- Corresponding Author:
- S. Grover
Department of Psychiatry, Postgraduate Institute of Medical Education and Research, Chandigarh-160 012
E-mail: drsandeepg2002@yahoo.com
| Date of Submission | 11 February 2014 |
| Date of Revision | 30 October 2015 |
| Date of Acceptance | 01 December 2015 |
| Indian J Pharm Sci 2015;77(6):771-779 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
Ethnic and regional variations have been found in the pharmacological treatment response. Though many efficacy studies have been conducted in India for antipsychotic treatment modalities of schizophrenia, there is a lack meta-analytic data of the existing literature from India. This study aimed to conduct a systematic review and meta-analysis of the antipsychotic treatment trials of schizophrenia in the Indian context. All controlled trials from India evaluating the clinical efficacy of antipsychotics in patients with schizophrenia were evaluated and 28 trials were included in the metanalysis. Effect sizes were computed using Cohen's 'd' and risk of bias was evaluated. Meta analysis revealed superiority of first generation antipsychotics over placebo (mean effect size of 1.387, confidence interval of 1.127 to 1.648). Second generation antipsychotics were marginally better than first generation antipsychotics (effect size 0.106, confidence intervals 0.009 to 0.204). There was improvement in the methodology of the trials over time (Kendall tau=0.289, P=0.049), though no statistically significant increase in trial duration and sample size was noted. There is lack of data on long term efficacy of antipsychotic in schizophrenia from India. First generation antipsychotics have demonstrated benefits over placebo in patients with schizophrenia in the Indian context, though marginally lesser than second generation ones.
Keywords
Schizophrenia, India, antipsychotics, meta-analysis
Schizophrenia is a severe mental illnesses associated with significant morbidity and poor quality of life [1-4]. It is not only associated with significant personal distress [5], but it also causes increased mortality due to suicides and associated medical illnesses [6]. Schizophrenia is also associated with increased rates of substance use disorders [7], high care giver burden [8] and occurrence of violence [9]. The social and economic costs of this disorder are considered to be substantial [10]. Adequate symptom control is considered to be paramount to reduce the morbidity and mortality associated with the disorder. Besides psychosocial interventions, use of antipsychotics is considered to be the most important treatment strategy to manage this disorder.
The last two decades has seen better understanding into the pharmacogenomics of medications including antipsychotics [11,12]. Ethnic differences in metabolism and action of drugs, which can have an impact on efficacy and thus important from standpoint of clinical decision making, are being gradually explored [12]. Hence, it becomes meaningful to ascertain how well do the interventions work in a particular ethnic background.
Over the years many studies have evaluated the efficacy/effectiveness of antipsychotics in patients from India [13]. However, it is at times not possible to reach to a conclusion about the usefulness of a particular antipsychotic medication based on a single trial. Meta-analytic studies have become the benchmark for compiling information from individual studies to make quantitative based recommendations. We were not able to identify any meta-analysis of studies originating from India evaluating the usefulness of antipsychotic medications, though there are 2 meta-analyses, which have evaluated the usefulness of electroconvulsive therapy (ECT) [14,15]. The results of these studies suggest that active ECT was more efficacious than sham ECT or placebo. Also, it has been found that ECT when combined with antipsychotics achieves better results than ECT alone. Addition of ECT may hasten the response to treatment in patients receiving antipsychotics [14,15].
This systematic review and meta-analysis was conducted with the objective of assessing the efficacy/ effectiveness of various antipsychotic medications in schizophrenia in the Indian context. Additionally, an attempt has been made to look at the deficiency of data originating from India, and as to how to plan future studies, which can be more meaningful.
Materials and Methods
Search strategy
Electronic searches for published trials were carried out using PubMed, Psych Info and Google Scholar search engines. The keywords were ‘schizophrenia’, ‘India’, ‘antipsychotic’ (also names of individual antipsychotics), ‘efficacy’, ‘effectiveness’ and ‘usefulness’. These key words were used in various combinations. The multiple searches were carried out through PubMed and other search engines in May 2013. After screening all the available data we found 296 relevant abstracts. Further studies were identified from the cross references and reference list of included studies. Searches were also made through Medknow publishers of journals from India that included Indian Journal of Psychiatry, Journal of Postgraduate Medicine, Indian Journal of Psychological Medicine, Indian Journal of Pharmacology and others. Unpublished work was not sought for as a part of this review and meta-analysis.
Study selection
The selection criteria for inclusion of various studies into this review and meta-analysis were, controlled trials evaluating an antipsychotic treatment modality for schizophrenia, the diagnosis of schizophrenia being made in accordance to any nosological system or through clinician’s interview, studies having atleast 2 treatment arms, reporting outcome measure of efficacy and published in English language peer reviewed journals. Studies evaluating the treatment modality in animal models and those evaluating the efficacy/effectiveness of antipsychotics in other conditions like bipolar depression, conduct disorder, and mental retardation were excluded. Studies with less than 5 participants in an individual treatment arm, or which had reported results in manner from which effect sizes could not be calculated were excluded from the meta-analysis. Multinational trials in which patients were recruited from India but the country specific data was not analyzed separately were also excluded.
Data extraction
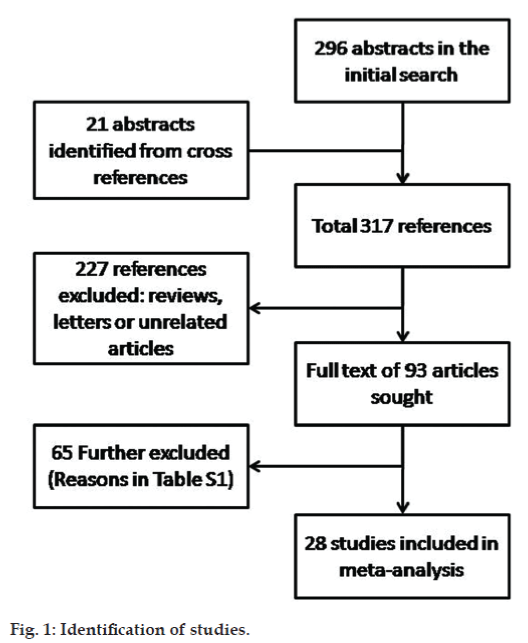
Data extraction from the identified abstracts was carried out by two investigators independently (SG and SS, fig. 1). Initial searches yielded 296 relevant articles. Cross references of these articles yielded additional 21 relevant articles. Of these articles, 93 studies were identified, which evaluated the use of antipsychotics in patients with schizophrenia. These articles were further evaluated on the inclusion and exclusion criteria for the metaanalysis. The full text of all identified studies were reviewed independently by both the investigators for the study characteristics (e.g. nature of the study, manner of randomization, blinding, duration of study, and intention to treat analysis), and clinical information (number of subjects, age range or mean, gender distribution, diagnoses made, medication groups, past treatment, efficacy/effectiveness measure, outcome and side effects) and risk of bias. Any discrepancies between the evaluators were resolved by mutual discussion. There was overall a high degree of concordance between the evaluators.
Based on the inclusion and exclusion criteria, 65 papers were excluded. The excluded studies are shown in supplemental table and the most common reason for exclusion of studies was lack of a control group in the study. The final meta-analysis included 28 studies.
For studies, which had reported more than one outcome measure, the primary efficacy measure was used for calculation of effect size. Wherever possible, the percentage of participants improved was used for calculation of effect size. Data from intention to treat analysis was used wherever possible. The number needed to treat (NNT) was also calculated for placebo controlled studies.
Risk of bias
The studies included in the meta-analysis were assessed for risk of bias. The elements that were studied for risk of bias included random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data and dropouts and selective reporting of results. Jadad scale [16] was used to quantify the risk of bias of trials included in the meta-analysis. The rating was done on the basis of reporting of randomization, blinding and reporting of withdrawals and drop-outs. The Jadad scale has been shown to have good validity and reliability [17].
Statistical analysis
Effect sizes were calculated for each antipsychotic medication. The effect size is a measure of the efficacy of an intervention. This allows easy comparison of studies using disparate methodology and efficacy measures. Effect sizes in the present study were calculated using the standardized mean difference (d). This was selected because it gives a robust measure for both categorical and continuous measures. For dichotomous variables of efficacy, logit method was used for deriving effect size and confidence intervals. In the present meta-analysis, random effects model was used for computing the mean effect sizes. Random effects model has been shown to be superior to the fixed effects model, especially when disparate studies are combined for analysis, which was expected for this meta-analysis. The I2 test of heterogeneity was used for assessing variation (heterogeneity) in the studies.
In studies, which had more than two interventions in defined groups, effect sizes were calculated for individual comparisons. Meta-analysis was conducted for comparisons, which had at least 3 trials. Mean effect sizes with confidence intervals were calculated for comparisons of first generation antipsychotics (FGAs) versus placebo, second generation antipsychotics (SGAs) versus FGAs.
Results and Discussion
Twenty eight studies and thirty five comparisons were included in the meta-analysis, as shown in Tables 1 to 3. Of the included studies, 10 were open labeled randomized controlled trials (RCTs), 7 were double blind RCTs, 4 were controlled trials, 3 were matched controlled trials, 2 were double blind controlled trials, and two were cross-over trials. Sixteen studies compared FGAs with another FGA or a placebo, 8 studies compared SGA with a FGA or another FGA, and 4 compared medications to other forms of treatment like ECT.
| Authors | Intervention | Number | Methodology | Efficacy measure | Duration | Effect sizes (CI) |
|---|---|---|---|---|---|---|
| Bagadiaet al. [18] | Chlorpromazine versus trifluoperazine | 50 versus 50 | Matched controlled trial | Clinician rated improvement | 3–4 weeks | 0.045 (−0.390–0.479) |
| Bagadiaet al. [19] | Pimozide versus trifluoperazine | 16 versus 14 | Crossover trial | Clinician rated improvement | 3 months | −0.179 (−1.256–0.898) |
| Channabasavanna and Michael [20] | Penfluridol versus placebo | 15 versus 15 | Controlled trial | SAPS, SANS | 12 weeks | 2.402 (1.010–3.794) |
| De Sousa and Nayani [21] | Trifluperidol versus trifluoperazine | 25 versus 25 | RCT | Clinician rated improvement | 6 weeks | −0.192 (−0.832–0.448) |
| Doongajiet al. [22] | Injectable prothipendyl versus placebo | 8 versus 5 | Controlled trial | Clinician rated improvement | 6 weeks | 1.046 (−0.521–2.613) |
| Kishore et al. [23] | Thiothixene versus prochlorperazine | 10 versus 10 | RCT | PSSRS | 90 days | −0.467 (−1.479–0.545) |
| Kishore et al. [23] | Thithixene versus trifluoperazine | 10 versus 10 | RCT | PSSRS | 90 days | −0.764 (−1.858–0.330) |
| Kishore et al. [23] | Thiothixene versus thioproperazine | 10 versus 10 | RCT | PSSRS | 90 days | 0 (−0.966–0.966) |
| Kishore et al. [24] | Trifluperidol versus prochlorperazine | 20 versus 20 | DBCT | PSSRS | 90 days | −0.744 (−1.707–0.218) |
| Kishore et al. [24] | Trifluperidol versus thiothixene | 20 versus 20 | DBCT | PSSRS | 90 days | 0 (−0.746–0.746) |
| Mahal and Janakiramaiah [25] | Pimozide versus placebo | 25 versus 24 | DBRCT | Mental status questionnaire | 6 months | 0.521 (−0.267–1.308) |
| Menon [26] | Trifluopreazine versus placebo | 30 versus 30 | Crossover | Behavior chart | 16 weeks | 0.413 (−0.227–1.053) |
| Menon [26] | Thiothixene versus placebo | 30 versus 30 | Crossover | Behavior chart | 16 weeks | 0.619 (−0.011–1.249) |
| Menon [26] | Trifluopreazine versus thiothixene | 30 versus 30 | Crossover | Behavior chart | 16 weeks | −0.206 (−0.779–0.367) |
| Menon [27] | Prochlorperazine versus placebo | 10 versus 10 | Matched control | Social interaction | 8 weeks | 1.976 (0.552–3.399) |
| Narayan et al. [28] | Prochlorperazine versus chlorpromazine | 10 versus 10 | RCT | Clinical ratings | 6 months | 0.297 (−0.837–1.431) |
| Ramachandran and Menon [29] | Trifluperidol versus placebo | 25 versus 25 | DBRCT | Clinician rating | 6 weeks | 2.445 (1.407–3.483) |
| Sethi and Bhiman [30] | 15 versus 15 | DBCT | BPRS | 4 weeks | 0.277 (−0.234–0.788) |
CGI: Clinical global impressions, DBCT: double blind controlled trial, DBRCT: double blind randomized controlled trial, PANSS: positive and negative syndrome scale, PSSRS: psychotic symptom severity rating scale, RCT: randomized controlled trial, SANS: scale for assessment of negative symptoms, SAPS: scale for assessment of positive symptoms, Time durations: days, weeks, months, CI: confidence interval
Table 1: Studies of first generation antipsychotics included in meta-analysis
| Authors | Intervention | Number | Methodology | Efficacy measure | Duration | Effect sizes (CI) |
|---|---|---|---|---|---|---|
| Avasthiet al. [34] | Olanzapine versus haloperidol | 17 versus 10 | Open RCT | BPRS, PANSS, CGI | 12 weeks | −0.153 (−1.222–0.917) |
| Chandra et al. [35] | Risperidone versus centbutindole | 22 versus 22 | DBRCT | PANSS, CGI | 8 weeks | −0.190 (−1.246–0.866) |
| Dharet al. [36] | Olanzapine versus haloperidol | 20 versus 20 | RCT | PANSS, ESRS | 6 months | 0.503 (−0.126–1.325) |
| Jindal et al. [37] | Aripiprazole versus olanzapine | 26 versus 27 | DBRCT | BPRS, PANSS | 6 weeks | 0.138 (−0.401–0.677) |
| Shah and Joshi [38] | Paliperidone versus olanzapine | 109 versus 105 | DBRCT | PANSS, CGI | 6 weeks | 0.007 (−0.370–0.384) |
| Shrivastava and Gopa [39] | Risperidone versus haloperidol | 50 versus 50 | RCT | PANSS, CGI | 1 year | −0.072 (−0.623–0.480) |
| Singamet al. [40] | Risperidone versus chlorpromazine | 50 versus 50 | RCT | PANSS | 1 year | 0.170 (−0.181–0.521) |
| Sagar and Chandrashekar [41] | Risperidone versus haloperidol | 23 versus 23 | DBRCT | PANSS, CGI | 6 weeks | 0.594 (0.004–1.185) |
BPRS: Brief psychiatric rating scale, CGI: clinical global impression, DBRCT: double blind randomized controlled trial, ESRS: extrapyramidal symptom rating scale, PANSS: positive and negative syndrome scale, RCT: randomized controlled trial, Time durations: weeks, months, years, CI: confidence interval
Table 2: Studies of second generation antipsychotics included in meta-analysis
| Authors | Intervention | Number | Methodology | Efficacy measure | Duration | Effect sizes (CI) |
|---|---|---|---|---|---|---|
| Bagadiaet al. [42] | ECT versus FGA | 50 versus 200 | Matched controlled trial | Clinician rated improvement | At least 3 weeks | 0.731 (0.195–1.267) |
| Das et al. [43] | Medication + ECT versus medication only | 23 versus 25 | Comparative study | GAs | Variable | 0.962 (0.457–1.467) |
| Janakiramaiah and Subbakrishnan [44] | ECT + chlorpromazine versus chorpromazine | 22 versus 22 | RCT | RP scale, CGI | 6 weeks | 0.091 (−0.501–0.682) |
| Ray [45] | ECT + chlorpromazine versus ECT | 20 versus 20 | Controlled trial | Clinician rating | Average 15 ECT sittings | 0.606 (−0.132–1.344) |
| Ray [45] | ECT + chlorpromazine versus chlorpromazine | 20 versus 20 | Controlled trial | Clinician rating | Average 15 ECT sittings | 0.606 (−0.132–1.344) |
| Bagadiaet al. [42] | Insulin subcoma versus FGA | 50 versus 200 | Matched controlled trial | Clinician rated improvement | At least 3 weeks | −0.257 (−0.611–0.097) |
CGI: Clinical global impression, ECT: electroconvulsive therapy, RCT: randomized controlled trial, RP scale: rockland Pollin scale, Time durations: weeks, CI: confidence interval, GAs: generation antipsychotics, FGA: first generation antipsychotic
Table 3: Studies involving electroconvulsive therapy included in meta-analysis
Among the studies involving only the FGAs, chlorpromazine, pimozide and trifluoperazine were the most common drugs that were studied. Other FGAs included penfluridol, trifluperidol, prothipendyl, thiothexine, thioproperazine, prochlorperazine, centbutindole, and haloperidol. Among the studies, which had used SGAs, olanzapine was the most common SGA. Others included risperidone, aripiprazole and paliperidone.
The most common structured efficacy measures included positive and negative syndrome scale (PANSS), brief psychiatric rating scale (BPRS) and clinical global impression. Many studies also had used clinician reported improvements. There were no overall statistically significant differences in the effect sizes obtained when structured instruments were used, vis-à-vis clinician rated improvement (student’s-t-test=1.568, P=0.129). The median duration of clinical trial was 8 w (inter-quartile range of 6 w to 13 w, range 2 w to one y). The sample sizes of the studies varied from 10 to 300, with a median of 45 (inter-quartile range of 30 to 60).
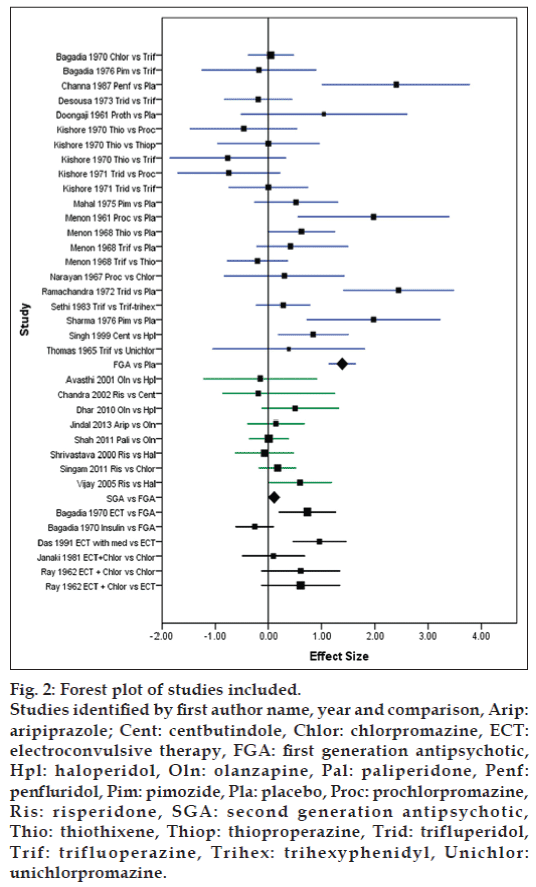
The random effect model was used for computation of effect sizes. Eight comparisons were available between FGA and placebo with a cumulative sample of 316 with a mean effect size 1.387 (confidence intervals (CI) of 1.127 to 1.648) favoring FGAs over placebo. The I2 value for this comparison was 59.1%. The mean effect size of comparison of SGA versus FGA involving 6 studies and a sample size of 240 was 0.106 (CI 0.009 to 0.204) favoring SGAs. Fig. 2 shows the forest plot of the studies and comparisons included in meta-analysis.
Figure 2: Forest plot of studies included.
Studies identified by first author name, year and comparison, Arip: aripiprazole; Cent: centbutindole, Chlor: chlorpromazine, ECT: electroconvulsive therapy, FGA: first generation antipsychotic, Hpl: haloperidol, Oln: olanzapine, Pal: paliperidone, Penf: penfluridol, Pim: pimozide, Pla: placebo, Proc: prochlorpromazine, Ris: risperidone, SGA: second generation antipsychotic, Thio: thiothixene, Thiop: thioproperazine, Trid: trifluperidol, Trif: trifluoperazine, Trihex: trihexyphenidyl, Unichlor: unichlorpromazine.
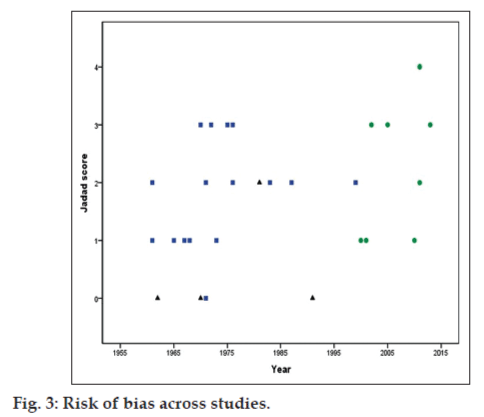
The risk of bias in the included studies is shown in Table 4. The Jadad scores ranged from 0 to 4 with a median of 2 (mean of 1.75, inter-quartile range of 1 to 3). Four studies had a Jadad score of 0, 8 studies each had a score of 1 and 2, 7 studies had score of 3 and one study had a score of 4. There was a statistically significant increase in the quality of the studies with time, with recent studies being associated with lesser risk of bias (Kendall tau=0.289, P=0.049). Fig. 3 shows the Jadad scores across the publication year of the studies. There was no statistically significant relationship of the risk of
| Author (s) | Random sequence | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Jadad score |
|---|---|---|---|---|---|---|
| Avasthiet al. [34] | + | ? | − | − | − | 1 |
| Bagadiaet al. [18] | − | − | − | − | NA | 0 |
| Bagadiaet al. [19] | + | ? | + | + | − | 3 |
| Bagadiaet al. [42] | − | − | − | − | NA | 0 |
| Chandra et al. [35] | + | ? | + | + | − | 3 |
| Channabasavanna and Michael [20] | ? | ? | + | + | − | 2 |
| Das et al. [43] | − | − | − | − | NA | 0 |
| De Sousa and Nayani [21] | + | ? | − | − | NA | 1 |
| Dharet al. [36] | + | ? | − | − | − | 1 |
| Doongajiet al. [22] | − | ? | + | + | NA | 2 |
| Janakiramaiah and Subbakrishnan [44] | + | ? | − | + | NA | 2 |
| Jindal et al. [37] | + | ? | + | + | − | 3 |
| Kishore et al. [23] | + | ? | + | + | NA | 3 |
| Kishore et al. [24] | − | − | + | + | NA | 2 |
| Mahal and Janakiramaiah [25] | + | ? | + | + | − | 3 |
| Menon [26] | + | ? | − | ? | NA | 1 |
| Menon [27] | − | − | + | ? | NA | 1 |
| Narayan et al. [28] | + | ? | − | − | NA | 1 |
| Ramachandran and Menon [29] | + | ? | + | + | NA | 3 |
| Ray [45] | ? | ? | ? | ? | NA | 0 |
| Sethi and Bhiman [30] | ? | ? | + | + | NA | 2 |
| Shah and Joshi [38] | + | ? | + | + | + | 4 |
| Sharma and Dutta [31] | + | ? | + | ? | − | 2 |
| Shrivastava and Gopa [39] | + | ? | − | − | − | 1 |
| Singamet al. [40] | + | ? | − | + | − | 2 |
| Singh et al. [32] | + | ? | + | ? | − | 2 |
| Thomas and Narayanan [33] | + | ? | − | − | NA | 1 |
| Sagar and Chandrashekar [41] | + | ? | + | + | NA | 3 |
| Sagar and Chandrashekar [41] | + | ? | + | + | NA | 3 |
+: attribute present, −: attribute not present, ?: unclear, NA: not applicable
Table 4: Risk of bias in the studies included in the metanalysis
The number needed to treat (NNT) for the placebo controlled studies for which this measure could be computed is depicted in Table 5. NNT represents the number of patients required to treat to get one patient as a ‘true’ responder to treatment. This measure is useful when placebo response is expected to be high. The NNT could be computed for placebo controlled studies of FGA and varied from 1.27 to 6.67. There was no significant correlation between the size of the comparison and the NNT.
| Authors | Active treatment | Number | Methodology | Duration (weeks) | Number needed to treat |
|---|---|---|---|---|---|
| Channabasavanna and Michael [20] | Penfluridol versus placebo | 15 versus 15 | Controlled trial | 12 | 1.27 |
| Doongajiet al. [22] | Injectable prothipendyl versus placebo | 8 versus 5 | Controlled trial | 6 | 5.00 |
| Menon [26] | Trifluopreazine versus placebo | 30 versus 30 | Crossover | 16 | 6.76 |
| Menon [26] | Thiothixene versus placebo | 30 versus 30 | Crossover | 16 | 4.22 |
| Menon [27] | Prochlorperazine versus placebo | 10 versus 10 | Matched control | 8 | 1.42 |
| Ramachandran and Menon [29] | Trifluperidol versus placebo | 25 versus 25 | DBRCT | 6 | 1.67 |
| Sharma and Dutta [31] | Pimozide versus placebo | 19 versus 15 | RCT | 4 | 1.63 |
DBCT: Double blind controlled trial, DB: double blind, HDRS: hamilton depression rating scale, RCT: randomized controlled trial, Time durations: weeks
Table 5: Number needed to treat in controlled studies
This is to the best to our knowledge the first meta-analysis evaluating the treatment modalities for schizophrenia from efficacy trials originating in India. The meta-analysis suggests that FGAs were superior to placebo and SGA are marginally superior to FGAs. The findings of the present analysis concur with that of the world literature. FGAs have proved to be efficacious in treatment of schizophrenia in well designed randomized controlled trials and meta-analysis [46]. However, the effect sizes of the studies included in the present meta-analysis were higher (suggesting more efficacy) reflecting in lower numbers needed to treat. SGAs as a whole has been found to be marginally better than FGAs (mean effect size of 0.106). Other meta-analytic studies have also suggested SGAs to be somewhat more efficacious than FGAs [47,48]. Amisulpiride, clozapine, olanzapine and risperidone have been suggested to be more efficacious than FGAs having small to medium effect sizes [48]. Apart from greater efficacy, SGAs also seem to have better tolerability and lesser discontinuation rates [46]. A comparison of the effect sizes and the confidence intervals from this study with that for ECT in the Indian context suggests that FGAs may be more effective than ECT [14]. However, this may be influenced by the small sampled studies included in the present meta-analysis. Also, the NNT of ECT was higher than that of placebo controlled studies of FGA included in the present meta-analysis, suggesting the advantage of FGAs over ECT.
Based on the findings of the systematic review, certain conclusions can be drawn. Firstly, though there had been quite a number of studies on FGAs, the number of studies with SGAs has been fairly limited. Prescription data from India shows that SGAs are more frequently used in the recent times [49]. However, there is a relative lack of data about SGAs from the country. Also, polypharmacy has been reported to be fairly common in India for the management of patients with schizophrenia due to clinical circumstances or psychiatrist’s preferences [50,51]. However, there are no studies, which deal with concomitant use of two or more antipsychotics for patients with schizophrenia from India.
Secondly, the sample sizes of most of the studies have been low, limiting the statistical approaches that could be utilized. A closer look of the sample size further reflects that some of the older studies used relatively larger sample size, but were limited by their methodology. Some the newer studies have also been underpowered for detecting a difference. Hence, it may be a prudent option to calculate requisite sample size prior to initiation of any study and conduct interim analysis to terminate study if required statistical superiority is achieved.
Thirdly, the studies have been of limited duration (median 8 weeks), and long duration studies spanning one year or more has been rare. As schizophrenia is usually a chronic psychotic condition and most patients require long term pharmacotherapy, longer studies can help to discern the efficacy of a medication for maintenance treatment too. Not all patients respond at a similar time to a given antipsychotic [52]. The efficacy of some of the antipsychotics (e.g. clozapine) can be best judged after a period of trial of about 6 months [53].
Fourth, many of the studies, which have been conducted in India have not tried to assess the dosage requirement. Further, many of the trials do not go up to the maximum tolerable doses, reflecting that the improvement achieved can potentially be accentuated by increasing the doses of antipsychotics.
Fifthly, the Jadad scores of most of the studies have been on the lower side, suggesting the need to improve the methodologies of the trials. This can be improved by explicitly using the randomized controlled design and stating the randomization procedure in fair detail. Blinding of the patients and assessors would help in minimizing the biases that can crop up due to expectancy effects. Also, data analysis should aim at an intention to treat analysis. This would help in minimizing the unbalancing of randomization due to premature dropouts. Still, it has been encouraging to see that with passage of time, the quality of the trials has been improving.
Sixthly, studies have usually been conducted at one centre. Multi-centric studies using the same methodology in different centers can reduce the regional and centre based differences in outcomes. This would also help in achieving a larger sample size in the study. Various scientific organizations like the Indian Psychiatric Society (IPS) can play an important role in facilitating such multi-centric studies by providing expertise, identifying potential sites and collaborators, generate funding through governmental and nongovernmental sources, and disseminate the results effectively. The Drug Controller General of India (DCGI) may consider making such trials mandatory while approving a newer antipsychotic in the Indian market.
Seventhly, studies till now have not explicitly looked at the factors like treatment acceptability and adherence to medications as an outcome measure or covariate. Acceptability of treatment and adherence to medication regimen can be an important prognostic marker for sustained efficacy of antipsychotics and could be studied through controlled trial design.
Lastly, it may be prudent to focus on certain areas with regards to antipsychotics, which have received limited attention. Controlled trials focusing on depot antipsychotics, efficacy and polypharmacy and treatment resistant schizophrenia can be attempted. Recent literature has also progressed to assessment of biological markers, which can predict response to treatment [54,55]. Such studies can be conducted in the Indian genetic stock to find potential markers of response.
To sum up, there is still a need to conduct well designed multi-centric effectiveness based randomized trials with good follow up especially with respect to SGAs. Presently, there is no systematic data from India on polypharmacy. Pharmacogenomic differences may predispose Indians to tolerate lower doses of antipsychotics. This may lead to increase in cumulative doses with polypharmacy, which may influence the side effect profile too. Present pharmacogenomic literature suggests that the alleles moderating the specific side effects like tardive dyskinesia may be different in the Indian population as compared to elsewhere [56,57]. Similar studies when extend to efficacy profile may also find unique differences.
Also it must be emphasized that psychopharmacology does not act in isolation. It can be best delivered in the context of an effective service model, which incorporates attention to psychosocial aspects along with clinician’s attempts to engage a patient towards recovery. Adjunct psychosocial interventions like psycho-education and family therapy may be quite helpful in engaging the patient and family into the treatment fold and expecting gradual and sustained improvement in the patient’s condition [58]. Hence, wherever feasible and appropriate, the additional use of psychosocial interventions would be beneficial.
Limitations of this systematic review and meta-analysis include that only studies published in peer reviewed English language journals were included and unpublished material (including dissertations) was not sought. Sensitivity analysis was not conducted due to wide variation in the characteristics of the studies and their focuses of reporting. Some of the studies did not report the findings that could be used to calculate standardized mean differences and were not included in quantitative analysis. Also, this meta-analysis focuses on efficacy and not tolerability (side effect profile) of antipsychotic agents. The differences in the efficacy measures of reporting improvement over time may result difficulty in drawing accurate inferences from the comparisons.
The systematic review suggests that evidence base needs to be further strengthened for intervention trials of schizophrenia in Indian context, especially with regards to SGAs. Future studies should aim at effectiveness based approach especially targeting the maintenance period. Pharmacogenomic link of the treatment response can be conducted to characterize allelic markers for favorable efficacy response and particular side effects. Documentation of the research and bringing it to the public domain to consolidate the evidence base can help others to enhance their practice and clinical decision making, with the overall aim of better patient outcomes.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Gaite L, Vázquez-Barquero JL, Borra C, Ballesteros J, Schene A, Welcher B, et al. Quality of life in patients with schizophrenia in five European countries: The EPSILON study. ActaPsychiatrScand 2002;105:283-92.
- Pinikahana J, Happell B, Hope J, Keks NA. Quality of life in schizophrenia: A review of the literature from 1995 to 2000. Int J Ment Health Nurs 2002;11:103-11.
- Lobana A, Mattoo SK, Basu D, Gupta N. Quality of life in schizophrenia in India: Comparison of three approaches. ActaPsychiatrScand 2001;104:51-5.
- Jablensky A. Epidemiology of schizophrenia: The global burden of disease and disability. Eur Arch Psychiatry ClinNeurosci 2000;250:274-85.
- Morrison AP, Nothard S, Bowe SE, Wells A. Interpretations of voices in patients with hallucinations and non-patient controls: A comparison and predictors of distress in patients. Behav Res Ther 2004;42:1315-23.
- Leucht S, Burkard T, Henderson J, Maj M, Sartorius N. Physical illness and schizophrenia: A review of the literature. ActaPsychiatrScand 2007;116:317-33.
- Blanchard JJ, Brown SA, Horan WP, Sherwood AR. Substance use disorders in schizophrenia: Review, integration, and a proposed model. ClinPsychol Rev 2000;20:207-34.
- Chan SW. Global perspective of burden of family caregivers for persons with schizophrenia. Arch PsychiatrNurs 2011;25:339-49.
- Fazel S, Gulati G, Linsell L, Geddes JR, Grann M. Schizophrenia and violence: Systematic review and meta-analysis. PLoS Med 2009;6:e1000120.
- Davies LM, Drummond MF. The economic burden of schizophrenia. Psychiatr Bull 1990;14:522-5.
- Kirchheiner J, Nickchen K, Bauer M, Wong ML, Licinio J, Roots I,et al. Pharmacogenetics of antidepressants and antipsychotics: The contribution of allelic variations to the phenotype of drug response. Mol Psychiatry 2004;9:442-73.
- Arranz MJ, Rivera M, Munro JC. Pharmacogenetics of response to antipsychotics in patients with schizophrenia. CNS Drugs 2011;25:933-69.
- Avasthi A, Aggarwal M, Grover S, Khan MK. Research on antipsychotics in India. Indian J Psychiatry 2010;52Suppl 1:S317-40.
- Tharyan P, Adams CE. Electroconvulsive therapy for schizophrenia. Cochrane Database Syst Rev 2005;2:CD000076.
- Painuly N, Chakrabarti S. Combined use of electroconvulsive therapy and antipsychotics in schizophrenia: The Indian evidence. A review and a meta-analysis. J ECT 2006;22:59-66.
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials 1996;17:1-12.
- Clark HD, Wells GA, Huët C, McAlister FA, Salmi LR, Fergusson D, et al. Assessing the quality of randomized trials: Reliability of the Jadad scale. Control Clin Trials 1999;20:448-52.
- Bagadia VN, Shastri PC, Dave KP, Shah LP. High doses of chlorpromazine and trifluoperazine in the treatment of patients suffering from schizophrenia. J Postgrad Med 1971;17:2-9.
- Bagadia V, Bhat R, Ghadiali HH, Pradhan PV, Shah LP. Comparative double blind of pimozide and trifluoperazine in maintenance treatment of schizophrenia. Indian J Psychiatry 1976;18:199-203.
- Channabasavanna SM, Michael A. Penfluridol maintenance therapy in schizophrenia: A controlled study. Indian J Psychiatry 1987;29:333-6.
- De Sousa A, Nayani G. A controlled trial of trifluperidol with trifluoperazine. Indian J Psychiatry 1973;15:290-3.
- Doongaji D, Bagadia V, Vahia N. Clinical experience with prothipendyl hydrochloride (Dominal)* – A new tranquiliser. Indian J Psychiatry 1961;3:228-34.
- Kishore B, Rajkumar, Kaur A. Thiothixene in hospitalized chronic schizophrenic patients. Indian J Psychiatry 1970;12:225-37.
- Kishore B, Jain C, Bhatia A. Trifluperidol, a butyrophenone, in hospitalised chronic schizophrenic patients. Indian J Psychiatry 1972;14:65-75.
- Mahal A, Janakiramaiah N. A double-blind placebo controlled trial of pimozide (R6238) on 49 hospitalized chronic schizophrenics. Indian J Psychiatry 1975;17:45-55.
- Menon S. Clinical trial of thiothixene. Indian J Psychiatry 1968;10:57-68.
- Menon S. Prochlorperazine in the treatment of chronic withdrawn schizophernics. Indian J Psychiatry 1961;3:157-9.
- Narayan H, Natarajan C, Bai S. Stemetil in chronic schizophrenia. Indian J Psychiatry 1967;9:234-8.
- Ramachandran V, Menon S. Trifluperidol – A controlled clinical trial on a group of schizophrenic patients. Indian J Psychiatry 1972;14:11-8.
- Sethi BB, Bhiman A. A clinical study of trifluperazinevstrifluperazinebenzhexol combination. Indian J Psychiatry 1983;25:155-6.
- Sharma S, Dutta D. A double-blind study of pimozide in the treatment of schizophrenic patients. Indian J Psychiatry 1976;18:34.
- Singh H, Srivastava JS, Raghuvanshi C, Dalal PK, Asthana OP. A comparative efficacy study of centbutindole and haloperidol in schizophrenia. Indian J Psychiatry 1999;41:325-8.
- Thomas G, Narayanan H. Trifluoperazine (Eskazine) in the treatment of chronic paranoid schizophrenics – (A comparative study with unichlorpromazine). Indian J Psychiatry 1965;7:175-80.
- Avasthi A, Kulhara P, Kakkar N. Olanzapine in the treatment of Schizophrenia: An open label comparative clinical trial from North India. Indian J Psychiatry 2001;43:257-63.
- Chandra R, Singh H, Dalal PK, Asthana OP, Srivastava JS. Comparative efficacy of centbutindole&risperidone in schizophrenia. Indian J Psychiatry 2002;44:365-71.
- Dhar R, Chavan BS, Sidana A. Comparative efficacy of haloperidol and olanzapine in patients of schizophrenia: A 6 month follow up trial. JMHHB 2010;15:31-9.
- Jindal KC, Singh GP, Munjal V. Aripiprazole versus olanzapine in the treatment of schizophrenia: A clinical study from India. Int J Psychiatry ClinPract 2013;17:21-9.
- Shah S, Joshi D. Tolerability and efficacy of paliperidone ER compared to olanzapine in the treatment of schizophrenia: A randomized, double-blind, multicentric trial. Ind Psychiatry J 2011;20:25-31.
- Shrivastava A, Gopa S. Comparative study of risperidone and haloperidol on clinical and psychosocial parameters in treatment of schizophrenia: A randomised open trial. Indian J Psychiatry 2000;42:52-6.
- Singam AP, Mamarde A, Behere PB. A single blind comparative clinical study of the effects of chlorpromazine and risperidone on positive and negative symptoms in patients of schizophrenia. Indian J Psychol Med 2011;33:134-40.
- Sagar KV, Chandrashekar CR. A double-blind randomized trial between risperidone and haloperidol in drug-naive patients with paranoid schizophrenia. Indian J Psychiatry 2005;47:30-2.
- Bagadia VN, Dave KP, Shah LP. A comparative study of physical treatments in schizophrenia. Indian J Psychiatry 1970;12:190-204.
- Das PS, Saxena S, Mohan D, Sundaram KR. Adjunctive electroconvulsive therapy for schizophrenia. Natl Med J India 1991;4:183-4.
- Janakiramaiah N, Subbakrishan DK. Ect-chlorpromazine combination compared with chlorpromazine only in schizophrenia. Indian J Psychiatry 1981;23:230-3.
- Ray SD. Relative efficacy of electroconvulsive therapy and chlorpromazine in schizophrenia. J Indian Med Assoc 1962;38:332-3.
- Leucht S, Cipriani A, Spineli L, Mavridis D, Orey D, Richter F, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: A multiple-treatments meta-analysis. Lancet 2013;382:951-62.
- Zhang JP, Gallego JA, Robinson DG, Malhotra AK, Kane JM, Correll CU. Efficacy and safety of individual second-generation vs. first-generation antipsychotics in first-episode psychosis: A systematic review and meta-analysis. Int J Neuropsychopharmacol 2013;16:1205-18.
- Leucht S, Corves C, Arbter D, Engel RR, Li C, Davis JM. Second-generation versus first-generation antipsychotic drugs for schizophrenia: A meta-analysis. Lancet 2009;373:31-41.
- Grover S, Kumar V, Avasthi A, Kulhara P. An audit of first prescription of new patients attending a psychiatry walk-in-clinic in North India. Indian J Pharmacol 2012;44:319-25.
- Ramadas S, Kuttichira P, Sumesh TP, Ummer SA. A study of an antipsychotic prescription pattern of patients with schizophrenia in a developing country. Indian J Psychol Med 2010;32:13-6.
- Rajkumar AP, Jebaraj P, Tharyan P. Multi-drug overdose risperidone, ziprasidone, valproate, trihexyphenidyl, and clonazepam. J Assoc Physicians India 2007;55:146-8.
- Case M, Stauffer VL, Ascher-Svanum H, Conley R, Kapur S, Kane JM, et al. The heterogeneity of antipsychotic response in the treatment of schizophrenia. Psychol Med 2011;41:1291-300.
- Meltzer HY. Treatment of the neuroleptic-nonresponsive schizophrenic patient. Schizophr Bull 1992;18:515-42.
- Garver DL, Holcomb JA, Christensen JD. Heterogeneity of response to antipsychotics from multiple disorders in the schizophrenia spectrum. J Clin Psychiatry 2000;61:964-72.
- Baeza I, Castro-Fornieles J, Deulofeu R, de la Serna E, Goti J, Salvà J, et al. Plasma homovanillic acid differences in clinical subgroups of first episode schizophrenic patients. Psychiatry Res 2009;168:110-8.
- Foster A, Wang Z, Usman M, Stirewalt E, Buckley P. Pharmacogenetics of antipsychotic adverse effects: Case studies and a literature review for clinicians. Neuropsychiatr Dis Treat 2007;3:965-73.
- Tiwari AK, Deshpande SN, Rao AR, Bhatia T, Lerer B, Nimgaonkar VL, et al. Genetic susceptibility to tardive dyskinesia in chronic schizophrenia subjects: III. Lack of association of CYP3A4 and CYP2D6 gene polymorphisms. Schizophr Res 2005;75:21-6.
- Kulhara P, Chakrabarti S, Avasthi A, Sharma A, Sharma S. Psychoeducational intervention for caregivers of Indian patients with schizophrenia: A randomised-controlled trial. ActaPsychiatrScand 2009;119:472-83.