- *Corresponding Author:
- N. D. Satyanarayan
Department of Pharmaceutical Chemistry, Kuvempu University, Postgraduate Centre, Kadur, Chikmagalur-577 548, India
E-mail: satya1782005@gmail.com
| Date of Submission | 30 June 2016 |
| Date of Revision | 19 May 2017 |
| Date of Acceptance | 25 January 2018 |
| Indian J Pharm Sci 2018;80(2):374-378 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The present investigation dealt with the antiproliferative, DNA-cleaving and preliminary phytochemical screening of n-hexane, dichloromethane and methanol extracts of Balanites roxburghiana Linn. The 3-(4,5-dimethylthiazole-2yl)-2,5-diphenyltetrazolium bromide assay was used to assess the antiproliferative activity against chronic myelogenous leukemia, hepatocellular carcinoma, breast cancer, cervical cancer, colorectal adinocarcinoma and normal human kidney embryonic cell lines. Calf-thymus DNA cleavage analysis was performed by agarose gel electrophoresis at various concentrations from 10 to 100 µg and preliminary phytochemical screening has been performed using standard procedure. The antiproliferative assay results of the extracts revealed that the n-hexane and dichloromethane extracts were more active compared to the methanol extract. The methanol extract showed 21.7 % activity against breast cancer cell lines only. n-Hexane extract (V1) exhibited DNA cleavage at all concentrations, which could be due to binding to DNA. The dichloromethane extract (V2) exhibited DNA cleavage at all concentrations but a very prominent low molecular weight DNA band formation could be observed with increasing concentration of the extract, With the methanol extract (V3), the DNA cleavage increased with concentration of the sample. A partial cleavage could be found at 10 µg concentration under a UV transilluminator. The preliminary phytochemical screening revealed presence of flavonoids, steroids, tannins and saponins.
Keywords
Balanites roxburghiana, antiproliferative, DNA cleavage, phytochemical screening, agarose gel electrophoresis, MTT assay, UV transilluminator
Cancer is one of the most severe disease in which unregulated proliferation of abnormal cells invade and disrupt surrounding tissues [1]. It begins in the cells of the body, where the orderly process is disturbed by production of unwanted new cells without the lysis of old cells thus these extra cells lump together to form a growing tumor [2]. It is the leading cause of morbidity and mortality worldwide with approximately 14.1 million new cases, 8.2 million cancer related deaths and is expected to rise by 70 % over the next two decades as reported by GLOBOCAN (Global cancer) 2012 and the International Agency for Research on Cancer online database [3]. The partially successful clinical therapies include radiation, chemotherapy and surgery, indicating an urgent need of alternative strategies [4]. Medicinal plants have been used as a remedy for treating of human diseases for centuries, because of the presence of secondary metabolites of therapeutic value [5] such as alkaloids, flavonoids, tannins and phenolics [6], which are expected to play a vital role in the treatment of cancer [7]. The majority of these compounds are capable of scavenging free radicals. The major chronic health problems such as cancer, heart diseases, inflammation, neurodegeneration, aging and also food deterioration are due to oxidative stress [8]. For offering the required protection to avoid oxidative stress and related permanent alterations of biomolecules, in vitro and in vivo studies have shown the effective role of phenolics in the preclusion or inhibition of disorders such as oxidative damage to DNA, proteins and lipid, or many chronic diseases [9]. Flavonoids may act through inhibiting cytoplasmic membrane function and also by inhibiting of DNA gyrase and beta-hydroxyacyl-acyl carrier protein dehydratase activities [10]. The isoflavone, genistein was able to modify cell morphology by formation of filamentous cells and inhibited the synthesis of DNA and RNA of Vibrio harveyi [11]. Plant secondary metabolites could maintain the health and cure various diseases including cancer with less harm full effects [12]. Balanites roxburghiana Del (family: Zygophyllaceae), known as desert date in English is found abundantly in dry lands of Africa and South Asia and is most common but neglected wild species [13]. It is a multi-branched, thorny shrub or tree grows up to l0 m tall. Crown is spherical in one or more distinct masses. Trunk is short and often branching from near the base. Bark is dark brown to grey and completely fissured. Branches are armed with yellow or green thorns up to 8 cm long. Leaves are with two separate leaflets; leaflets are obovate, asymmetric, 2.5 to 6 cm long, bright green, leathery, with fine hairs when young. Flowers are fragrant, yellowish green growing in fascicles of the leaf axils. Fruit is rather long, narrow drupe, 2.5 to 7 cm long, 1.5 to 4 cm in diameter. Immature fruits are green and tormentose, turning yellow and glabrous when mature. Pulp of the fruit is bitter-sweet to taste and edible. Seed is the pyrene (stone), measures as 1.5 to 3 cm long, light brown, fibrous, and tremendously hard [14]. This plant exhibited different pharmacological activities such as cardioprotective, antioxidant [15], anthelmintic [16], antibacterial [17], antivenin [18], antiinflammatory, analgesic [19], antioxidant, xanthine oxidase and acetyl cholinesterase inhibitory [20], antinociceptive, antioxidant [21], mosquito larvicidal [22], hepatoprotective [23], antiviral [24], wound healing [25], hypocholesterolemia [26], diuretic [27], aldose reductase inhibitory [28] and antidiabetic [29]. Compounds isolated from this plant such as balanitin-6,7-saponin [30] and steroidal saponin [31] have shown anticancer activity. Fruit extract has inhibited proliferation of Ehrlich ascitic tumor [32] and methanol extract of fruit has shown antiproliferative activity against breast, colon, and liver cancer cells in a sulforhodamine B assay [33]. The above documented literature revealed that B. roxburghiana is a medicinally important plant and has to be considered for detailed study to understand its mechanism of action against cancer and also to check against other cell lines, which are not previously investigated. Hence, the present study was aimed to determine antiproliferative activity along with DNAcleaving activity using 3-(4,5-dimethylthiazole-2yl)- 2,5-diphenyltetrazolium bromide (MTT) assay and agarose gel electrophoresis of various solvent extracts of B. roxburghiana fruit, respectively.
The chemicals used were of analytical grade. Doxorubicin (Sigma-Aldrich, USA), n-hexane, dichloromethane, methanol, dimethyl sulfoxide (DMSO; HiMedia), calf-thymus DNA (Genei, Bengaluru) other chemicals such as agarose gel and ethylene bromide used for the study were also purchased from (HiMedia). The fruits of B. roxburghiana were collected in the month of February to March-2014 around Kadur town of Chikmagalur, India and were authenticated at the Herbarium, Department of Botany, Kuvempu University, Shankaraghatta, Shimoga, India. The collected fruits were immediately sprayed with alcohol to cease enzymatic degradation of secondary metabolites. The fruits were stored in cool, dry place before extraction.
The shade-dried fruits (79.3 g) of B. roxburghiana were chopped into small fragments of 1-2 inches in length and extracted with different solvents viz. n-hexane, dichloromethane and methanol successively in a Soxhlet extractor for about 72 h each. The solvent was evaporated under reduced pressure and controlledtemperature using a Buchi evaporator. The solventevaporated mass of n-hexane, dichloromethane and methanol extracts were 0.68, 0.76 and 10.52 g, respectively. The extracts were stored in a freezer (–4°) until further use. Preliminary phytochemical screening of B. roxburghiana fruit was performed using standard procedures [34,35]. The results were shown in Table 1.
| Test | Phytochemical test | BRH | BRD | BRM |
|---|---|---|---|---|
| Alkaloids | Mayer’s test | - | - | - |
| Wagner’s test | - | - | - | |
| Flavonoids | Ferric chloride test | - | - | + |
| Alkaline test | - | + | + | |
| Glycosides | Killer-Killan’s test | - | - | - |
| Bromine water test | - | - | - | |
| Steroids |
Salkowaski’s test Liebermann Burchad test |
+ | + | + |
| + | + | + | ||
| Tannins | Ferric chloride test | - | - | + |
| Saponins | Foam test | + | + | + |
| Carbohydrates | Molisch’s test | - | - | + |
| Benedicts test | - | - | - | |
| Proteins | Xanthoprotein test Ninhydrin test |
- - |
- - |
- - |
BRH- B. roxburghiana n-hexane extract, BRD- B. roxburghiana dichloromethane extract and BRM- B. roxburghiana methanol extract
Table 1: Showing the result of preliminary phytochemical screening of B. roxburghiana fruit extract
Six different cell lines, colorectal adenocarcinoma (COLO 205); chronic myelogenous leukaemia (K562); breast adenocarcinoma (MCF 7); cervical cancer (HeLa); normal human kidney embryonic cell line (HEK293) and hepatocellular carcinoma (HepG2) were used in this investigation. All cell lines were obtained from the National Centre for Cell Sciences, Pune, India, and were cultured at a seeding density of 0.2×106 in Dulbecco’s modified Eagles medium/Roswell Park Memorial Institute (DMEM/RPMI) medium supplemented with 10 % fetal bovine serum, 100 U/ml penicillin and 100 μg/ml streptomycin, respectively maintained in a humidified atmosphere with 5 % CO2 at 37°. The samples were dissolved in DMSO (not exceeding the final concentration of 0.01 %) and further diluted in cell culture medium. The antiproliferative response of extract was assessed using the MTT assay [36]. Cells (∼10 000) were plated in 200 μl growth medium in the presence or absence of the extract (25, 50, 100, and 200 μg/ml) in 96-well culture plates for 24 h. Then the culture plates were centrifuged at 2000 rpm for 10 min at room temperature. Supernatant (100 μl) was discarded and 20 μl of MTT (5 mg/ml in PBS) was added to each well and incubated for 4 h at 37°. The viability of the cells was determined at 570 nm using a spectrophotometer.
The extract was added separately to the DNA sample. The sample mixtures were incubated at 37° for 2 h. The treatment of DNA samples and the electrophoresis of these samples were performed according to the following procedure [37]. Two hundred and fifty milligrams of agarose was dissolved in 25 ml of TAE buffer (4.84 g Tris base, pH 8.0, 0.5 M EDTA/1: l) with boiling. As the gel attained ~55°, it was poured into the gel cassette fitted with comb and the gel was allowed to solidify. The comb was carefully removed and the gel was placed in the electrophoresis chamber flooded with TAE buffer. DNA sample (20 μl) mixed with bromophenol blue dye at 1:1 ratio was carefully loaded into the wells along with DNA marker and a constant 50 V of electricity was passed for around 45 min. The gel was removed carefully, stained with ethydium bromide solution (10 μg/ml) for 10-15 min and the bands were observed under a UV transilluminator. The preliminary phytochemical screening of the n-hexane, dichloromethane and methanol extracts of the fruits of B. roxburghiana revealed the presence of saponins, tannins, steroids and flavonoids.
The result for antiproliferative activity by different extracts of B. roxburghiana fruit on different cell lines were shown in Table 2. The ongoing research is to seek out effective treatments for cancer including the use of medicinal plants. This treatment makes use of the compounds naturally present in plants that are known to inhibit or kill carcinogenic cells [38]. Phytochemical screening of the fruit extracts showed the presence of flavonoids, steroids, tannins and saponins (Table 1). Flavonoids have been reported to inhibit xanthine oxidase [39] and cyclooxygenase [40] enzymes. The molecular mechanism might involve the inhibition of the pro-oxidant process that causes tumor promotion. Growth promoting oxidants and reactive oxygen species are the major catalysts of the tumor promotion and progression stages and therefore antioxidants inhibit tumor cell proliferation. In addition, the mechanism of inhibition of polyamine biosynthesis can contribute to the antiproliferative activities of flavonoids. Ornithine decarboxylase is a rate-limiting enzyme in polyamine biosynthesis and is correlated with the rate of DNA synthesis and cell proliferation in several tissues.
| Cancer cell lines | Average inhibition | BRH | BRD | BRM | Doxorubicin concn 1uM |
|---|---|---|---|---|---|
| K562 | Average inhibition (%) | 9.99763 | 12.9898 | 11.5887 | 95.57 |
| SD | 3.6373 | 2.17129 | 2.17876 | 2.2256 | |
| MCF – 7 | Average inhibition (%) | 11.624 | 20.1765 | 21.7037 | 97.61 |
| SD | 1.59556 | 2.09651 | 2.73341 | 2.189 | |
| Hela | Average inhibition (%) | 13.5706 | 13.2369 | 12.6808 | 97.16 |
| SD | 1.97422 | 2.22469 | 3.33519 | 2.27 | |
| Colo | Average inhibition (%) | 21.7153 | 10.4015 | 11.2226 | 91.55 |
| SD | 3.50687 | 1.21466 | 3.61641 | 1.8723 | |
| HepG2 | Average inhibition (%) | 9.18919 | 15.9214 | 16.6585 | 97.355 |
| SD | 2.82746 | 2.57552 | 2.08484 | 1.5699 | |
| HEK | Average inhibition (%) | -1.55109 | 0.44668 | -6.48649 | 5.678 |
| SD | 3.1856 | 1.61342 | 3.22495 | 1.56 |
BRH- B. roxburghiana n-hexane extract, BRD- B. roxburghiana dichloromethane extract and BRM- B. roxburghiana methanol extract
Table 2: Antiproliferative activity of solvent extracts of B. roxburghiana fruit by MTT assay on with different cancer cell lines
Several studies reported that flavonoids could inhibit ornithine decarboxylase induced by tumor promoters causing a subsequent decrease in polyamine and inhibition of DNA and protein synthesis [41]. Steroids might bind to Na+/K+-ATPase resulting in a complex but well-documented changes in cell signalling events. The signalosome complex included the enzyme, Na+/K+-ATPase as well as, phosphoinositide-3 kinase and phospholipase each of which, in turn, set into action complex signalling events that resulted in tumor cell death through either apoptosis or autophagyrelated mechanisms [42].
The antiproliferative and DNA-cleaving potential of various extracts of B. roxburghiana fruit have shown less significant activity against different cell lines but methanol and dichloromethane extracts have shown significant activity against MCF-7 cell line and n-hexane against co COLO 205 cell lines. From the above result, it could be predicted that the inhibition of MCF-7 cells by methanol and dichloromethane extract would be most effective and the inhibition of COLO 205 cell line by n-hexane extract is the best among the extracts screened.
The results obtained have shown that the three extracts of B. roxburghiana fruit has moderate inhibition on different cancer cell lines. The evaluation is carried out by dissolving the extracts in DMSO (not exceeding the final concentration of 0.01 %) and further diluted in cell culture medium. The antiproliferative response of the extract was assessed by MTT assay using doxorubicin as a standard drug molecule. The viability of the cells was determined using a spectrophotometer at 570 nm. The IC50, that is, the concentration of the extract required to inhibit cell growth by 50 %, was determined.
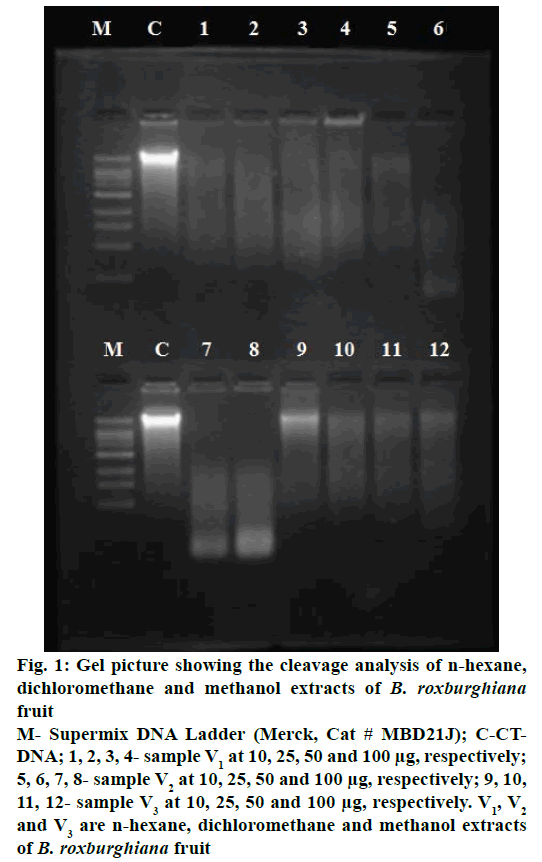
DNA-cleaving activity of n-hexane, dichloromethane and methanol extracts of B. roxburghiana fruit was studied using agarose gel electrophoresis method and the results were presented in Figure 1. DNA was the target for drug discovery as it regulated many biochemical reactions. Clinical efficacies of many drugs can be correlated with their ability to induce enzyme-mediated DNA-cleavage. The loci present in DNA are involved in regulatory processes such as gene expression, gene transcription, mutagenesis and carcinogenesis [43]. In particular, designing of the compound having ability to cleave DNA is utmost important not only from the primary biological point of view but also in terms of photodynamic therapeutic approach to develop potent drugs [44]. The extracts, which were found to be active in DNA-cleavage assay, were further screened for antiproliferative study. DNA-cleaving potential of the extracts were examined by comparing the band appeared in control and test samples at 100 μg concentration, Figure 1 clearly demonstrated that with n-hexane extract (V1), DNA cleavage could be seen at all concentrations, with dichloromethane extract (V2), DNA cleavage was observed at all concentrations but a very prominent low molecular weight DNA band formation could be seen with increasing concentration of sample and with methanol extract (V3), DNA cleavage increased with the concentration of sample. A partial cleavage could be found at 10 μg and cleavage activity increased along with sample concentrations.
Figure 1: Gel picture showing the cleavage analysis of n-hexane,
dichloromethane and methanol extracts of B. roxburghiana
fruit
M- Supermix DNA Ladder (Merck, Cat # MBD21J); C-CTDNA;
1, 2, 3, 4- sample V1 at 10, 25, 50 and 100 μg, respectively;
5, 6, 7, 8- sample V2 at 10, 25, 50 and 100 μg, respectively; 9, 10,
11, 12- sample V3 at 10, 25, 50 and 100 μg, respectively. V1, V2 and V3 are n-hexane, dichloromethane and methanol extracts
of B. roxburghiana fruit
The current study is to assess the preliminary phytochemical screening, DNA cleavage and antiproliferative activity of various extract of B. roxburghiana fruit, which showed the presence of flavonoids, steroids, tannins and saponins. Out of these secondary metabolites some are responsible for DNA cleavage and antiproliferative activity. DNA cleavage could be seen at all concentrations of the n-hexane extract 10, 25, 50 and 100 μg, the intensity of DNA attached to the wells was observed more. This might be because of sample binding to DNA, dichloromethane extract showed very prominent low molecular weight DNA band formation and methanol extract has shown a partial cleavage at 10 μg concentration. The results revealed that the n-hexane and dichloromethane extracts were the most active compared to the methanol extract. The methanol extract has shown activity only against breast cancer cell line. Thus B. roxburghiana fruit appeared to be significantly active against cancer cell lines in the MTT assay and active in the DNA-cleavage agarose gel electrophoresis assay. Further research is needed to isolate specific phytochemicals from the extracts that were responsible for the antiproliferative activity against MCF-7 and COLO 205 cell lines.
Acknowledgement
The authors are grateful to the authorities of Kuvempu University for providing necessary facilities to carry out the present work. Authors also thank the Biogenics, Hubli and one of the authors S. P. Soul Shekhar would like to thank the OBC Cell Kuvempu University for providing fellowship to carry out Ph. D. programme.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Gennari C, Castoldi D, Sharon O. Natural products with taxol-like antitumor activity: synthetic approaches to eleutherobin and dictyostatin. Pure Appl Chem 2007;79:173-80.
- Nishanthi M, Prasanthi P, Muni Teja K, Makesh Kumar Reddy K, Nishanthi P, et al. A Cancer Disease: A Review. Asian J Pharm Res 2013;3:47-52.
- Ferlay J, Soerjomataram I, Dixit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:359-86.
- Dai J, Mumper RJ. Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules 2010;15:7313-52.
- Nostro A, Germano MP, D’Angelo V, Marino A, Cannatelli MA. Extraction methods and bioautography for evaluation of medicinal plant antimicrobial activity. Lett Appl Microbiol 2000;30:379-84.
- Edeoga HO, Okwu DE, Mbaebie BO. Phytochemical constituents of some Nigerian medicinal plants. Afr J Biotechnol 2005;4:685-8.
- Kaur R, Kapoor K, Kaur H. Plants as a source of anticancer agents. J Nat Prod Plant Resour 2011;1:119-24.
- Tapiero H, Tew KD, Nguyen Ba G, Mathe G. Polyphenols: do they play a role in the prevention of human pathologies. Biomed Pharmacother 2002;56:200-7.
- Malins DC, Johnson PM, Barker EA, Polissar NL, Wheeler TM, Anderson KM. Cancer-related changes in prostate DNA as men age and early identification of metastasis in primary prostate tumors. PANS 2003;100:5401-06.
- Cushnie TP, Lamb AJ. Antimicrobial activity of flavonoids. Int J Antimicrob Agents 2005;26:343-56.
- Ulanowska K, Tkaczyk A, Konopa G, Wegrzyn G. Differential antibacterial activity of genistein arising from global inhibition of DNA, RNA and protein synthesis in some bacterial strains. Arch Microbiol 2006;184:271-78.
- Harun-ur-Rashid MD, Gafur MA, Sadik MG, Rahman MAA. Biological activities of a new acrylamide derivative from Ipomoea turpethum. Pak J Biol Sci 2002;5:968-69.
- Hall JB, Waljer DH. Balanites aegyptiaca Del. A monograph. Cardiff, Wales: School of Agricultural and Forest Science. University of Wales; 1991.
- Daya LC, Vaghasiya HU. A review on Balanites aegyptiaca Del (desert date): phytochemical constituents, traditional uses and pharmacological activity. Pharmacogn Rev 2011;5:55-62.
- El Mastry SM, Ebeed MM, El Sayed IH, Nasr MY, El Halafawy KA. Protective effect of Balanites aegyptiaca on antioxidant defense system against adriamycin induced cardiac toxicity in expermental mice. Egypt J Biochem Mol Biol 2010;28:1.
- Koko WS, Galal M, Khalid HS. Fasciolicidal efficacy of Albizia anthelmintica and Balanites aegyptiaca compared with albendazole. J Ethnopharmacol 2000;71:247-52.
- Gnoula C, Guissou P, Duez P, Frederich M, Dubois J. Nematocidal compounds from the seeds of Balanites aegyptiaca isolation and structure elucidation. Int J Pharmacol 2007;3:280-4.
- Wufen BM, Adamu HM, Cham YA, Kela SL. Preliminary studies on the antivenin potential and phytochemical analysis of the crude extracts of Balanites aegyptica (Linn.) Delile on albino rats. Nat Prod Rad 2007;6:18-21.
- Gaur K, Nema RK, Kori ML, Sharma CS, Singh V. Anti-inflammatory and analgesic activity of Balanites aegyptiaca in experimental animal models. Int J Green Pharma 2008;2:214-17.
- Meda NT, Lamien-Meda A, Kiendrebeogo M, Lamien CE, Coulibaly AY, Millogo-Rasolodimby J. In vitro antioxidant, xanthine oxidase and acetylcholinesterase inhibitory activities of Balanites aegyptiaca (L.) Del. (Balanitaceae). Pak J Biol Sci 2010;13:362-68.
- Speroni E, Cervellati R, Innocenti G, Costa S, Guerra MC, Dall Acqua S. Anti-inflammatory, anti-nociceptive and antioxidant activities of Balanites aegyptiaca (L.) Delile. J Ethnopharmacol 2005;98:117-25.
- Zarroug IM, Nugud AD, Bashir AK, Mageed AA. Balanites aegyptiaca as a mosquito larvicide. Pharma Biol 1990;28:267-71.
- Mohamed AH, Eltahir KE, Ali MB, Galal M, Ayeed IA, Adam SI. Some pharmacological and toxicological studies on Balanites aegyptiaca bark. Phytother Res 1999;13:439-41.
- Hamid OA, Wahab ME, Abdu ZZ, Idris SM. Balanites aegyptiaca extracts for treatment of HIV/AIDS and leukemia. Patent no: WO2001049306 A1; 2001.
- Annan K, Dickson R. Evaluation of wound healing actions of Hoslundia opposita vahl, Anthocleista nobilis G. Don and Balanites aegyptiaca L. J Sci Technol 2008;28:26-33.
- Abdel-Rahim EA, El-Saadany SS, Wasif MM. Biochemical dynamics of hypocholesterolemic action of Balanites aegyptiaca fruit. Food Chem 1986;20:69-78.
- Wani NS, Kabade JB, Kabade MV, Joshi SM, Patil AD. Diuretic activity of leaves of Balanites roxburghii Linn. Int J Pharma Res Dev 2010;2:4.
- Amira AM, Heshma EA, Sara C, Olaf K, Basma S, Sharif S, et al. Aldose reductase inhibition of a saponin-rich fraction and new furostanol saponin derivatives from Balanites aegyptiaca. Phytomedicine 2015;22:829-36.
- Mansour HA, Newairy AA. Amelioration of impaired renal function associated with diabetes by Balanites aegyptiaca fruits in streptozotocin-induced diabetic rats. J Med Res 2000;21:115-25.
- Gnoula C, Mégalizzi V, De Nève N, Sauvage S, Ribaucour F, Guissou P. Balanitin-6 and -7: Diosgenyl saponins isolated from Balanites aegyptiaca Del. display significant anti-tumor activity in vitro and in vivo. Int J Oncol 2008;32:5-15.
- Pettit GR, Doubek DL, Herald DL. Isolation and structure of cytostatic steroidal saponins from the African medicinal plant Balanites aegyptiaca. J Nat Prod 1991;54:1491-502.
- Issa NM, Mansour FK, El-Safti FA, Nooh HZ, El-Sayed IH. Effect of Balanites aegyptiaca on Ehrlich Ascitic carcinoma growth and metastasisin Swiss mice. Exp Toxicol Pathol 2015;67:435-41.
- Samir AMZ, Ezzat IAE, Ramadan MA, Sayed B, Ahmed BMM. Anticarcinogenic activity of Methanolic Extract of Balanites aegyptiaca against breast, colon, and liver cancer cells. Int J Adv Res 2015;3:255-66.
- Evans WC. Trease and Evans Pharmacognosy. 14th ed. London: Bailliere Tindall Ltd.; 1996.
- Harborne JB. Phytochemical methods: a guide to modern techniques of plant analysis. 3rd ed. London: Chapman and Hall; 1998.
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assay. J Immunol Methods 1983;65:55-63.
- Sambrook J, Fritsch EF, Maniatis T. Molecular cloning, A laboratory Manual. 2nd ed. New York: Cold Spring Harbor Laboratory; 1989.
- Umadevi M, Sampath Kumar KP, Debjit Bhowmik, Duraivel S. Traditionally used anticancer herbs in India. J Med Plant Stud 2013;1:56-74.
- Chang WS, Lee YJ, Lu FJ, Chiang HC. Inhibitory effects of flavonoids on xanthine oxidase. Anticancer Res 1993;13:2165-70.
- Mutoh M, Takahashi M, Fukuda K, Komatsu H, Enya T, Matsushima HY, et al. Suppression by flavonoids of cyclooxygenase-2 promoter-dependent transcriptional activity in colon cancer cells: Structure-activity relationship. Jpn J Cancer Res 2000;91:686-91.
- Tanaka T, Makita H, Kawabata K, Mori H, Kakumoto M, Satoh K, et al. Chemoprevention of azoxymethane-induced rat colon carcinogenesis by the naturally occurring flavonoids, diosmin and hesperidin. Carcinogenesis 1997;18:957-65.
- Robert AN, Peiying Y, Alison DP, Keith IB. Cardiac glycosides as novel cancer therapeutic agents. Mol Interv 2008;8:36-49.
- Sreelatha S, Padma PR, Umadevi M. Protective effects of Coriandrum sativum extracts on carbon tetrachloride-induced hepatotoxicity in rats. Food Chem Toxicol 2009;47:702-8.
- Kumar V, Kaur K, Karelia DN, Beniwal V, Gupta GK, Sharma AK, et al. Synthesis and biological evaluation of some 2-(3, 5-dimethyl-1Hpyrazol- 1-yl)-1 arylethanones: Antibacterial, DNA photocleavage, and anticancer activities. Eur J Med Chem 2014;81:267-76.