- *Corresponding Author:
- Wei Zhao
Department of Neurosurgery, Yuhuangding Hospital of Yantai, Yantai, Shandong 264000, China
E-mail: ytyhdns2@126.com
| This article was originally published in a special issue, “Recent Developments in Biomedical Research and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2022:84(4) Spl Issue “51-56” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Several researchers have suggested that the antisense non-coding ribonucleic acid in the INK4 locus long non-coding ribonucleic acid (cyclin-dependent kinase inhibitor 2B antisense ribonucleic acid 1) plays a key role in the pathogenesis of the intracranial aneurysm. However, because single studies are often underpowered due to inadequate sample sizes, the role of the antisense non-coding ribonucleic acid in the INK4 locus rs1333040 polymorphism in intracranial aneurysms has been inconclusive among Asian population. To obtain a comprehensive conclusion, we conducted a meta-analysis with 4260 cases and 14 536 controls to estimate the association between the rs1333040 polymorphism and intracranial aneurysms susceptibility. The electronic databases of PubMed and Embase were searched before May 2022, to identify the relevant literature. Cochran’s Q statistics and the I2 test were applied to assess heterogeneity among the included investigations. The summary pooled odds ratios and 95 % confidence intervals were evaluated for the strength of association using the random or fixed-effects model. A total of 6 eligible studies were enrolled in this meta-analysis, involving 4260 cases and 14 536 controls. The pooled results indicated a significant association between the rs1333040 polymorphism and the intracranial aneurysm in Asians under 3 genetic models (TT vs. CC: Odds ratio=1.48, 95 % confidence interval=1.30-1.70; TC vs. CC: Odds ratio=1.20, 95 % confidence interval=1.05-1.38; dominant model-TT+TC vs. CC: Odds ratio=1.34, 95 % confidence interval=1.18-1.53). The findings of this meta-analysis emphasize the rs1333040 polymorphism as a common risk factor for intracranial aneurysms in Asian people.
Keywords
Intracranial aneurysms, meta-analysis, polymorphism, antisense non-coding ribonucleic acid in the INK4 locus
Intracranial aneurysms is a common cerebrovascular abnormality disease characterized by localized dilation or ballooning of intracranial arteries[1]. Aneurysmal Subarachnoid Haemorrhage (aSAH) due to ruptured intracranial aneurysm is a catastrophic neurological condition; aSAH is associated with high morbidity and neurological deficit rate. Intracranial aneurysms can be repaired by microsurgical clipping or endovascular coiling[2]. Genetic component and environmental risk factors are thought to play important roles in the etiology of aneurysms[3-5].
Several Genome-Wide Association Studies (GWAS) and candidate gene studies have identified common genetic variations on chromosomes 2q, 8q and 9p contributing to the risk of the intracranial aneurysm formation. Antisense Non-Coding Ribonucleic Acid in the INK4 Locus (ANRIL) is located on chromosome 9p21 and encodes a large Antisense (AS) non-coding Ribonucleic Acid (RNA) in the INK4 locus. ANRIL is adjacent to the Cyclin-Dependent Kinase Inhibitor 2A-Cyclin-Dependent Kinase Inhibitor 2B (CDKN2A- CDKN2B) locus, which is robustly associated with coronary artery disease via regulating vascular cell proliferation. The aberrations of CDKN2A and CDKN2B are responsible for abnormal regulation of vascular cell proliferation and senescence[6]. ANRIL rs1333040 Single-Nucleotide Polymorphism (SNP) in the 9p21.3 locus has been reported to have an effect on intracranial aneurysms pathogenesis. A number of studies have been conducted to investigate the association between the rs1333040 polymorphism and intracranial aneurysms with conflicting conclusions[7-11]. The objective of our study was to clarify the effects of the rs1333040 polymorphism on the risk of intracranial aneurysms in larger samples by meta-analysis.
Materials and Methods
Search strategy:
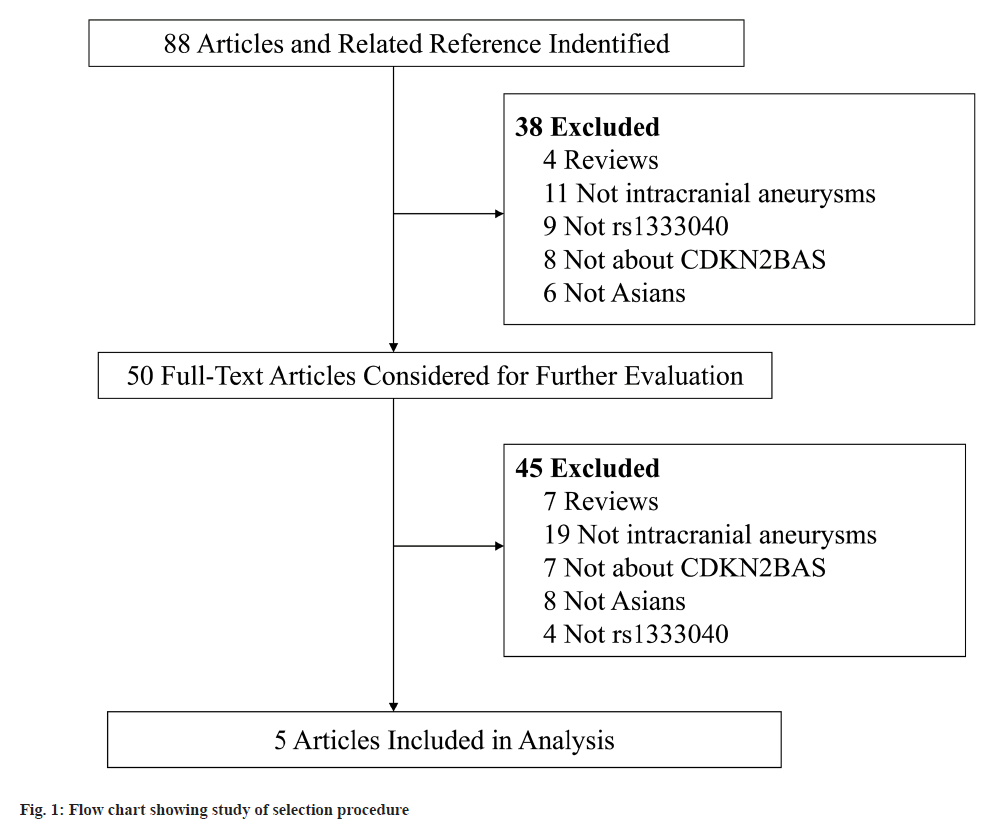
The meta-analysis was carried out in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. Two authors independently performed a systematic literature search with PubMed and Embase up to May 2022. The following keywords were used in isolation and combination with one another: “CDKN2B-AS”, “ANRIL”, “SNPs” and “intracranial aneurysms”. Besides, the references lists of relevant articles were also manually searched to identify additional eligible articles. The process of the search strategy is shown in fig. 1.
Inclusion and exclusion criteria:
Eligible studies included in the meta-analysis were selected according to the following entry criteria: Epidemiological studies with case-control study design; studies investigating the association between the rs1333040 polymorphism and the risk of intracranial aneurysms; studies providing sufficient information for extraction. The exclusion criteria were as follows: Not case-control studies and studies without detailed data of genotype distributions.
Data extraction and synthesis:
Data of eligible studies was extracted from the included studies by two authors independently. The extracted information includes: The first author’s name of the literature, publication year, country, source of controls, number of cases and controls for the rs1333040 polymorphism and evidence of Hardy-Weinberg Equilibrium (HWE) for controls.
Statistical analysis:
The obtained data were analyzed using Statistical software for data science (STATA) 13.0 software. The strength of association between the rs1333040 polymorphism and intracranial aneurysms risk were analyzed by pooling Odds Ratio (ORs) with 95 % Confidence Interval (CIs) in three models including dominant genetic model (TT+TC vs. CC), heterozygous genetic model (TC vs. CC) and homozygous genetic model (TT vs. CC). The significance of the pooled OR was determined via the Z test and p<0.05 was considered significant. HWE for each study was tested in control subjects using Chi-square test at a significance level of p<0.05. Heterogeneity was assessed by the Chi-square-based Q-test and I2 statistics. I2>50 % indicated heterogeneity across studies, the random-effect model was used to analyze the pooled OR[12,13]. Subgroup analyses by country were performed. In addition, the one-way sensitivity analyses were performed by removing each study one by one to reflect the influence of a single data set on the pooled OR. Furthermore, Begg and Egger’s test were performed to evaluate the potential publication bias[14,15], where p<0.05 was considered statistically significant.
Results and Discussion
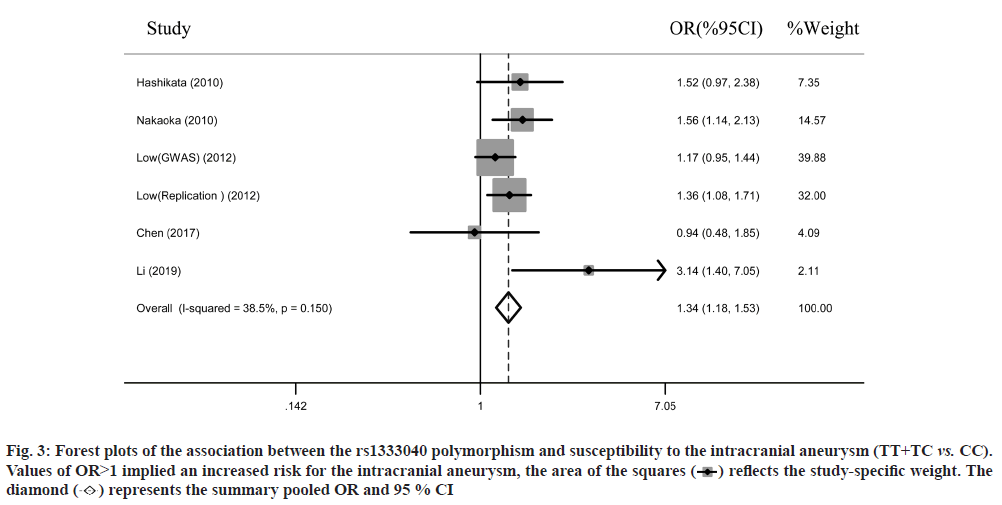
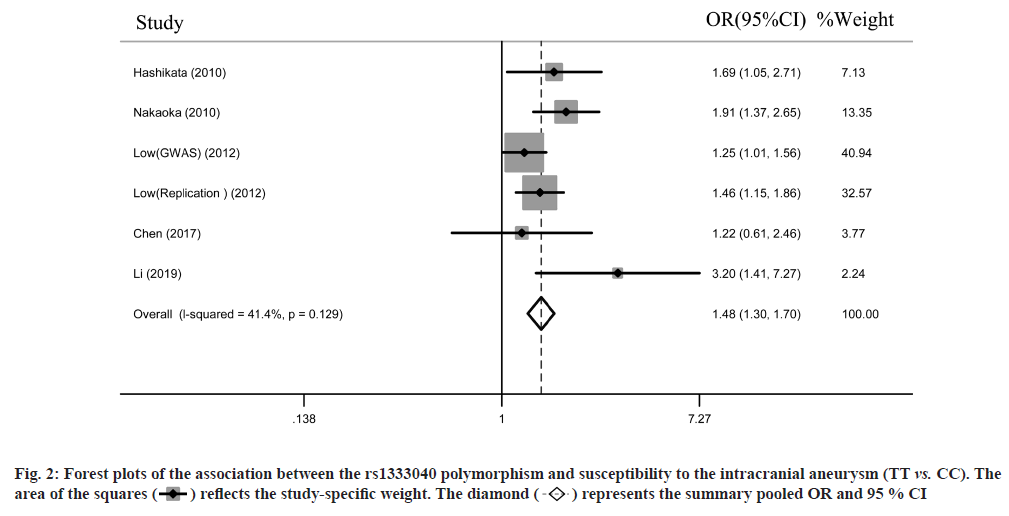
Overall analyses of the study were shown below. The study selection was carried out as shown in fig. 1. Low et al.[9] performed a genome-wide association study and a replication study in 1 publication. Finally, we identified 6 individual studies involving 4260 cases and 14 536 controls in the present meta-analysis. 2 studies were conducted among Chinese, 4 studies were conducted among Japanese. The characteristics of the included 6 studies are listed in Table 1. The overall meta-analysis results suggested a significant association between the rs1333040 polymorphism under homozygous genetic model (TT vs. CC: OR=1.48, 95 % CI=1.30- 1.70, I2=41.4 %) (fig. 2), heterozygous genetic models (OR=1.20, 95 % CI=1.05-1.38, I2=42.8 %), and dominant genetic models (OR=1.34, 95 % CI=1.18- 1.53, I2=38.5 %) (fig. 3).
| First author | Year | Country | Source of controls | Case | Control | Case | Control | HWE | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CC | CT | TT | CC | CT | TT | |||||||
| Hashikata | 2010 | Japan | HB | 419 | 408 | 36 | 180 | 203 | 51 | 187 | 170 | 0.97 |
| Nakaoka | 2010 | Japan | HB | 981 | 699 | 85 | 379 | 517 | 90 | 322 | 287 | 0.98 |
| Low (GWAS) | 2012 | Japan | HB | 1383 | 5484 | 117 | 570 | 696 | 535 | 2409 | 2540 | 0.30 |
| Low (Replication) | 2012 | Japan | HB | 1047 | 7210 | 87 | 435 | 525 | 790 | 3165 | 3255 | 0.62 |
| Chen | 2017 | China | HB | 200 | 200 | 19 | 65 | 116 | 18 | 92 | 90 | 0.42 |
| Li | 2019 | China | PB | 230 | 535 | 7 | 97 | 126 | 48 | 217 | 270 | 0.64 |
Note: PB: Population Based; HB: Hospital Based and HWE: p values for Hardy-Weinberg Equilibrium (HWE) for each study’s control group
Table 1: Main Characteristics of All Studies Included In the Meta-Analysis
Fig. 3: Forest plots of the association between the rs1333040 polymorphism and susceptibility to the intracranial aneurysm (TT+TC vs. CC). Values of OR>1 implied an increased risk for the intracranial aneurysm, the area of the squares  reflects the study-specific weight. The diamond
reflects the study-specific weight. The diamond  represents the summary pooled OR and 95 % CI
represents the summary pooled OR and 95 % CI
Subgroup analyses were as follows. We also performed stratified analyses by geographical location, significant associations were found among Chinese (2 studies) in homozygous genetic model (OR=1.96, 95 % CI=1.17- 3.27, I2=68.0 %) and Japanese (4 studies) in all genetic models (TT vs. CC: OR=1.45, 95 % CI=1.26-1.67, I2=37.6 %; TC vs. CC: OR=1.19, 95 % CI=1.03-1.37, I2=0 %; dominant model-TT+TC vs. CC: OR=1.32, 95% CI=1.16-1.51, I2=0 %) but not among Chinese in two genetic models (TC vs. CC: OR=1.41, 95 % CI=0.31- 6.35, I2=86.7 %; dominant model-TT+TC vs. CC: OR=1.68, 95 % CI=0.51-5.55, I2=80.5 % ) (Table 2).
| Rs1333040 | n | Cases/Controls | TT vs. CC | TC vs. CC | Dominant modela | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95 % CI) | phet | I2 | OR (95 % CI) | phet | I2 | OR (95 % CI) | phet | I2 | |||
| Total | 6 | 4260/14 536 | 1.48 (1.30-1.70) | 0.129 | 41.40 % | 1.20 (1.05-1.38) | 0.12 | 42.80 % | 1.34 (1.18-1.53) | 0.15 | 38.50 % |
| Country | |||||||||||
| China | 2 | 430/735 | 1.96 (1.17-3.27) | 0.077 | 68.00 % | 1.41 (0.31-6.35) | 0.006 | 86.70 % | 1.68 (0.51-5.55) | 0.024 | 80.50 % |
| Japan | 4 | 3830/13 801 | 1.45 (1.26-1.67) | 0.186 | 37.60 % | 1.19 (1.03-1.37) | 0.739 | 0 % | 1.32 (1.16-1.51) | 0.426 | 0 % |
Note: phet: p value of Q-test for heterogeneity test and aDominant model: TT+TC vs. CC
Table 2: Stratified Analyses of the Rs1333040 Polymorphism on Intracranial Aneurysm
Heterogeneity analysis was shown below. In the current meta-analysis, there was no obvious between- study heterogeneity under all genetic models in overall population (Table 2).
Sensitivity analysis was performed by deleting each independent study at a time to assess the influence of any single study. The results of the sensitivity analysis did not materially changed by removing any of each individual study, suggesting the stability of our findings.
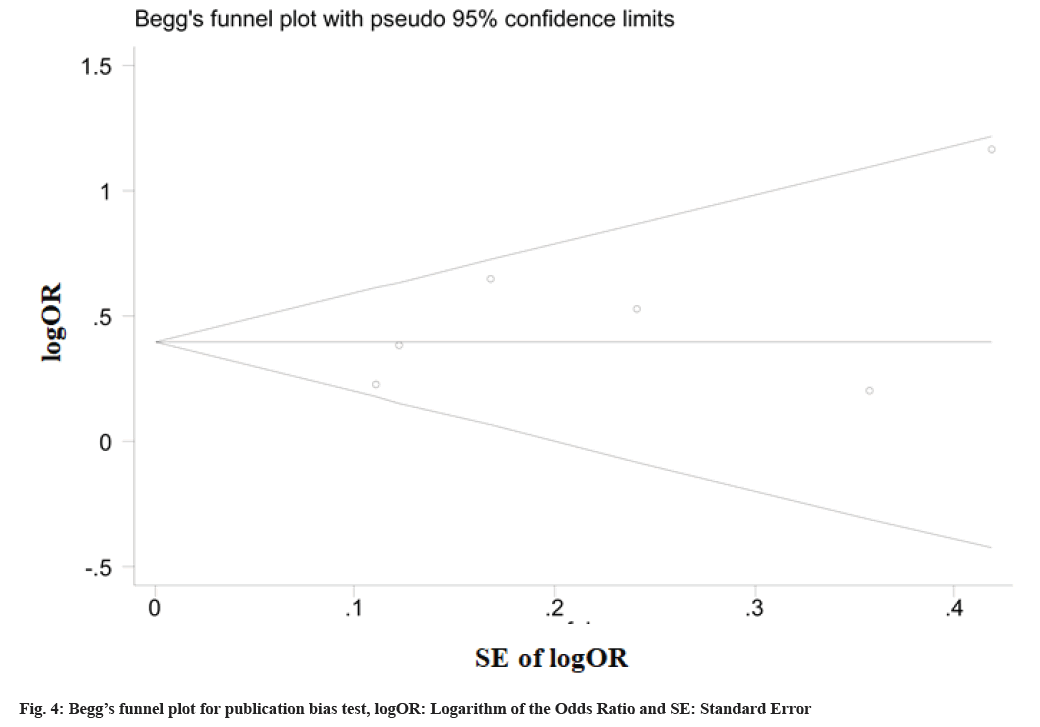
Assessment of bias is explained as follow. Begg’s funnel plot and Egger’s linear regression test were done to assess the publication bias. The results did not show any evidence of publication bias (t=1.47, p=0.216 TT vs. CC) and 95 % CI: -1.48, 4.81 included zero, indicating no apparent publication bias in our analysis (fig. 4).
We performed a meta-analysis to assess the effects of rs1333040 polymorphism on the intracranial aneurysm risk in Asians. Pooled results of the overall population showed that there was significant association between the rs1333040 polymorphism and the intracranial aneurysm risk in Asians.
ANRIL participates in the atherosclerotic processes by influencing the expression of adjacent protein-coding genes expression. The genetic variants of ANRIL is involved in the pathogenesis of vascular remodeling or repair[16]. Previous studies demonstrated that Caspase Recruitment Domain-containing protein 8 (CARD8) is a downstream target gene regulated by altering ANRIL expression[5,17]. CARD8 pathway may act in the process of atherosclerosis by impacting vascular remodeling. It is believed that functional ANRIL may mediate susceptibility to intracranial aneurysms through regulation of the messenger RNA (mRNA) level of CARD8. Although the detailed mechanism remains unclear, these functional studies supported the notion that ANRIL might play a general role in the vascular pathology and intracranial aneurysms.
Variants of 9p21 locus regulate vascular remodeling by influencing the expression of two protein-coding genes p16 (INK4a) and p15 (INK4b)[6,18]. The sequence variant on 9p21 has been found to play a pivotal role in the development of intracranial aneurysm by altering vascular cell proliferation. Moreover, evidence has shown that this locus is composed of vascular disease and diabetes blocks linkage disequilibrium blocks. The vascular disease block was tagged by two common sequence variants rs1333040 or rs10757278, which are in strong linkage disequilibrium[19].
Rs1333040 can affect the expression of ANRIL and neighboring associated protein-coding mRNA expression through multiple mechanisms. The identified SNP rs1333040 C>T has been shown to correlate (0.7<r2<0.8) with 24 SNPs located within or three prime Untranslated Region (3’-UTR) downstream of ANRIL gene[20]. The rs1333040 TT genotype has been shown to be associated with higher level of serum total cholesterol and lower level of High-Density Lipoprotein-Cholesterol (HDL-C) levels. Lower level of HDL-C will not be able to remove excess cholesterol molecules that cause atherosclerotic plaques[21]. Further well-designed studies should be conducted to investigate the biological mechanism of this association in the process of an intracranial aneurysm formation.
However, several drawbacks of our meta-analysis were impossible to avoid. First, intracranial aneurysm is a complex disease affected by gene-to-environment interactions. We were unable to explore gene-gene and gene-environment interactions. Second, the included publications were majorly limited to English databases, which may affect our results. Third, more functional studies are needed to explain our results.
In summary, the present study had successfully elaborated that the rs1333040 polymorphism might be related to the intracranial aneurysm risk. Larger populations than those included in our meta-analyses will be required to confirm the current findings.
Acknowledgements:
This work is supported by Shandong Medical and Healthy Science Technology Development Plan (202004040974).
Conflict of interests:
The authors declare that they have no conflict of interests.
References
- Vlak MH, Algra A, Brandenburg R, Rinkel GJ. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: A systematic review and meta-analysis. Lancet Neurol 2011;10(7):626-36.
[Crossref] [Google Scholar] [PubMed]
- Darsaut TE, Findlay JM, Magro E, Kotowski M, Roy D, Weill A, et al. Surgical clipping or endovascular coiling for unruptured intracranial aneurysms: A pragmatic randomised trial. J Neurol Neurosurg Psychiatry 2017;88(8):663-8.
[Crossref] [Google Scholar] [PubMed]
- Ruigrok YM, Rinkel GJ, Wijmenga C. Genetics of intracranial aneurysms. Lancet Neurol 2005;4(3):179-89.
[Crossref] [Google Scholar] [PubMed]
- Adeyemo A, Rotimi C. Genetic variants associated with complex human diseases show wide variation across multiple populations. Public Health Genomics 2010;13(2):72-9.
[Crossref] [Google Scholar] [PubMed]
- Bai Y, Nie S, Jiang G, Zhou Y, Zhou M, Zhao Y, et al. Regulation of CARD8 expression by ANRIL and association of CARD8 single nucleotide polymorphism rs2043211 (p. C10X) with ischemic stroke. Stroke 2014;45(2):383-8.
[Crossref] [Google Scholar] [PubMed]
- Visel A, Zhu Y, May D, Afzal V, Gong E, Attanasio C, et al. Targeted deletion of the 9p21 non-coding coronary artery disease risk interval in mice. Nature 2010;464(7287):409-12.
- Hashikata H, Liu W, Inoue K, Mineharu Y, Yamada S, Nanayakkara S, et al. Confirmation of an association of single-nucleotide polymorphism rs1333040 on 9p21 with familial and sporadic intracranial aneurysms in Japanese patients. Stroke 2010;41(6):1138-44.
[Crossref] [Google Scholar] [PubMed]
- Nakaoka H, Takahashi T, Akiyama K, Cui T, Tajima A, Krischek B, et al. Differential effects of chromosome 9p21 variation on subphenotypes of intracranial aneurysm: Site distribution. Stroke 2010;41(8):1593-8.
[Crossref] [Google Scholar] [PubMed]
- Low SK, Takahashi A, Cha PC, Zembutsu H, Kamatani N, Kubo M, et al. Genome-wide association study for intracranial aneurysm in the Japanese population identifies three candidate susceptible loci and a functional genetic variant at EDNRA. Hum Mol Genet 2012;21(9):2102-10.
[Crossref] [Google Scholar] [PubMed]
- Chen Y, Li G, Fan H, Guo S, Li R, Yin J, et al. CDKN2BAS gene polymorphisms and the risk of intracranial aneurysm in the Chinese population. BMC Neurol 2017;17(1):1-8.
- Li B, Hu C, Liu J, Liao X, Xun J, Xiao M, et al. Associations among genetic variants and intracranial aneurysm in a Chinese population. Yonsei Med J 2019;60(7):651-8.
[Crossref] [Google Scholar] [PubMed]
- DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: An update. Contemp Clin Trials 2007;28(2):105-14.
[Crossref] [Google Scholar] [PubMed]
- Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959;22(4):719-48.
[Crossref] [Google Scholar] [PubMed]
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315(7109):629-34.
[Crossref] [Google Scholar] [PubMed]
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088-101.
[Crossref] [Google Scholar] [PubMed]
- Holdt LM, Teupser D. Recent studies of the human chromosome 9p21 locus, which is associated with atherosclerosis in human populations. Arterioscler Thromb Vasc Biol 2012;32(2):196-206.
[Crossref] [Google Scholar] [PubMed]
- Cunnington MS, Santibanez Koref M, Mayosi BM, Burn J, Keavney B. Chromosome 9p21 SNPs associated with multiple disease phenotypes correlate with ANRIL expression. PLoS Genet 2010;6(4):e1000899.
[Crossref] [Google Scholar] [PubMed]
- Burd CE, Jeck WR, Liu Y, Sanoff HK, Wang Z, Sharpless NE. Expression of linear and novel circular forms of an INK4/ARF-associated non-coding RNA correlates with atherosclerosis risk. PLoS Genet 2010;6(12):e1001233.
[Crossref] [Google Scholar] [PubMed]
- Helgadottir A, Thorleifsson G, Magnusson KP, Grétarsdottir S, Steinthorsdottir V, Manolescu A, et al. The same sequence variant on 9p21 associates with myocardial infarction, abdominal aortic aneurysm and intracranial aneurysm. Nat Genet 2008;40(2):217-24.
[Crossref] [Google Scholar] [PubMed]
- Timofeeva MN, Hung RJ, Rafnar T, Christiani DC, Field JK, Bickeböller H, et al. Influence of common genetic variation on lung cancer risk: Meta-analysis of 14 900 cases and 29 485 controls. Hum Mol Genet 2012;21(22):4980-95.
[Crossref] [Google Scholar] [PubMed]
- Temel ŞG, Ergören MÇ. The association between the chromosome 9p21 CDKN2B-AS1 gene variants and the lipid metabolism: A pre-diagnostic biomarker for coronary artery disease. Anatol J Cardiol 2019;21(1):31-8.
[Crossref] [Google Scholar] [PubMed]
 reflects the study-specific weight. The diamond
reflects the study-specific weight. The diamond  represents the summary pooled OR and 95 % CI
represents the summary pooled OR and 95 % CI