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) in mice serum [28]. Therefore, one possible mechanism might relate to the enhancement in macrophage activity and antioxidative effect of earthworm extract. There are growing evidences that the antitumor effect of EFE is associated with the apoptosis of cells [14,17,29]. Consistent with these reports, our results indicated that cell apoptosis was induced by EFEA with significant morphological changes. However, we did not investigate apoptosis related proteins (caspases and Bax/Bcl-2) and signal pathways, which would become a focus of our subsequent research.
A wound scratch assay indicated that the migration of MCF-7, which was treated with EFEA (40 μg/ml) was inhibited when compared to the untreated control group. It could be inferred that the difference was a consequence of the diminished adhesion and movement of cells. The invasion, progression and metastasis of tumor cells was closely related to cell adhesion between the extracellular matrix and other cells [30]. Cell adhesion was dependent on cell adhesion molecules and kinase [31,32]. FAK, a non-receptor binding to tyrosine kinase, which could regulate focal adhesion formation and disassembly, is involved in tumour initiation and progression, including cell survival and apoptosis, adhesion, migration and invasion [33-36]. FAK is a key mediator of intracellular signaling by integrins, a major family of cell surface receptors in the extracellular matrix, in the regulation of different cellular functions in a variety of cells [37]. An earlier report illustrated that EFE showed significant antitumor activity in hepatoma cells and suggested this might be caused by EFE inducing apoptosis of hepatoma cells and inhibiting the expression of the matrix metalloproteinase-2 (MMP-2) [17]. Our data showed that EFEA can down-regulate the expression of FAK in MCF-7 in a concentration-dependent manner. It is possible that there might be a change in MMP expression via the activation of FAK signalling. This warranted further investigation. A variant isoform of CD44, CD44v6 is a multi-structural and multifunctional transmembrane glycoprotein involved in cell-cell and cell-matrix adhesion interactions as well as cell migration [38]. Immunohistochemistry of CD44v6 has been performed on formalin-fixed paraffin wax-embedded breast cancer tissue samples by Chinese researchers. They demonstrated that CD44v6 played opposing roles in the development of breast cancer [39]. In addition, it has been reported that EFE could obviously inhibit the expression of CD44v6 in hepatocellular carcinoma mice [27]. In our present study, we found that EFEA decreased the expression of CD44v6 in a concentration-dependent manner compared with an untreated control group. The results showed that EFEA can resist the migration of MCF-7 cells. This might be due to the reductions in adhesion factors CD44v6 and FAK, which decrease in a dosedependent manner at concentrations lower than IC50.
In summary, EFEA was successfully isolated and purified, a distinct protein fraction from the crude extract, of which a 25 kDa fraction was found to possess a strong fibrinolytic function and aid in the suppression of the proliferation and migration of MCF-7 tumour cells. The present results suggested that EFEA could serve as a novel agent for the treatment of breast cancer. The anticancer effects in vivo, as well as the potential clinical effectiveness of EFEA is worthy of further investigation.
Acknowledgements
This work was supported by Shandong Provincial Natural Science Foundation, China (ZR2015PH050).
Conflicts of interest
There are no conflicts of interest.
References
- Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, et al. Cancer treatment and survivorship statistics. CA Cancer J Clin 2016;66:271-89.
- Karimian H, Fadaeinasab M, Moghadamtousi SZ, Hajrezaei M, Zahedifard M, Razavi M, et al. The chemopreventive effect of Tanacetum polycephalum Against LA7-Induced breast cancer in rats and the apoptotic effect of a cytotoxic sesquiterpene lactone in MCF7 cells: A Bioassay-Guided Approach. Cell Physiol Biochem 2015;36:988-1003.
- Verma MK, Xavier F, Verma YK, Sobha K. Evaluation of cytotoxic and antitumor activity of partially purified serine protease isolate from the Indian earthworm Pheretima posthuma. Asian Pac J Trop Med 2013;3:896-901.
- Zahedifard M, Faraj F, Paydar M, Looi C, Hasandarvish P, Hajrezaie M, et al. Synthesis of apoptotic new quinazolinone-based compound and identification of its underlying mitochondrial signalling pathway in breast cancer cells. Curr Pharm Des 2015;21:3417-26.
- Matausic-Pisl M, Tomicic M, Micek V, Grdisa M. Influences of earthworm extract G-90 on haematological and haemostatic parameters in Wistar rats. Eur Rev Med Pharmacol Sci 2011;15:71-8.
- Matausijc-Pisl M, Cupic H, Kasuba V, Mikecin AM, Grdisa M. Tissue extract from Eisenia foetida as a wound-healing agent. Eur Rev Med Pharmacol Sci 2010;14:177-84.
- Balamurugan M, Parthasarathi K, Cooper EL, Ranganathan LS. Earthworm paste (Lampito mauritii, Kinberg) alters inflammatory, oxidative, haematological and serum biochemical indices of inflamed rat. Eur Rev Med Pharmacol Sci 2007;11:77-90.
- Hua Z, Wang YH, Cao HW, Pu LJ, Cui YD. Purification of a protein from coelomic fluid of the earthworm Eisenia foetida and evaluation of its hemolytic, antibacterial and antitumor activities. Pharm Biol 2011;49:269-75.
- Fiolka MJ, Lewtak K, Rzymowska J, Grzywnowicz K, Hulas-Stasiak M, Sofinska-Chmiel W, et al. Antifungal and anticancer effects of a polysaccharide-protein complex from the gut bacterium Raoultella ornithinolytica isolated from the earthworm Dendrobaena veneta. Pathog Dis 2013;69:49-61.
- Liu Z, Wang J, Zhang J, Yu B, Niu B. An extract from the earthworm Eisenia fetida non-specifically inhibits the activity of influenza and adenoviruses. J Tradit Chin Med 2012;32:657-63.
- Mihara H, Sumi H, Yoneta T, Mizumoto H, Ikeda R, Seiki M, et al. A novel fibrinolytic enzyme extracted from the earthworm, Lumbricus rubellus. Jpn J Physiol 1991;41:461-72.
- Nakajima N, Mihara H, Sumi H. Characterization of potent fibrinolytic enzymes in earthworm, Lumbricus rubellus. Biosci Biotechnol Biochem 1993;57:1726-30.
- Wu JX, Zhao XY, Pan R, He RQ. Glycosylated trypsin-like proteases from earthworm Eisenia fetida. Int J Biol Macromol 2007;40:399-406.
- Ge X, Bo Q, Hong X, Cui J, Jiang X, Hong M, et al. A novel acidic serine protease, ASPNJ inhibits proliferation, induces apoptosis and enhances chemo-susceptibility of acute promyelocytic leukemia cell. Leuk Res 2013;37:1697-703.
- Verma MK, Pulicherla KK. Broad substrate affinity and catalytic diversity of fibrinolytic enzyme from Pheretima posthumous purification and molecular characterization study. Int J Biol Macromol 2017;95:1011-21.
- Verma MK, Pulicherla KK. Enzyme promiscuity in earthworm serine protease: substrate versatility and therapeutic potential. Amino Acids 2016;48:941-8.
- Chen H, Takahashi S, Imamura M, Okutani E, Zhang ZG, Chayama K, et al. Earthworm fibrinolytic enzyme: antitumor activity on human hepatoma cells in vitro and in vivo. Chin Med J 2007;120:898-904.
- Wang C, Wang F, Li M, Tang Y, Zhang JP, Gui LL, et al. Structural basis for broad substrate specificity of earthworm fibrinolytic enzyme component A. Biochem Biophys Res Commun 2004;325:877-82.
- Park JK, Moon JH, Kim JH, Kim EE. Crystallization and preliminary X-ray crystallographic analysis of peptide deformylase (PDF) from Bacillus cereus in ligand-free and actinonin-bound forms. Acta Crystallogr Sect F Struct Biol Cryst Commun 2005;61:150-2.
- Astrup T, Mullertz S. The fibrin plate method for estimating fibrinolytic activity. Arch Biochem Biophys 1952;40:346-51.
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970;227:680-5.
- Cui C, Wang P, Cui N, Song S, Liang H, Ji A. Stichopus japonicus polysaccharide, fucoidan or heparin enhanced the SDF-1alpha/CXCR4 axis and promoted NSC migration via activation of the PI3K/Akt/FOXO3a signaling pathway. Cell Mol Neurobiol 2016;36:1311-29.
- Cui C, Cui N, Wang P, Song S, Liang H, Ji A. Neuroprotective effect of sulfated polysaccharide isolated from sea cucumber Stichopus japonicus on 6-OHDA-induced death in SH-SY5Y through inhibition of MAPK and NF-kappaB and activation of PI3K/Akt signaling pathways. Biochem Biophys Res Commun 2016;470:375-83.
- Rodriguez LG, Wu X, Guan JL. Wound-healing assay. Methods Mol Biol 2005;294:23-9.
- Cui C, Cui N, Wang P, Song S, Liang H, Ji A. Sulfated polysaccharide isolated from the sea cucumber Stichopus japonicus against PC12 hypoxia/reoxygenation injury by inhibition of the MAPK signaling pathway. Cell Mol Neurobiol 2015;35:1081-92.
- Xie J, He W, Weng N, Guo Z, Yu M, Liu N, et al. Extraction and Isolation of the antitumor protein components from earthworm (Eisenia fetida andrei) and the antitumor activity. Clim Res 2003;39:227-34.
- Wang J, Chen H, Ji H, Zhang Z. Effect of earthworm fibrinolytic enzyme on growth of xenografted tumor of hepatocellular carcinoma (HCC) and expression of CD44v6. Cancer Res Prev Treat 2009;36:375-9.
- Lin S, Zou K. Affecting tumorigenic mouse immune function and antioxidase of QY -Ι from earthworm. Str Pharm J 2002;14:9-11.
- Macsik LL, Somogyi I, Opper B, Bovari-Biri J, Pollak E, Molnar L, et al. Induction of apoptosis-like cell death by coelomocyte extracts from Eisenia andrei earthworms. Mol Immunol 2015;67:213-22.
- Stroka KM, Konstantopoulos K. Physical biology in cancer. 4. Physical cues guide tumor cell adhesion and migration. Am J Physiol Cell Physiol 2014;306:C98-C109.
- Fogh BS, Multhaupt HA, Couchman JR. Protein kinase C, focal adhesions and the regulation of cell migration. J Histochem Cytochem 2014;62:172-84.
- Mackesy DZ, Goalstone ML. Extracellular signal-regulated kinase-5: Novel mediator of insulin and tumor necrosis factor alpha-stimulated vascular cell adhesion molecule-1 expression in vascular cells. J Diabetes 2014;6:595-602.
- Tancioni I, Miller NL, Uryu S, Lawson C, Jean C, Chen XL, et al. FAK activity protects nucleostemin in facilitating breast cancer spheroid and tumor growth. Breast Cancer Res 2015;17:47.
- Xu H, Tian Y, Yuan X, Wu H, Liu Q, Pestell RG, et al. The role of CD44 in epithelial-mesenchymal transition and cancer development. Onco Targets Ther 2015;8:3783-92.
- Stone RL, Baggerly KA, Armaiz-Pena GN, Kang Y, Sanguino AM, Thanapprapasr D, et al. Focal adhesion kinase: an alternative focus for antiangiogenesis therapy in ovarian cancer. Cancer Biol Ther 2014;15:919-29.
- Lechertier T, Hodivala-Dilke K. Focal adhesion kinase and tumour angiogenesis. J Pathol 2012;226:404-12.
- Guan JL. Integrin signaling through FAK in the regulation of mammary stem cells and breast cancer. IUBMB Life 2010;62:268-76.
- Chatterji T, Varkaris AS, Parikh NU, Song JH, Cheng CJ, Schweppe RE, et al. Yes-mediated phosphorylation of focal adhesion kinase at tyrosine 861 increases metastatic potential of prostate cancer cells. Oncotarget 2015;6:10175-94.
- Wu X, Li X, Zhang H, Zhang X, Ning Z, Yin Y, et al. Clinical significance of CD44s, CD44v3 and CD44v6 in breast cancer. J Int Med Res 2015;43:173-9.