- *Corresponding Author:
- Kusum V. Devi
Al–Ameen College of Pharmacy, Near Lal–Bagh Main Gate, Hosur Road, Bangalore-560 027, India
E-mail: kusumal62@yahoo.com
| Date of Submission | 28 February 2005 |
| Date of Revision | 23 August 2005 |
| Date of Acceptance | 2 February 2006 |
| Indian J Pharm Sci, 2006, 68 (1): 1-6 |
Abstract
Human Immunodeficiency Virus (HIV) is a retrovirus that causes irreversible destruction of the immune system, leading to the occurrence of opportunistic infections and malignancies. During the last decade, even though attempts were being made to eradicate HIV, it was found that eradication of HIV is highly unlikely, and effective antiretroviral therapy is required on a long-term basis to maintain viral suppression and reduce disease progression. During this decade, effective therapies aimed at continued suppression of HIV replication and targeted at resting HIV reservoirs such as brain, lymphatic systems will be critical to prolong survival and renewing hopes for a cure. Currently available antiHIV drugs can be classified into three categories: nucleoside reverse transcriptase inhibitors, non-nucleoside reverse transcriptase inhibitors and protease inhibitors. Most of these drugs bear some significant drawbacks such as relatively short half-life, low bioavailability, poor permeability and undesirable side effects. Efforts have been made to design drug delivery systems for antiHIV agents to: a) reduce the dosing frequency, b) increase the bioavailability and decrease the degradation/metabolism in the gastrointestinal tract, c) improve the CNS penetration and inhibit the CNS efflux, and d) deliver them to the target cells selectively with minimal side effects. This article is an attempt to compile all major research work towards drug delivery for AIDS therapy and channel future attempts in the area of more effective controlled delivery of antiHIV agents.
Human Immunodeficiency Virus (HIV) is a retrovirus that can be subdivided into HIV-1 and HIV-2. Both types of HIV infection depletes the helper T-lymphocytes (CD4 cell/mm3), resulting in continued destruction of the immune system, leading to the occurrence of opportunistic infections and malignancies. A person infected with HIV is defined by Centers for Disease Control and Prevention (CDC) as having positive antibodies against HIV (positive HIV test), with 200 or more helper T-lymphocytes, and the absence of an Acquired Immunodeficiency Syndrome (AIDS) defining illness. By definition then, an HIV infected person with AIDS has fewer than 200 cells/mm3 CD4 cells or the presence of AIDS defining illness.
During the last decade, though attempts were being made to eradicate HIV, it was found that eradication of HIV is highly unlikely, and effective antiretroviral therapy is required on a long-term basis to maintain viral suppression and reduce disease progression. During this decade, effective therapies aimed at continued suppression of HIV replication and targeted at resting HIV reservoirs such as brain, lymphatic systems will be critical to prolong survival and renewing hopes for a cure. Thus goals of antiretroviral therapy include, reducing the symptoms of HIV infection and delay disease progression to AIDS, reducing viral load to undetectable levels or lowest level possible for sufficiently longer duration, maintenance of durability of viral suppression, eliminating resting reservoirs of HIV, reducing viral resistance and drug failure, designing effective therapeutic regimens that minimize the drug adherence problem, reducing total pill burden and minimizing interference with quality life.
Diagnosis and commencement of treatment
Established HIV infection is diagnosed by finding antibodies to HIV in the plasma using various serological testing methods such as ELISA (Enzyme Linked Immuno Sorbent Assays), Orasure western blot, SUDS (Single Used Diagnostic System), Orasure HIV-1. Generally according to the United States Food and Drug Administration and World Health Organization guidelines, the antiretroviral therapy is commenced with these observations, such as when patients experience severe symptoms of HIV infection or have been diagnosed with AIDS or when the viral load in the blood sample is found to be 50,000 copies/ml or more or when the CD4 cell count is less than 200-350 cells/mm3.
Therapy
The major classes of antiretroviral agents that are in common use include the nucleoside reverse transcriptase inhibitors (NRTI), protease inhibitors (PI), non-nucleoside reverse transcriptase inhibitors (NNRTI).
Nucleoside reverse transcriptase inhibitors (NRTI)
These are the first class of antiretrovirals. All compounds in this class are prodrugs which need to be converted intracellularly in the cytoplasm to their active form before exerting their antiviral activity. The active forms of these drugs are substrates for reverse transcriptase enzyme, and they result in termination of DNA chain elongation of the retrovirus. They include zidovudine (AZT), didanosine (ddI), zalcitabine (ddC), stavudine (d4T), lamivudine (3TC), abacavir, tenofovir DF and emtricitabine (Table 1). A few other molecules in this class are under investigation such as alovudine, amdoxovir (DAPD), DPC817, elvucitabine, lodanosine, lobucavir and racivir.
| Name and Date of FDA approval | Bioavailability (%) | Half-life (h) | Tmax (h) | Primary routes of *elimination | Dosage forms | |
|---|---|---|---|---|---|---|
| Zidovudine (AZT) 19th March 1987 | 60-70 | 1.1 | 0.5-1.5 | Glucuronidation; renal excretion of the parent drug and glucuronide | Capsule, tablet, syrup, injection | |
| Didanosine (ddI) 9th October 1991 | 25-43 | 1.3-1.6 | 0.6-1 | Renal excretion | Tablet, solution | |
| Zalcitabine (ddC) 19th June 1992 | 88 | 1.2 | 0.8-1.5 | Renal excretion | Tablet | |
| Stavudine (d4T) 24th June 1994 | 82-99 | 0.9-1.2 | 0.5-0.75 | Renal excretion | Capsule, powder | |
| Lamivudine (3TC) 17th Nov 1995 | 86-88 | 8.5 | 0.9 | Renal excretion | Tablet, solution | |
| Abacavir 17th Dec 1998 | 83 | 1.5 | - | Renal excretion and hepatic metabolism | Film coated tablet | |
| Tenofovir 26th Oct 2001 | 25% in Fasting increases with food | - | 1.0 | Renal excretion | Tablet | |
| Emtricitabine 2nd 2003 | Rapid and extensively absorbed | 10 | 1-2 | Renal excretion | Capsules | |
*Elimination includes both metabolism and excretion of drug.
Table 1: Nucleoside Reverse Transcriptase Inhibitors Approved By Usfda For Hiv
Protease inhibitors (PI)
They act primarily at the end of the HIV life cycle to cause the formation of non-infectious immature virions. These agents represent a major advance in the management of HIV disease and have dramatically altered disease progression to AIDS. They include saquinavir, ritonavir, indinavir, nelfinavir, amprenavir, lopinavir (always used in combination with ritonavir), atazanavir, fosamprenavir (Table 2); and a few newer molecules under investigation are tipranavir, DMP-450 and TMC 114.
| Name and Date of FDA approval | Bioavailability (%) | Half-life (h) | Tmax (h) | Primary routes of *elimination | Dosage forms |
|---|---|---|---|---|---|
| Saquinavir 6th Dec 1995 | 4-12 | 13.2 | 3 (with food) | Intestinal and hepatic metabolism | Capsule |
| Ritonavir 1st Mar 1996 | 60 | 3-5 | 3.4 | Hepatic metabolism | Capsule, solution |
| Indinavir 13th Mar 1996 | Well absorbed | 2 | 0.8 | Hepatic metabolism | Capsule |
| Nelfinavir 14th Mar 1997 | Well absorbed | 3.5-5 | 3.4-4 | Hepatic metabolism | Tablet, powder |
| Amprenavir 15th Apr 1999 | Not well absorbed | 7.1-10.6 | 1.9 | Hepatic metabolism and renal excretion | Capsule, solution |
| Atazanavir 20th June 2003 | Rapidly absorbed | 7 | 2.5 | Extensive hepatic metabolism and renal excretion | Capsule |
| Fosamprenavir 20th Oct 2003 | Not yet established | 7.7 | 1.5-4 | Hepatic metabolism | Tablet |
*Elimination includes both metabolism and excretion of drug.
Table 2: Protease Inhibitors Approved By Usfda
Non-Nucleoside reverse transcriptase inhibitors (NNRTI)
These inactivate the HIV-1 reverse transcriptase enzyme by non-competitively binding directly to the HIV-1 reverse transcriptase structure likely at amino acid positions 100 and 103. These are not active against HIV- 2. Unlike NRTIs, these do not require activation by cellular phosphorylation. They include nevirapine, delavirdine, efavirenz (Table 3); and a number of newer molecules are under investigation such as DPC 083, DMC 961, DMC 963 and TMC 125 [1-5].
| Name and Date of FDA approval | Bioavailability (%) | Half-life (h) | Tmax (h) | Primary routesof *elimination | Dosage forms |
|---|---|---|---|---|---|
| Nevirapine 21st June 1996 | 90 | >22 | 1.5 | Possible hepatic metabolism | Tablet |
| Delavirdine 4th Apr 1997 | 85 | 2.4 | 1.2 | Hepatic metabolism | Tablet |
| Efavirenz 17th Sept 1998 | Well absorbed | 52-76 | 5 | Hepatic metabolism | Capsule film coated tablet |
*Elimination includes both metabolism and excretion of drug.
Table 3: Non Nucleoside Reverse Transcriptase Inhibitors Approved By Usfda
Novel strategies
These will focus on essential steps in the life cycle of HIV, preventing HIV entry and infection of CD4 lymphocytes and other target cells.
Peptide fusion inhibitors
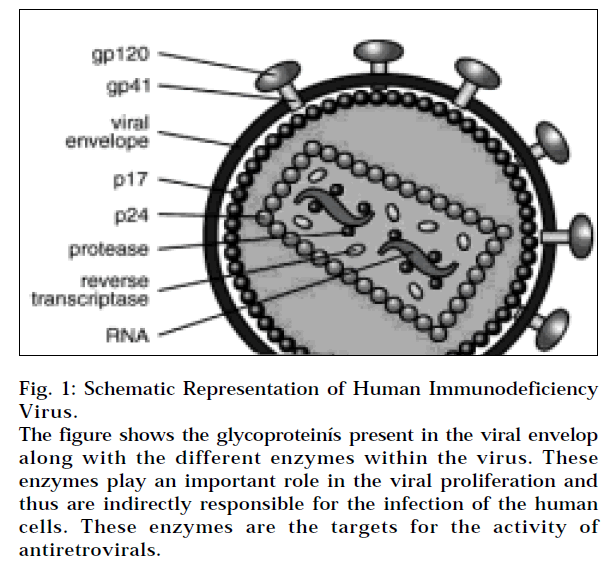
These inhibit HIV from entering the target cells by hindering the gp41 protein on the virus (Fig 1). Enfuvirtide is the first representative of this new group of antiretrovirals - fusion inhibitors, which is approved for clinical use. Due to its attachment to the HR1 domain of HIV glycoprotein gp 41, enfuvirtide blocks the fusion between the virus and the target cell. Enfuvirtide is chemically a synthetic peptide consisting of 36 amino acids and is not stable in GIT and must be administered only subcutaneously. Enfuvirtide has been registered for use as salvage therapy of patients with multi-resistance, for whom it is not possible to constitute an effective combination of other available antiretrovirals [6-8]. Other fusion inhibitors under investigation are BMS-488043, SCH-D.
Vaccines
Vaccines are being developed for prevention as well as for treatment of HIV infection/AIDS. Preventive vaccines are for HIV-negative individuals; they are to prevent HIV infection. Therapeutic vaccines are for HIV-positive individuals; they are to improve the immune system. Currently no HIV/AIDS vaccines are approved for use; however, many are in clinical trials.
Combinational therapy
Mono-therapy is no longer recommended because incomplete viral suppression can encourage development of resistance. Similarly magnitude and durability of viral suppression is lower with dual antiretroviral combinations compared with combinations containing three or more agents. For example, generally two NRTIs are combined with antiretrovirals from PI or NNRTI class. Similarly, mono-therapy with PI or NNRTI is also not advisable in order to prevent the emergence of resistance and subsequent drug failure. The current strategy for the treatment of HIV infection is called highly active antiretroviral therapy (HAART) and is based on cocktails of drugs that are currently approved by the Food and Drug Administration. These drugs include compounds that target the viral entry step and the enzymes reverse transcriptase or protease. The introduction of HAART has dramatically changed the landscape of HIV disease. Death from AIDS-related diseases has been reduced significantly since HAART came into use. Nevertheless, it is not clear how long clinical benefit will last taking into account the emergence of multiple drug-resistant viral strains. Addition of new anti-HIV drugs targeting other steps of the viral replication cycle may increase the potency of inhibition and delay resistance development. HIV integrase is an essential enzyme in the HIV life cycle and is an attractive target for new drug development. Despite years of intensive research, only two classes of compounds that block integrase are identified until now, namely, the diketo acids and the pyranodipyrimidines [9]. The molecule under investigation in this class is L-000870810.
Drawbacks of conventional Route
Majority of the currently marketed antiHIV agents are formulated as solid dosage forms, viz., tablets and capsules for oral use; or liquid dosage forms, viz., solutions, suspensions for oral and parenteral use. While the oral dosage forms offer convenience, delivery of drugs via this route suffers from significant first pass effect, variation of absorption and degradation in the gastrointestinal tract due to enzymes and extreme pH conditions. For example, zidovudine the first antiretroviral developed, although rapidly absorbed from the intestine, loses considerable potency by the hepatic first pass metabolism (40%) and then rapid elimination from the body with a biological half life of only 1 h. As a shortfall for all the conventional oral dosage forms, the duration of action is limited since the absorption of the drug depends on the resident time of the drug in the gastrointestinal tract [10]. Also, many of these compounds exhibit poor or low bioavailability due to various other factors, namely, physicochemical properties such as dissolution rate and solubility, or biological properties such as permeability (didanosine exhibits low intestinal permeability) and metabolism. The performance of a drug is a function of its physicochemical properties, such as aqueous solubility and drug stability. Studies have shown that solution stability and acid liability have become a significant concern in the dosage form development of dideoxynucleosides. Thus the causes for poor and variable absorption are vast, but they can primarily be related to physicochemical properties such as dissolution rate and solubility. For example, the oral bioavailability of NNRTIs is limited due to their low aqueous solubility. Thus the bioavailability variability of antiretrovirals may be a significant factor in the failure of some of the drug regimens. Also, after an antiHIV drug is absorbed and enters the blood circulation, metabolism/elimination and transport barriers will substantially decrease the effective amount of drug reaching the target action site. In order to succeed in an effective therapy for AIDS, it is crucial to maintain the systemic drug concentration consistently above their target antiretroviral concentration throughout the course of the treatment and to enhance localization and intracellular delivery of the drug. However, because of the short biological half life of number of these drugs, conventional routes are inherently limited in that they can not maintain a constant plasma level with the target therapeutic range for a prolonged duration [11]. Due to virustatic nature of the drugs, they must be administered for the life of the patient. All these therapeutic moieties exhibit dose-dependent toxic side effects such as hepatotoxicity, hyperglycaemia, hyperlipidemia, lactic acidosis, lipodystropy, osteonecrosis, osteoporosis, osteopenia, skin rashes, resulting from excessive systemic concentration, and they often require dosage reduction or even cessation of treatment, since conditions like lactic acidosis may even be fatal. Thus despite their undisputed effectiveness, several complicated clinical issues are associated with the use of these agents. Adherence of the patient to the treatment is critical, as loss of antiviral efficacy has been correlated with poor pill taking resulting in loss of viral suppression. The high pill burden [examples: fosamprenavir-1400 mg twice daily, nelfinavir-1250 mg twice daily (10 tablets daily), amprenavir 1200 mg three times daily (16 tablets daily)] and coordination with the meals make these agents laborious to take [12].
Novel Approach
Researchers are being challenged to find new treatment strategies as the currently used drug therapies begin to fail in some patient population, reports on termination of therapy due to drug side effects are mounting, and new drug resistant strains of HIV are emerging. These strategies include a ‘‘multi-drug cocktail’’ therapy that attacks at several stages of HIV life cycle; a therapeutic vaccine which can boost the immune response against the virus; the development of a preventive vaccine based on a weakened strain of HIV, and the successful maintenance of HIV-inhibitory concentrations at target sites with minimal side effects. To avoid hepatic first pass metabolism and intestinal degradation, efforts are being made to alter the mode and route of delivery of the drug [13]. Delivery of nucleoside analogues through percutaneous, rectal, buccal, nasal, intrathecal routes and as coated dosage form by oral route, are being studied. Percutaneous absorption has been one of the most reported routes for non-oral administration of antiHIV agents [14]. Also efforts have been made to design drug delivery systems for antiHIV agents to reduce the dosing frequency, to enhance the bioavailability, to improve the CNS penetration and inhibit the CNS efflux and to deliver them to the target cells selectively with minimal side effects. Amongst the recent approaches controlled and targeted delivery are the noted ones.
Controlled drug delivery
In order to fulfil the need of a long-term treatment with antiHIV agents, where most of them suffer from the drawbacks of frequent administration, plasma concentration fluctuation, significant adjustment in the lifestyle, it is desirable to have controlled- or sustained-release drug delivery systems to improve the overall therapeutic benefit and to achieve an ideal therapy. By sustained or controlled delivery, it is possible to achieve effective plasma concentration without significant fluctuation, to avoid sub-therapeutic and toxic plasma concentrations, to facilitate release of the medication in a controlled manner to obtain a continuous delivery, to achieve an effective therapy with low dosage of the drug, to reduce the frequency of medication and thus to improve patient adherence, by preventing the interference of therapy with the day-to-day lifestyle.
Thus it was proposed that a non-invasive zero-order delivery such as the transdermal route is desirable, as controlled delivery via oral route retains most of the drawbacks of conventional oral delivery. Transdermal delivery can provide sustained delivery of antiHIV agents for a predetermined period at a predetermined rate. It can enhance the antiviral activity and reduce the dosing frequency and severity of toxic side effects by optimizing the blood concentration profiles within the therapeutic range for longer duration. Transdermal delivery improves the bioavailability by circumventing hepatic first pass, acid instability, intestinal permeability factor; which are the most common problems faced with the oral route. Percutaneous absorption of a number of antiretrovirals has been studied indicating a promising future for this route for the delivery of antiretrovirals [15-18].
Novel drug delivery carriers such as liposomes, microparticles and drug-encapsulated erythrocytes are also under investigation. Liposomes as drug delivery systems are very versatile in that they can be tailored to suit the delivery of various drug molecules. The ability of liposomes to alleviate the toxicity of various drugs has been established. Liposomal formulations of toxic drugs like doxorubicin and amphotericin B were significantly less toxic compared to the free drug. The effect of liposomal encapsulation, on the haematopoietic toxicity and antiviral efficacy of AZT has been studied in mice. Liposomal encapsulated AZT was more effective than the AZT in preventing the development of plasma reverse transcriptase activity after infection of mice with the retrovirus. Thus the liposomal AZT resulted in a significant reduction in the toxicity, a substantial enhancement of antiviral activity coupled with enhanced localization of the drug in the liver and spleen. Furthermore, it is observed that encapsulation of dideoxycytidine-5’-triphosphate (ddCTP) in liposomes exhibited good chemical stability of the drug molecule. Results obtained with this encapsulated ddCTP in the murine acquired immunodeficiency syndrome model indicate that ddCTP encapsulated in liposomes can reduce proviral DNA in cells of the mononuclear phagocyte system (MPS) in both spleen and bone marrow. Also the liposomal form increases the efficacy, reduces the toxicity and increases the half life of the drug [19-21].
Targeted/intracellular delivery
A more advanced version of controlled delivery is the targeted delivery. Targeted delivery exhibits all the advantages of the controlled delivery and at the same time facilitates delivery of the drug to the target site. It has been found that viral load in certain type of cells, such as lymphocytes and macrophages, are much higher than the other cells. These high viral load cells can serve as reservoir for HIV. Therefore it is obvious that targeting the antiHIV agents specifically to these cells would increase the efficacy of the therapy. To enhance localization and intracellular delivery, these agents have been encapsulated in liposomes or antibody-bearing liposomes. The antiviral effects of 2’,3’-dideoxycytidine (ddC), 2’,3’-dideoxycytidine-5’-triphosphate (ddCTP) and liposome-encapsulated ddCTP were compared in cultured human monocyte-macrophages infected with HIV-1. These treatments inhibited virus replication at nanomolar drug levels. Studies on drug stability and uptake suggest that a large part of the free ddCTP is dephosphorylated before entering the cells, whereas the liposome encapsulated remains stable over days and is taken up probably by endocytosis [22]. The study of pharmacokinetic profiles and tissue distribution of long circulating and conventional liposomes of 2’,3’-dideoxyinosine (ddI) was determined using male Sprague-Dawley rat as animal model. The study indicated that the long circulating liposomes of ddI exhibited decreased clearance and reduced accumulation of the drug in several tissues and thus may act as a better drug delivery and also may provide prolonged exposure of HIV infected tissues like CNS, thymus, liver, kidney, heart, lungs, salivary glands, eyes, foetal tissue to didanosine [23]. Nanoparticles also have been used as carriers for the delivery of zidovudine, saquinavir and zalcitabine to monocytes or macrophages. Polyhexylcyanoacrylate nanoparticles loaded with saquinavir and zalcitabine were prepared by emulsion polymerization and tested for antiviral activity in primary human monocytes/macrophages in vitro. A significantly higher efficacy was observed with saquinavir-loaded nanoparticles. Using nanoparticles as a drug carrier system could improve the delivery of antiviral agents to the mononuclear phagocyte system in vivo, overcoming pharmacokinetic problems and enhancing the activities of drugs for the treatment of HIV infection and AIDS [24]. Liposomal encapsulated zalcitabine inhibited virus replication at nanomolar drug level, which suggests the capability of liposomes for targeting can be exploited [25,26]. Liposomes have also been shown to decrease bone marrow toxicity and enhance activity against murine AIDS induced immunosuppressant [27].
To conclude, although remarkable achievements in AIDS therapy have been attained since this fatal disease was first recognized more than a decade ago, enormous challenges remain for scientists to ultimately halt the progression and find a cure for AIDS. Currently available antiHIV agents have relatively short half life, low bioavailability, poor CNS penetration and retention, and undesirable side effects. These drawbacks give researchers tremendous opportunities to design and develop novel drug delivery systems to overcome the transport barriers and inherent elimination and metabolism problems associated with these antiHIV drugs. Delivery systems for these drugs are being developed to compensate the shortcomings; however, the benefits of technological advancements are yet to reach the poor and needy patients.
Acknowledgements
The authors wish to thank Prof. B. G. Shivananda, Principal, Al-Ameen College of Pharmacy Bangalore, for his continuous support rendered to us in the preparation of this article. Also, we are thankful to Al-Ameen College of Pharmacy for providing us with excellent facilities. The authors are thankful to AICTE its help in the succesful completion of the article.
References
- Betty, J.D., In; Herfindal, T.E., Eds., Text Book of Therapeutics Drug and Disease Management, 7th Edn., Lippincott Williams and Wilkins, USA, 2000,1555.
- Stephen, R. and David, W.H., In; Rall, T.W., Taylor, P., Nies, A.S. and Gilman, A.G., Eds., Goodman and Gilman’s The Pharmacological Basis of Therapeutics, 8th Edn., Vol. II., Pergamom Press Inc., 1991,1182.
- Gulick, R.M., Clin. Microbiol. Infect., 2003, 9, 186.
- Mcnicholl, I.R., Curr. Infect. Dis. Rep., 2004, 6, 159.
- Tavel, I.A., Expert Opin. Invest. Drugs., 2000, 9, 917.
- Machala, L., Klin. Mikrobiol. Infekc. Lek., 2004, 10, 114.
- Greenberg, M., Cammack, N., Salgo, M. and Smiley, L., Rev. Med.Virol., 2004, 14, 321.
- Jamjian, M.C. and McNicholl, I.R., Amer. J. Health Syst.Pharm., 2004, 61, 1242.
- Witvrouw, M., Van Maele, B., Vercammen, J., Hantson, A., Engelborghs, Y., De Clercq, E.,Pannecouque, C. and Debyser, Z., Curr. Drug Metab., 2004, 5, 291.
- Yong, H. L. and Patrick, J. S., Adv. Drug. Del. Rev., 1999, 39, 1.
- Xiaoling, L., William, K. C., Adv. Drug. Del. Rev., 1999, 39, 81.
- Bruce, J. A., Adv. Drug. Del. Rev., 1999, 39,105.
- Haresh, M., Chien, Y.W., Int. J. Pharm., 1993, 95, 1.
- Dae, D.K., Chien, Y.W., J. Pharm. Sci., 1995, 85, 214.
- Dae, D.K., Chien, Y.W., J. Control. Rel., 1996, 40, 67.
- Dae, D.K., Chien, Y.W., Drug Develop. Ind. Pharm., 1996, 22, 1047.
- Yi, J., Toshinobu, S., Yasunori, M., Kazuhiko, J., Drug Develop.Ind. Pharm., 2000, 26, 193.
- Thomas, N.S., Panchagnula, R., Eur. J. Pharm. Sci., 2003, 18, 71.
- Oussoren, C., Magnani, M., Fraternale, A., Casabianca, A., Chiarantini, L., Ingebrigsten, R., Underberg, W.J., Storm, G., Int. J.Pharm., 1999, 180, 261.
- Duzgunes, N., Pretzer, E., Simoes, S., Slepushkin, V., Konopka, K., Flasher, D. de., Lima, M.C., Mol. Membrane Biol., 1999, 16, 111.
- Desormeaux, A., Bergeron, M.G., J. Drug Target., 1998, 6,1.
- Szebeni, J., Wahl, M.S., Betagiri, V.G., Wahl, M.L., Gartner, S., Popovic, M., Parker, J.R., Black, D.V.C., Weinstein, J.N., AIDSRes. Hum. Retrovirus.,1990, 6, 691.
- Kim, S., Scheerer, S., Geyer, M.A., Howell, S.B., J. Infect. Dis., 1990, 162,750.
- Andreas R.B., Hagen V.B., Jo R.K, Ian B.D., Antimicrob. Agents Chemother.,1996, 40, 1467.
- Dipali, R. S., Singh, M., Betagiri, G. V., Drug Del., 1996, 3, 279.
- Dipali, R. S., Lin, Y.J., Ravis, W. R., Betagiri, G.V., Int. J. Pharm., 1997, 152, 89.
- Phillips, N.C., Tsoukas, C., Blood, 1992, 79, 1137.