- *Corresponding Author:
- Kinnari N. Mistry
Ashok and Rita Patel Institute of Integrated Study and Research in Biotechnology and Allied Sciences (ARIBAS), affiliated to Sardar Patel University, Vallabh Vidyanagar, Anand-388 120, India
E-mail: kinnarinmistry@yahoo.com
| Date of Submission | 30 March 2017 |
| Date of Revision | 14 February 2018 |
| Date of Acceptance | 14 August 2018 |
| Indian J Pharm Sci 2018;80(5):875-882 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The present study was conducted to study phytochemical composition, antioxidative and antiproliferative effect of methanol extract of Butea monosperma leaf. Superoxide scavenging assay, metal chelating assay, DPPH and MTT assay were employed. MTT assay was performed on A-549 human lung carcinoma cells and chick embryo fibroblasts were used as the control. Deoxyribonucleic acid fragmentation and real time assay was performed to check apoptosis and gene expression level. Results obtained indicated that the methanol extract of Butea monosperma exhibited high level of antioxidant activity compared to standard antioxidant. Methanol extract and different fraction of the extract exhibited significant antiproliferative activity against lung cancer cell line. The chloroform fraction was found to be most active in MTT assay against A-549 cells while it was less toxic to normal cells. Cells exposed to LD50 concentration of the chloroform fraction exhibited breakdown of DNA. Increased expression of p53, Bax and caspase-3 gene and reduced expression of Bcl-2 gene gave evidence that the chloroform fraction of Butea monosperma might induce apoptosis. These results indicated that the methanol extract and its fractions of Butea monosperma leaf possessed immense potential for tumour treatment. Therefore, it would be necessary to carry out further studies to isolate and identify the active principles responsible for these activities.
Keywords
Apoptosis, A-549 cell line, Butea monosperma plant, cytotoxic activity, DNA fragmentation
Cancer is one of the most dreadful diseases globally and it appears to be due to extreme free radical damage, which eventually causes damage to the DNA, lipids and protein. In cell cycle, growth and division of normal cells occur in an well-ordered manner, however in cancerous cells, defective caspase-mediated cell death (apoptosis) leads to increased cell proliferation [1]. Some proteins manage the temporal order within the cell cycle highly regulated to confirm that division of cells occur only when required. Inaccuracy in this directive becomes the characteristic of cancer [2,3]. Tumour cells evolved mechanisms to resist cell death and this has motivated researchers to investigate many plants, which might selectively induce apoptosis in cancer cells [3,4]. It was reported that presence of some phytochemicals in herbs could be the main reason for antitumor activity exerted through inducing apoptosis in cancer cells [5-8]. In programmed cell death, activation of caspases (cysteinyl, aspartate-specific proteases) leads to activation of target substrates within the cell [6]. In chemotherapy, drugs target quickly growing cells thus these drugs might also target normal cells that proliferate quickly like those in the hair follicles, gastrointestinal tract and bone marrow along with cancer cells. Attributable to this some facet effects occur, together with hair loss, canal distress and low white corpuscle count [9-11]. For a long time, natural remedies and their preparations were shown to be effective in treating many sorts of disorders [5,11,12]. Although herbal treatments are becoming more and more popular worldwide, we all know very little concerning the active ingredients and molecular mechanisms in lots of these remedial plants [5,13].
Cytotoxicity studies on plant-based resources could lead on to a discovery of recent herbal-based antitumor drugs [14].
India has distinctive plant diversities, which have been intensively studied for anticancer elements. Butea monosperma (subfamily- Caesalpinioideae, family- Fabaceae) grows all over the South Asian peninsula (Figure 1). In central India it is generally called as Palash tree [15,16]. It had been selected to study apoptotic effect of B. monosperma leaf extract on the lung cancer cell line, A-549 and compare these results with those obtained on chick embryo fibroblasts. Preliminary work carried out with the methanol extract established that the extract possessed antioxidant and antiinflammatory activity [17]. To date the mechanism of anticancer activity of B. monosperma plant extract has not been studied against lung cancer cell line. Thus, the methanol extract of B. monosperma and its fractions were investigated for anticancer activity on A-549 cell line along with levels of expression of p53, Bax, Bcl-2 and caspase-3 gene in comparison to chick embryo fibroblasts.
Materials and Methods
Fresh sample of B. monosperma leaves were collected from Pune, in August 2013 under the supervision of a botanist and all materials were submitted in original form to the Botany Department, Pune. Authentication of B. monosperma leaves was done using macroscopic, microscopic, histochemical and phytochemical characteristics of the plant.
Preparation of plant extract
Leaves of B. monosperma plant were washed, shadedried and ground to a fine powder using a grinder mixer. Initially 50 g of the powdered plant material was extracted with 500 ml methanol at room temperature with maceration (6×24 h), after which the extract was filtered and concentrated in a rotary evaporator (at 40- 50°) under reduced pressure. The percent yield was calculated from the weight of powder before and after extraction [18]. % yield = (B/A)×100, where, B is the weight of dried powder/leaves after extraction and A is the weight of dried powder/leaves before extraction. Methanol extract of dried leaves of B. monosperma yielded 8.16 g %. This extract was subjected to qualitative phytochemical analysis using standard protocols [19-21].
Estimation of total phenolic and flavonoid contents
For determining the total phenolic content, a modified Folin-Ciocalteu method was used [22]. This assay was performed by mixing 0.5 ml of extract, 0.1 ml Folin- Ciocalteu reagents (0.5 N) and keeping it at room temperature for 15 min. Then 2.5 ml of saturated Na2CO3 was added and allowed to stand for 30 min. Absorbance was measured at 760 nm using a UV/Vis spectrophotometer. Standard curve of gallic acid was created in a range of 0-28 μg/ml using the same procedure (r2=0.9454). Total phenolic content was expressed as milligram gallic acid equivalent (GAE)/g plant extract.
Total flavonoid content was studied using the modified aluminium chloride method [23]. The reaction mixture comprised of 1.0 ml of plant extracts, 0.5 ml of potassium acetate (120 mM), 0.5 ml of aluminium chloride (1.2 %) and kept for 30 min at room temperature. Absorbance was measured at 415 nm using a UV/Vis spectrophotometer. The total flavonoid content was computed from a calibration curve made with quercetin as standard (0-200 mg/ml in ethanol). The concentration of total flavonoids was then expressed as milligram quercetin equivalents/gram crude extract (r2=0.955).
Superoxide radical scavenging activity
The principal behind this assay was the capability to inhibit reduction of nitro blue tetrazolium (NBT) in the NBT system [24]. For determination of superoxide dismutase activity, a method developed by Martinez et al. was used with a slight modification [25]. Each 3 ml reaction mixture comprised of 50 mM sodium phosphate buffer, pH 7.8, 13 mM methionine, 2 mM riboflavin, 100 mM EDTA, NBT (75 mM) and 1 ml sample solution. The formation of blue colour formazan was followed by perceptive the rise in absorbance after 10 min lighting from a fluorescent lamp at 560 nm. The whole reaction assembly was surrounded in a box, covered with aluminium foil. Tubes with reaction mixture were kept in the dark and served as blanks. Super oxide scavenging activity (%) = (A0–A1)/A0×100, where A0 is absorption of control, A1 is absorption of tested extract solution.
Metal chelating activity
Metal chelating activity was studied using the protocol as described previously by Chan et al. [26]. Briefly, 1 ml of plant extract was incubated together with equal volume of FeSO4 (0.1 mM) and ferrozine (0.25 mM) with vortexing for 10 min. Absorbance was measured at 562 nm. The % chelating activity was calculated using the Eqn., metal chelating ability (%) = (A0–A1)/ A0×100, where, A0 is the absorbance of control and A1 is the absorbance of sample.
2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity
DPPH radical scavenging activity test was performed as described by Blois [27]. To 1 ml of a 0.2 mM DPPH solution in ethanol, 1 ml of the fraction solutions at different concentration was added. The mixture was kept for 30 min at room temperature and then absorbance was measured at 517 nm. For every fraction tested the DPPH scavenging action was examined by matching its absorbance with solution having no sample. The DPPH radical scavenging activity of the plant extract was expressed as IC50. IC50 means the amount of extract necessary to inhibit the development of DPPH radicals by 50 %. Results obtained were compared to those obtained with ascorbic acid, the standard antioxidant. Following Eqn. was used to calculate free scavenging activity; DPPH scavenging ability (%) = (A0–As)/ A0×100, where A0 is absorption of control sample, AS is the absorption of tested extract solution.
Partial purification of B. monosperma plant
Based on the anticancer activity observed the methanol extract of B. monosperma was further partitioned into different fractions using an extractor funnel and solvents in increasing order of polarity. Methanol extract was dissolved in water (500 ml) and successively extracted with hexane (100 ml×3), chloroform (100 ml×3), ethyl acetate (100 ml×3), butanol (100 ml×3) and water. The hexane (1.51 g), chloroform (1.23 g), ethyl acetate (1.8 g), butanol (1.94 g) and water (5.43 g) fractions were concentrated and weighed. Stock solutions of these fractions were prepared by dissolving in 1 ml dimethyl sulfoxide (DMSO), which were further diluted with DMEM medium. Then, test solutions were filtered through a 0.22 μm membrane filter (Axiva, SciChem Biotech) and stored at –20° for more experiments.
Culture medium and cell lines
A-549 (human lung cancer) cell line was acquired from NCCS, Pune. Primary culture of chick embryo fibroblast cells and cell lines were grown in DMEM containing 100 U/ml penicillin, 10 % heat-inactivated fetal bovine serum and 100 μg/ml streptomycin solutions. Cell lines were cultured at 37° in a CO2 incubator. Cell culture media and reagents were purchased from Gibco Company (Germany).
Cytotoxic study
MTT colorimetric assay was used to evaluate the antiproliferative activity of methanol crude extract and its fractions of B. monosperma. In MTT assay mitochondrial enzyme reduce soluble MTT into an insoluble colour formazan product in viable tumour cells, which may be measured spectrophotometrically [28]. Briefly, 200 μl of cells (1×104 cells/ml) were seeded in 96 well plates and kept for 24 h (37°, 5 % CO2). After 24 h, prepared concentrations of every sample (25-500 μg/ml) was added. Plant samples were dissolved in DMSO and control cells contained DMSO at the equivalent concentration (0.5 % v/v) of treated cells. After 24 h of incubation, 20 μl of MTT solution (5 mg/ml in phosphate buffer solution) was added and kept the plate for another 4 h. To dissolve formazan crystals formed, medium containing MTT were gently replaced by DMSO. Absorbance was measured at 560 nm using an ELISA plate reader (Bio-Rad). Three independent assays were performed to calculate the results. Then 50 % cell viability of B. monosperma was calculated using the Eqn., cytotoxicity (%) = OD of control sample–OD of treated sample/OD control sample×100.
DNA fragmentation assay (apoptosis)
The induction of cell death in treated A-549 cells was determined by studying DNA fragmentation. Cells (2×106 per ml) were treated with chloroform fraction of B. monosperma at LD50 concentrations and control cells were treated with 0.5 % DMSO (v/v) for 24 h. The cells were washed twice with phosphate buffer solution after stimulation. Then cells were centrifuged for 1500 rpm for 10 min at 14°. The pellet was resuspended in a lysis buffer for 10 s (1 % NP-40, 50 mM Tris-HCI, 10 mM NaCl, 20 mM EDTA, pH 7.5). Supernatant was collected and extraction is repeated with lysis buffer. The supernatant was brought to 1 % SDS and kept for 60 min with RNAase-A (5 μg/ml) at 56° and then digestion with proteinase K (2.5 μg/ml) for at least 30 min at 56°. After addition of half of the volume of 10 M ammonium acetate, the DNA is precipitated with 2.5 volumes of ethanol. DNA samples were electrophoretically separated on 1.8 % agarose gel in 1X Tris-acetate-EDTA buffer. DNA fragmentation was observed under UV transilluminator. As a positive control, camptothecin was used in the present study.
Real-time assay of apoptosis-related genes
The expression levels of well-known apoptosis-related genes p53, Bax, Bcl-2 and caspase were studied using two-step quantitative polymerase chain reaction (qPCR) assay as described [29,30]. Chloroform fraction at LD50 concentration was used in treatment over the period of 4, 12 and 24 h. The sequence of primers used in qPCR were listed in Table 1.
| Gene | Sequence | Product size |
|---|---|---|
| p53 FP | TGCTCAAGACTGGCGCTAAA | 145 b.p |
| p53 RP | CAATCCAGGGAAGCGTGTCA | 145 b.p |
| Bax FP | CAG AGG ATG ATT GCC GCCG | 113 b.p |
| Bax RP | AAA AGG GCG ACA ACC CGG CC | 113 b.p |
| Bcl-2 FP | TTTGTGGAACTGTACGGCCC | 136 b.p |
| Bcl-2RP | GTTGACTTCACTTGTGGCCC | 136 b.p |
| Casp-3FP | TGTGAGGCGGTTGTAGAAGA | 158 b.p |
| Casp-3RP | GCACACCCACCGAAAACCAG | 158 b.p |
| GAPDH-FP | TGGTATCGTGGAAGGACTCA | 258 b.p |
| GAPDH-RP | ATGCCAGTGAGCTTCCCGTT | 258 b.p |
Table 1: The sequences of primers used in real time PCR
Results and Discussion
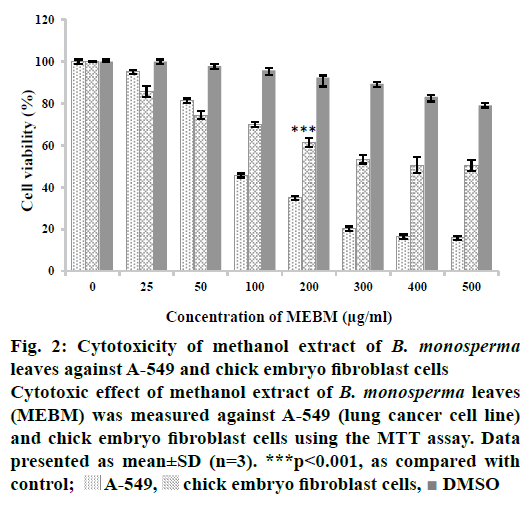
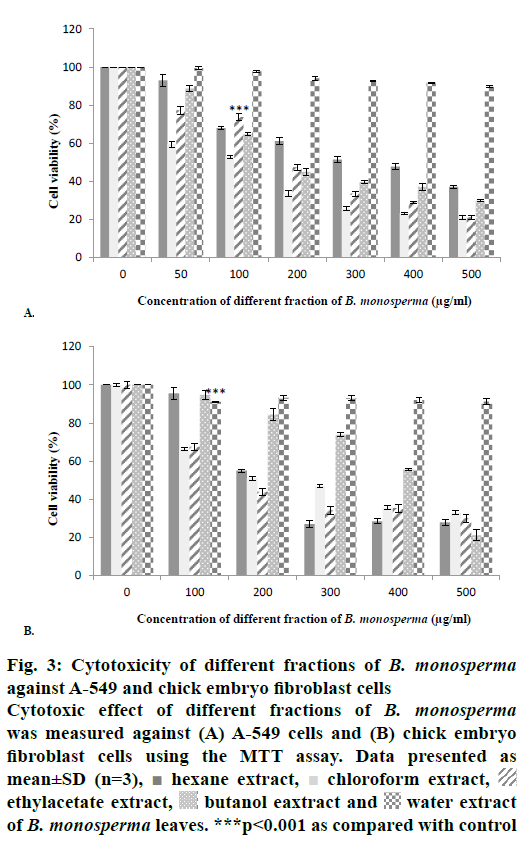
Methanol extract of B. monosperma leaves was subjected to preliminary phytochemical analysis that disclosed the presence of numerous secondary metabolites like tannins, flavonoids, alkaloids, carbohydrates, protein and terpenoids (active compounds) and absence of starch, saponins, glycosides and steroids (Table 2). The crude methanol extract of B. monosperma was further fractioned in different solvents and the fractions obtained were studied to identify the most cytotoxic fraction using the MTT assay. Results of antioxidant activity of methanol extract of B. monosperma were presented in Table 3. The total phenolic content was found to be 196.4±5.4 mg GAE/g for the methanol extract of B. monosperma. On the other hand, the total flavonoid content was 96.8±6.6 mg quercetin equivalent/g crude methanol extract of B. monosperma. Cytotoxicity possessed by the crude methanol extract of B. monosperma was evaluated against A-549 and chick embryo fibroblast cells. Results showed that the crude methanol extract inhibited the viability of A-549 cell when these cells were treated with LD50 concentration for 24 h. Crude methanol extract was tested at concentrations of 25, 50, 100, 200, 300, 400 and 500 μg/ml. As shown in Figure 2, extract had considerable concentration-dependent inhibition of cell viability and proliferation of the A-549 cells and chick embryo fibroblast cells. According to these results, cytotoxic activity of the extract on A-549 cells was more than that on the chick embryo fibroblast cells. Among the fractions tested, the chloroform fraction exhibited selective cytotoxicity against cancer cells relative to normal cells (Figure 3).
| Test | Coloration | BM |
|---|---|---|
| Starch: weak iodine solution | Blue black | - |
| Flavonoids: to test solution few drops of 1 % FeCl3 was added | blackish red colour | + |
| Saponins: 1 ml of filtrate with 2 ml distilled water shaken vigorously and allowed to stand for 10 min. | Development of foam on the surface of the mixture, lasting for 10 min | - |
| Glycosides: to 1 ml of the filtrate; 1 ml of FeCl3 reagent (mixture of 1 vol. of 5 % FeCl3 solution+99 vol. of glacial acetic acid) was added followed by a few drops of conc. H2SO4 | Formation of two layers: lower layer is reddish and upper layer is bluish-green | - |
| Terpenoids: 1 ml of the filtrate with 2 ml CHCl3 and careful addition of a few drops of conc. H2SO4 | An interface with a reddish-brown coloration | + |
| Tannins: 1 ml of filtrate with 2 ml of FeCl3 | Dark green | + |
| Alkaloids: 1 ml of the filtrate with 2 ml of Dragendroff's reagent | Turbid orange colour | + |
| Carbohydrates: 2 ml of Fehling's (A+B)+few drops of sample heated on a burner | Formation of a red or dull violet colour at the interface of the two layers | + |
| Steroids: conc. H2SO4 was added to ice cold extract | Colour change: violet to blue | - |
| Protein: few drops of 10 % NaOH was added to test solution followed by few drops of 0.1 % CuSO4 | The pink or violet colour |
+ |
BM: B. monosperma
Table 2: Secondary metabolites in methanol extract of B. monosperma
| Concentration (µg/ml) | % Chelating effect | % Superoxide scavenging activity | DPPH activity |
|---|---|---|---|
| 50 | 4.61 ± 1.4 | 5.62 ± 2.6 | 5.34 ± 0.9 |
| 100 | 12.90 ± 3.9 | 14.64 ± 1.9 | 16.97 ± 2.4 |
| 200 | 20.51 ± 0.6 | 17.85 ± 0.4 | 22.67 ± 1.1 |
| 300 | 29.83 ± 0.7 | 22.34 ± 2.9 | 35.73 ± 2.7 |
| 400 | 39.48 ± 1.9 | 35.16 ± 0.7 | 43.86 ± 0.5 |
| 500 | 42.13 ± 2.5 | 58.54 ± 1.8 | 53.13 ± 1.9 |
| IC50 of MEBM | 548.44 R²=0.979 |
496.78 R²=0.9236 |
455.83 R²=0.9853 |
| IC50 of standard (µg/ml) |
EDTA=288.37 R²=0.9914 |
Gallic acid=89.368 R²=0.9715 |
Ascorbic acid=11.59 R²=0.9769 |
N=3. All the values are expressed as mean ± SD; MEBM: methanol extract of B. monosperma
Table 3: Antioxidant activity of methanol extract of B. Monosperma
Figure 2: Cytotoxicity of methanol extract of B. monosperma leaves against A-549 and chick embryo fibroblast cells
Cytotoxic effect of methanol extract of B. monosperma leaves
(MEBM) was measured against A-549 (lung cancer cell line)
and chick embryo fibroblast cells using the MTT assay. Data
presented as mean±SD (n=3). ***p<0.001, as compared with
control;  A-549,
A-549,  chick embryo fibroblast cells,
chick embryo fibroblast cells,  DMSO
DMSO
Figure 3: Cytotoxicity of different fractions of B. monosperma against A-549 and chick embryo fibroblast cells
Cytotoxic effect of different fractions of B. monosperma was measured against (A) A-549 cells and (B) chick embryo
fibroblast cells using the MTT assay. Data presented as
mean±SD (n=3),  hexane extract,
hexane extract,  chloroform extract,
chloroform extract,  ethylacetate extract,
ethylacetate extract,  butanol eaxtract and
butanol eaxtract and  water extract
of B. monosperma leaves. ***p<0.001 as compared with control
water extract
of B. monosperma leaves. ***p<0.001 as compared with control
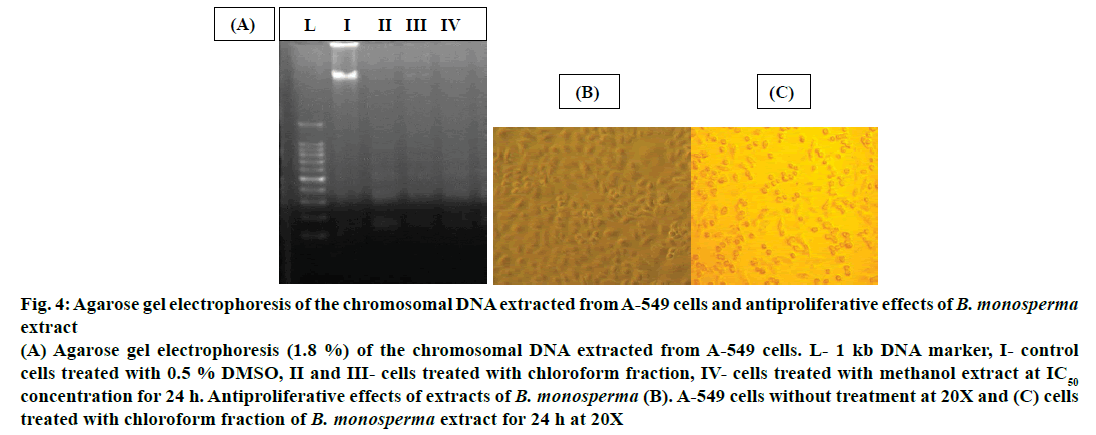
To analyse DNA fragmentation in cells, DNA is extracted from the apoptotic cells and separated on an agarose gel. Degradation of chromosomal DNA into small inter nucleosomal fragments occurred when cells treated with chloroform fraction and methanol extract, which was regarded as a biochemical hallmark of cells undergoing apoptosis, thus fragmented DNA confirmed antiproliferative effect of the extract (Figure 4A). Cells without any treatment did not show any fragmentation (Figure 4B). A-549 cells exhibited morphological changes after treatment like chromatin condensation, cell shrinkage and sphere-shaped form (Figure 4C), which were descriptive of apoptotic cells.
Figure 4: Agarose gel electrophoresis of the chromosomal DNA extracted from A-549 cells and antiproliferative effects of B. monosperma extract
(A) Agarose gel electrophoresis (1.8 %) of the chromosomal DNA extracted from A-549 cells. L- 1 kb DNA marker, I- control
cells treated with 0.5 % DMSO, II and III- cells treated with chloroform fraction, IV- cells treated with methanol extract at IC50 concentration for 24 h. Antiproliferative effects of extracts of B. monosperma (B). A-549 cells without treatment at 20X and (C) cells
treated with chloroform fraction of B. monosperma extract for 24 h at 20X
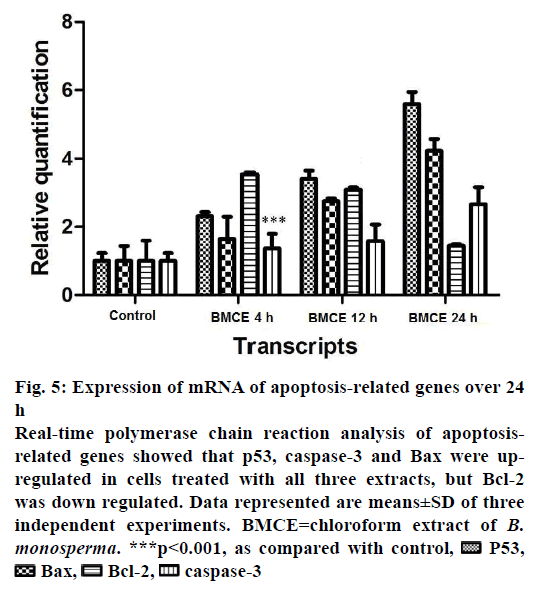
The expression levels of some apoptosis-related genes such as p53, Bax, Bcl-2 and caspase-3 were examined to understand the molecular mechanism behind chloroform fraction-induced programmed cell death in A-549 cells. The relative quantification of p53, Bax, Bcl-2 and caspase-3 mRNA levels were performed. In A-549 cells treated with chloroform fraction of B. monosperma for 4, 12 and 24 h and expression levels of these genes where compared to untreated control cells. Changes in expression level of p53, Bax, Bcl-2 and caspase-3 genes were summarised in Figure 5. After treatment, several-fold increase was observed in the number of transcripts of p53, Bax and caspase-3. A decrease in Bcl-2 expression was observed with chloroform fraction-treated cells and Bax expression was significantly increased (Figure 5), which showed that the treatment induced programmed cell death by changing the Bax and Bcl-2 ratio in favour of apoptosis. Chemotherapy and chemoprevention used to treat cancer by causing caspase-mediated cell death could represent an appropriate methodology to restrain the proliferation of cancerous cells. Many anticancer agents are developed to treat cancer but critical side effects and resistance are the severe issues. Therefore, there is a need for development of more effective anticancer agents. Over the last few years, screening of traditional plants and their parts for cytotoxic properties has gained interest in drug industry. Therapeutically important bioactive components generally occur as secondary metabolites in all plants but their concentration varies consistent with the plant parts, season, climate, and growth phase. Cold percolation method used in the present study has advantages over the Soxhlet extraction that refutes the chances of loss of heat labile components during the extraction. Moreover, the bioactivity of various parts of B. monosperma in terms of antiinflammatory, antioxidant, antimicrobial, antiviral, anticonvulsant, anticonceptive and hepatoprotective properties have been reported. According to Sahare et al. B. monosperma leaves and roots significantly inhibited the motility of microfilariae in a concentrationdependant manner (IC50- 83 ng/ml) [31]. B. monosperma leaves showed considerable hypoglycaemic and antioxidant effects [32,33]. Borkar et al. reported antiinflammatory activities of petroleum ether, hexane, ethanol, ethyl acetate and chloroform extracts of B. monosperma leaves [17].
Figure 5: Expression of mRNA of apoptosis-related genes over 24
h
Real-time polymerase chain reaction analysis of apoptosisrelated
genes showed that p53, caspase-3 and Bax were upregulated
in cells treated with all three extracts, but Bcl-2
was down regulated. Data represented are means±SD of three
independent experiments. BMCE=chloroform extract of B.
monosperma. ***p<0.001, as compared with control, P53,
P53,  Bax,
Bax, Bcl-2,
Bcl-2,  caspase-3
caspase-3
A good anticancer agent should destroy or incapacitate cancer cells without causing unnecessary damages and side effects to normal cells. This perfect condition is attainable by causing apoptosis in malignant cells [34]. Generally cells undergoing this condition exhibit some morphological changes like cell shrinkage, blebbing, DNA fragmentation, loss of cell-membrane attachment and chromatin condensation [35]. Cells treated with plant extract would change their morphology from adherent epithelial like shape and also lose their attachment which is characteristics of apoptotic cells (Figure 4C). Lastly, the plant extract elicited programmed cell death in treated cells, not necrosis. Treatment with the plant extract resulted in breakdown of chromosomal DNA into smaller fragments (Figure 4A), which was a biochemical trademark of cells undergoing programmed cell death [5]. Electrophoresis of cells DNA resulted in a smudge, but not a ladder and this confirmed that cells were killed due to apoptosis and not necrosis.
In the present study, to know the possible anticancer mechanisms of chloroform fraction, the expression of apoptotic genes was analysed. After treatment with plant extract, expression of caspase-3 was increased and decrease in Bcl-2 expression level (Figure 5) was observed. Thus expression of Bcl-2 mRNA was decreased but the expression of p53, Bax and caspase-3 mRNA was increased. Therefore, the Bax/Bcl-2 ratio was also elevated. The results obtained in this study indicated that chloroform fraction induced programmed cell death through the mitochondrial apoptotic pathways, which was confirmed by the observed enhanced expression levels of caspase-3 after treatment. These pro- and antiapoptotic genes have been the main regulators of the intrinsic pathway of cell death. According to earlier reports, ratio of Bax to Bcl-2 decided the vulnerability of cells undergoing apoptosis [36]. Bcl-2 expression was significantly inhibited whereas the expression of p53, Bax, and caspase-3 increased after the treatments in a time-dependant manner. Chloroform fraction might encourage programmed cell death through p53 balancing the ratio of Bax to Bcl-2. Bcl-2 family is one of major classes of regulators in the intrinsic pathway [37]. The possible method by which p53 control the apoptosis by increasing gene expression of pro-apoptosis genes and inhibiting antiapoptotic genes [38,39]. Apoptosis occurred due to extrinsic or intrinsic or mitochondrial pathway [40]. Based on the above activity exhibited by the chloroform fraction, identification of active substances would play a significant role in the safe, effective use of chloroform fraction therapeutically. In the end, the current study confirmed that the chloroform fraction killed A-549 cells by induction of apoptosis.
This investigation established for the first time that the chloroform fraction of B. monosperma up regulated p53, Bax, caspase-3 gene and down regulated Bcl- 2 gene as. Therefore, the chloroform fraction of B. monosperma could serve as a potential source to find new molecules for cancer treatment. Since the above results demonstrated the cytotoxic potential of the chloroform fraction of B. monosperma in vitro, additional studies are required to investigate possible in vivo cytotoxic activity of the chloroform extract and to find out the molecular mechanisms of such activity.
Acknowledgement
Authors are grateful to Charutar Vidya Mandal and Anand Agriculture University, Vallabh Vidyanagar, Gujarat for providing a platform for this research work. Authors wish to thank Dr. Subhash S. Deokule, Head of the Botany Department, Pune for authenticating the plat parts collected. Authors also thank Dr. Nilanjan Roy, Director of Ashok and Rita Patel Institute of Integrated Study & Research in Biotechnology and Allied Sciences (ARIBAS), New Vallabh Vidyanagar, for providing facilities and for making valuable suggestions during this research work.
Conflict of interest
We declare that we have no conflict of interest.
References
- Zimmerman MA, Huang Q, Li F, Liu X, Li CY. Cell death–stimulated cell proliferation: A tissue regeneration mechanism usurped by tumors during radiotherapy. Semin Radiat Oncol 2013;23(4):288-95.
- Sclafani RA, Holzen TM. Cell cycle regulation of DNA replication. Annu Rev Genet 2007;41:237-80.
- Foster DA, Yellen P, Xu L, Saqcena M. Regulation of G1 cell cycle progression: distinguishing the restriction point from a nutrient-sensing cell growth checkpoint (s). Genes Cancer 2010;1:1124-31.
- Pistritto G, Trisciuoglio D, Ceci C, Garufi A, D'Orazi G. Apoptosis as anticancer mechanism: function and dysfunction of its modulators and targeted therapeutic strategies. Aging 2016;8(4):603-19.
- Gao H, Lamusta J, Zhang WF, Salmonsen R, Liu Y, O’Connell E, et al. Tumor cell selective cytotoxicity and apoptosis induction by an herbal preparation from Brucea javanica. N Am J Med Sci 2011;4(2):62-6.
- Valiyari S, Baradaran B, Delazar A, Pasdaran A, Zare F. Dichloromethane and methanol extracts of Scrophularia oxysepala induces apoptosis in MCF-7 human breast cancer cells. Adv Pharm Bull 2012;2(2):223-31.
- Yamamoto M, Miura N, Ohtake N, Amagaya S, Ishige A, Sasaki H, et al. Genipin, a metabolite derived from the herbal medicine Inchin-ko-to, and suppression of Fas-induced lethal liver apoptosis in mice. Gastroenterology 2000;118(2):380-9.
- Badgujar N, Mistry KN, Chudasama P, Patel JS. In vitro Antioxidant and Cytotoxic Effects of Methanol Extracts of Vitex negundo, Lantana camara, Bauhinia variegata and Bauhinia racemosa on Human Cancer Cell lines. Indian J Pharm Sci 2017;79:431-37.
- Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer 2004;4(4):253-65.
- Kawabe T. G2 checkpoint abrogators as anticancer drugs. Mol Cancer Ther 2004;3(4):513-9.
- Millimouno FM, Dong J, Yang L, Li J, Li X. Targeting apoptosis pathways in cancer and perspectives with natural compounds from mother nature. Cancer Prev Res 2014;7(11):1081-107.
- Ning Z, Lu C, Zhang Y, Zhao S, Liu B, Xu X, et al. Application of plant metabonomics in quality assessment for large-scale production of traditional Chinese medicine. Planta Med 2013;79:897-908.
- Zahri S, Razavi SM, Niri FH, Mohammadi S. Induction of programmed cell death by Prangos uloptera, a medicinal plant. Biol Res 2009;42:517-22.
- Yousefzadi M, Heidari M, Akbarpour M, Mirjalili MH, Zeinali A, Parsa M. In vitro cytotoxic activity of the essential oil of Dorema ammoniacum D. Don. Middle-East J Sci Res 2011;7:511-4.
- Shah GM, Khan MA, Ahmad M, Zafar M, Khan AA. Observations on antifertility and abortifacient herbal drugs. Afr J Biotechnol 2009;8(9):1959-64.
- Khan AU. Evaluating the last remnants of Butea monosperma (Lam.) Kuntze Forest for their in situ conservation: a case study. Environ Monit Assess 2010;170:171-84.
- Borkar VS, Gangurde HH, Gulecha VS, Bhoyar PK, Mundada AS. Evaluation of in vitro antihelmintic activity of leaves of Butea monosperma. Int J Phytomed 2010;2:31-5.
- Mandal S, Patra A, Samanta A, Roy S, Mandal A, Mahapatra TD, et al. Analysis of phytochemical profile of Terminalia arjuna bark extract with antioxidative and antimicrobial properties. Asian Pac J Trop Biomed 2013;3(12):960-6.
- Edeoga HO, Okwu DE, Mbaebie BO. Phytochemical constituents of some Nigerian medicinal plants. Afr J Biotechnol 2005;4:685-8.
- Veerachari U, Bopaiah A. Phytochemical investigation of the ethanol, methanol and ethyl acetate leaf extracts of six cassia species. Int J Pharma Sci 2012;3:34-9.
- Nag S, Paul A, Dutta R. Phytochemical Analysis of Methanolic extracts of leaves of some medicinal plants. IJSRP 2013;3(4):1-5.
- Mongkolsilp S, Pongbupakit I, Sae-Lee N, Sitthithaworn W. Radical scavenging activity and total phenolic content of medicinal plants used in primary health care. J Pharm Sci 2004;9:32-5.
- Pourmorad F, Hosseinimehr SJ, Shahabimajd N. Antioxidant activity, phenol and flavonoid contents of some selected Iranian medicinal plants. Afr J Biotechnol 2006;5(11):1142-45.
- Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 1971;44(1):276-87.
- Martinez CA, Loureiro ME, Oliva MA, Maestri M. Differential responses of superoxide dismutase in freezing resistant Solanum curtilobum and freezing sensitive Solanum tuberosum subjected to oxidative and water stress. Plant Sci 2001;160:505-15.
- Chan E, Lim Y, Omar M. Antioxidant and antibacterial activity of leaves of Etlingera species (Zingiberaceae) in Peninsular Malaysia. Food Chem 2007;104:1586-93.
- Blois MS. Antioxidant determinations by the use of a stable free radical. Nature 1958;181:1199-200.
- Sylvester PW. Optimization of the tetrazolium dye (MTT) colorimetric assay for cellular growth and viability. Methods Mol Biol 2011;716:157-68.
- Samarakoon SR, Ediriweera MK, Nwokwu CDU, Bandara CJ, Tennekoon KH, Piyathilaka P, et al. A Study on Cytotoxic and Apoptotic Potential of a Triterpenoid Saponin (3-O--L-Arabinosyl Oleanolic Acid) Isolated from Schumacheria castaneifolia Vahl in Human Non-Small-Cell Lung Cancer (NCI-H292) Cells. BioMed Res Int 2017;2017:9854083.
- Bong I, Lim P, Balraj P, Sim Ui Hang E, Zakaria Z. Quantitative analysis of the expression of p53 gene in colorectal carcinoma by using real-time PCR. Trop Biomed 2006;23(1):53-9.
- Sahare KN, Anandharaman V, Meshram VG, Meshram SU, Gajalakshmi D, Goswami K, et al. In vitro effect of four herbal plants on the motility of Brugiamalayi microfilariae. Indian J Med Res 2008;127(5):467-71.
- Sharma N, Garg V. Antihyperglycemic and antioxidative potential of hydroalcoholic extract of Butea monosperma Lam flowers in alloxan-induced diabetic mice. Indian J Exp Biol 2009;47:571-6.
- Sharma N, Garg V. Antidiabetic and antioxidant potential of ethanolic extract of Butea monosperma leaves in alloxan-induced diabetic mice. Indian J Biochem Biophys 2009;46(1):99-105.
- Tacar O, Sriamornsak P, Dass CR. Doxorubicin: an update on anticancer molecular action, toxicity and novel drug delivery systems. J Pharm Pharmacol 2013;65:157-70.
- Kepp O, Galluzzi L, Lipinski M, Yuan J, Kroemer G. Cell death assays for drug discovery. Nat Rev Drug Discov 2011;10(3):221-37.
- Marzo I, Naval J. Bcl-2 family members as molecular targets in cancer therapy. Biochem Pharmacol 2008;76(8):939-46.
- Chang HK, Shin MS, Yang HY, Lee JW, Kim YS, Lee MH, et al. Amygdalin induces apoptosis through regulation of Bax and Bcl-2 expressions in human DU145 and LNCaP prostate cancer cells. Biol Pharm Bull 2006;29:1597-602.
- Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science 2004;305:626-9.
- Oren M. Decision making by p53: life, death and cancer. Cell Death Diffe 2003;10:431-42.
- Fulda S, Debatin K. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene 2006;25:4798-811.