- *Corresponding Author:
- S. N. Preethamol
Department of Botany, Cell and Molecular Biology Division, University of Calicut, Kerala 673635,India
E-mail: preethamolsn92@gmail.com
| Date of Submission | 09 June 2020 |
| Date of Revision | 18 August 2021 |
| Date of Acceptance | 31 March 2022 |
| Indian J Pharm Sci 2022;84(2):407-414 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Ophiorrhiza jacobii, is a therapeutically less explored species, belonging to the family Rubiaceae and endemic to the Western Ghats. The present study was aimed to evaluate the antioxidant potential and chemical composition of the methanolic extract of the plant Ophiorrhiza jacobii using various in vitro methods and gas chromatography-mass spectrometric analysis respectively. Phytochemical screening of the extract revealed the presence of numerous bioactive secondary metabolites like phenols, terpenoids, sterols and tannins that might contribute to the medicinal properties of the plant. The maximum percentage inhibition obtained in 2,2-diphenyl-1-picrylhydrazyl, hydroxyl, nitric oxide and superoxide radical scavenging assays were 72.01 %±0.79 %, 70.24 %±0.90 %, 67.37 %±1.20 % and 76.00 %±1.07 % respectively. In all the assays, a dose-dependent increase in free radical scavenging activity was exhibited by the plant extract and the percentage inhibition of the radicals showed that the plant possesses significant antioxidant capacity. Gas chromatography-mass spectrometry analysis revealed several antioxidant compounds like phytol, vitamin E, stigmasterol, solanesol and n-hexadecanoic acid suggesting that the plant can be a potential source of natural antioxidant to treat various ailments related to oxidative stress. Till date, no literature is available on the evaluation of the medicinal properties of Ophiorrhiza jacobii and this study forms the first report on the antioxidant potential and chemical composition of the plant. The presence of terpenoids, phytosterols, terpene alcohols, fatty acids and their esters, also suggests that the plant can be a novel source for the development of new therapeutic agents to treat various health disorders.
Keywords
Ophiorrhiza jacobii, phytochemical, antioxidant, free radical, gas chromatography-mass spectrometry
Free radicals or Reactive Oxygen Species (ROS) are highly unstable molecules produced by metabolic reactions in our body. They attack almost all thDPPH radical scavenging potential of the plant extract was determined by following the methodologye parts of the body including lipids, proteins, Deoxyribonucleic Acid (DNA), fats and even brain cells. They create oxidative stress that results in serious ailments like premature ageing, cardiovascular diseases, neurodegenerative diseases, asthma, arthritis, diabetes and stroke[1]. Some of the most potent ROS include superoxide anions, Hydrogen Peroxide (H2O2), hydroxyl radicals, nitric oxide radicals and peroxynitrile[2]. They attack other compounds to seize electrons and make them free radicals and initiate chain reactions that cause cell death[3]. Though the immune system in our body protects cells from oxidative stress, age and individual health conditions can affect this process[2] and accumulation of ROS occurs. High level of ROS can cause changes in membrane fluidity, protein crosslinking, DNA damage and finally cell death[4].
Antioxidants are substances that counteract free radicals and neutralize them thus inhibiting oxidative stress and protect cells[5]. Though synthetic antioxidants are widely used, they are suspected to cause serious health hazards and thus plant-based natural products have gained much attention as safer antioxidants[6].
Medicinal plants are the major sources of natural medicine[7] but only 15 % of these are scientifically explored and evaluated for their medicinal properties[8]. The present study was designed to evaluate the antioxidant potential and chemical composition of the methanolic extract of the plant, Ophiorrhiza jacobii (O. jacobii) by Hareesh et al. belonging to the family Rubiaceae. The plant is a decumbent herb, endemic to Southern Western Ghats of India. It is characterized by densely hairy vegetative as well as reproductive parts with short peduncle and pink coloured flowers[9]. The genus Ophiorrhiza L. comprises of 321 species, 5 varieties and 1 subspecies[10] and many members of the genus are well known for their medicinal properties. They have been used in folklore medicine for the treatment of various ailments like ulcers, leprosy, gastropathy and amenorrhea[11]. The present study was aimed to assess the antioxidant potential of the methanolic plant extract of O. jacobii. Numerous plant extracts have been subjected to experiments related to their antioxidant potential and the results reveal that their bioactivity is the contribution of their phytoconstituents[1]. Thus the chemical constituents of the extract were analysed by preliminary phytochemical screening and Gas Chromatography-Mass Spectrometry (GC-MS) methods to validate their antioxidant efficacy. Though according to previous reports, plants within the genus have cytotoxic properties[12], there is no documented report on any bioactivity of O. jacobii. Thus the present study is the first report on the antioxidant potential and GC-MS analysis of the plant.
Materials and Methods
Collection of plant material:
Fresh whole plant materials of O. jacobii were collected from Idukki district of Kerala in India. The collected plant materials were identified by Dr. M. Sabu (Professor, Department of Botany, University of Calicut, Kerala). The voucher specimen was deposited in the herbarium of University of Calicut with an accession number viz., CALI: 143973.
Preparation of the extract:
The collected plant materials were thoroughly washed to remove the soil and dirt and were shade dried. The dried plant materials were chopped to small pieces and blended to powder and then stored in airtight container. 20 g of powder was weighed and extracted with 200 ml of 100 % methanol for 6 h at 50°-60° using Soxhlet apparatus. The obtained extract was first allowed to cool and then filtered using Whatman filter paper No.1 and later concentrated at 60°. The final extract was then stored in an amber coloured bottle at 4° for further use.
Phytochemical screening:
The preliminary phytochemical screening of the methanolic extract of the plant for the presence of various secondary metabolites was carried out using standard protocols[13-18].
1,1-Diphenyl-2-Picryl-hydrazyl (DPPH) radical scavenging activity:
DPPH radical scavenging potential of the plant extract was determined by following the methodology of Chang et al.[19] with slight modifications. From the stock concentration of 10 mg/ml Dimethyl Sulfoxide (DMSO), different concentrations (12.5 μg/ml, 25 μg/ ml, 50 μg/ml, 100 μg/ml, 200 μg/ml) of the sample were taken and made up to a volume of 20 μl with DMSO and then 1.48 ml of 0.1 mM DPPH (0.1 mM) solution was added to this. The reaction mixture was then incubated for 20 min at room temperature in dark conditions. After incubation, the absorbance of the reaction mixture was read at 517 nm against a suitable blank. Ascorbic acid was taken as the standard. A solution devoid of the test sample, but an equal quantity of distilled water was considered as the control. The percentage inhibition of DPPH radical was calculated using the below equation.
Percentage inhibition=Ac-As/Ac×100, where Ac is the absorbance of the control and As is the absorbance of the sample.
Hydroxyl radical scavenging activity:
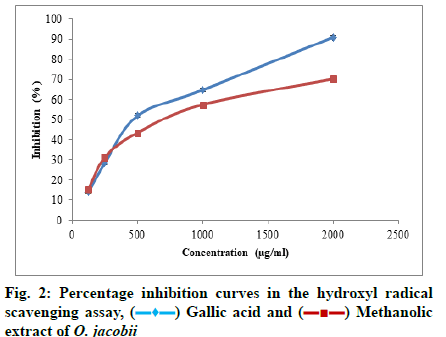
The ability of the plant extract to scavenge hydroxyl radicals was determined using the method of Kunchandy and Rao[20]. Following the method, the reaction mixture consisting of deoxyribose (2.8 mM), Ferrous Chloride (FeCl2-0.1 mM), Ethylene Diamine Tetraacetic Acid (EDTA-0.1 mM), H2O2-1 mM, ascorbate (0.1 mM) and phosphate buffer (20 mM, pH 7.4) was prepared. Aliquots of the sample (12.5 μl, 25 μl, 50 μl, 100 μl, 200 μl) with different concentrations were taken from the stock solution and added to 500 μl of reaction mixture and then made up to a volume of 1 ml. This solution was then incubated for 1 h at 37°. After incubation, 1 ml of Trichloroacetic Acid (TCA-2.8 %) and 1 ml of aqueous Thiobarbituric Acid (TBA-1 %) was added and the solution was again incubated for 15 min at 90°. The solution was then allowed to cool and the absorbance was measured at a wavelength of 532 nm against a suitable blank. A solution devoid of the test compound, but an equal amount of distilled water was taken as the control and gallic acid was used as the standard.
The ability to scavenge hydroxyl radical was calculated using the following equation.
Percentage inhibition=Ac-As/Ac×100, where Ac is the absorbance of the control and As is the absorbance of the sample.
Nitric oxide radical scavenging activity:
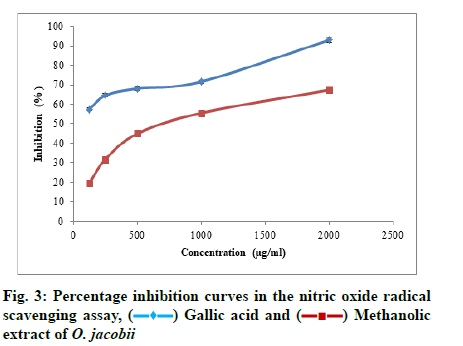
Nitric oxide radical scavenging potential of the plant extract was estimated using the method of Kumaran and Karunakaran[21], with slight modifications.
From the stock solution; different concentrations of the sample (125 μg/ml, 250 μg/ml, 500 μg/ml, 1000 μg/ ml, 2000 μg/ml) were taken and mixed with sodium nitroprusside (5 mmol/l) in Phosphate Buffer Saline (PBS) of pH 7.4 and incubated at 25° for 30 min. 1.5 ml of the incubated solution was then removed and diluted with 1.5 ml of Griess reagent. Griess reagent consisted of 1 % sulphanilamide, 2 % phosphoric acid and 0.1 % N-1-naphthyl ethylene diamine dihydrochloride. The absorbance of the reaction mixture was measured against a suitable blank at 546 nm. Gallic acid was used as the standard and control without the sample but an equivalent amount of distilled water was taken. The percentage inhibition of nitric oxide radical was calculated using the following equation.
Percentage inhibition=Ac-As/Ac×100, where Ac is the absorbance of the control and As is the absorbance of the sample.
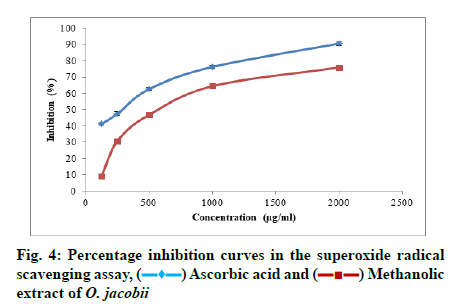
Superoxide radical scavenging activity:
The methodology of Valentao et al.[22] is followed for the estimation of superoxide radical scavenging potential of the plant extract. Reaction mixture was prepared by mixing different concentrations of the sample (125 μg/ ml, 250 μg/ml, 500 μg/ml, 1000 μg/ml, 2000 μg/ml) taken from the stock solution with 0.05 ml riboflavin solution (0.12 mM), 0.2 ml EDTA solution (0.1 M) and 0.1 ml Nitroblue Tetrazolium solution (NBT-1.5 mM). Then the mixture was diluted to 2.64 ml with phosphate buffer (0.067 M) and illuminated for 5 min with fluorescent light. The absorbance of the solution was measured at 560 nm. The solution was again illuminated for 30 min and absorbance was recorded at the same wavelength and the change in optical density was calculated. Ascorbic acid was used as the standard and control without the test sample, but an equal amount of distilled water was taken. The ability to scavenge superoxide radical was calculated using the equation.
Percentage inhibition=Ac-As/Ac×100, where Ac is the absorbance of the control and As is the absorbance of the sample.
The Half Maximal Inhibitory Concentration (IC50) for all the antioxidant assays carried out was calculated using the software ED50plus v1.0.
GC-MS analysis:
The chemical composition of the plant extract was determined by GC-MS analysis performed on Shimadzu GCMS-QP-2010 plus system. Thermal Desorption system TD 20, fitted with 60 m×0.25 mm×0.25 m Wall- Coated Open Tubular (WCOT) column coated with diethylene glycol was used for the analysis. Helium was the carrier gas used with a flow rate of 1.21 ml/min, column pressure was 77.6 KPa. Injector and detector temperatures were maintained at 260°, the volume of sample injected was 6 μl and the split ratio of the column was 10:0. The conditions set for component separation were: Linear temperature program of 70°- 260° at 3°/min and then held at 260° for 6 min, with a total run time of 44.98 min. The MS parameters used were: Electron Ionization (EI) voltage of 70 eV, peak width of 2 s, mass range of 40-850 m/z and detector voltage of 1.5 V.
Identification of compounds:
Compounds obtained were identified by comparison of their linear retention indices. The MS fragmentation patterns of the compounds were compared with other compounds of already known composition, with the patterns available in the spectral library of National Institute of Standards and Technology (NIST) and also by comparing data available from the literature. The quantitative estimation of the compounds was done on the basis of their respective GC peak areas.
Statistical analysis:
All the data were statistically analysed using the software Statistical Package for the Social Sciences (SPSS) Version 20. The results obtained were validated using one-way Analysis of Variance (ANOVA) and Duncan’s multiple range tests and those with p<0.05 were considered statistically significant. All the experiments were analysed in triplicates and results were expressed as mean±Standard Error (SE).
Results and Discussion
The phytochemical screening of the methanolic extract of the plant revealed the presence of various bioactive constituents like phenols, flavonoids, sterols, terpenoids, tannins, alkaloids, anthraquinones, coumarins,glycosides, cardiac glycosides, carbohydrates, proteins and fats. Saponins and phlobatannins were found to be absent in the methanolic extract.
Methanolic extract of O. jacobii exhibited a dosedependent increase in its free radical scavenging potential in all the four assays done. The inhibition percentage of the radicals was found to increase with increasing concentration of the extract.
In DPPH radical scavenging assay, at the highest concentration, the percentage inhibition was observed as 72.01 %±0.79 % and the IC50 value obtained was 92.642 μg/ml. Significantly, the IC50 value comes in the range of the selected concentrations and hence the plant extract has potential scavenging effects on DPPH radical with a considerable percentage of inhibition. Fig. 1 shows the dose-dependent inhibition effects of the plant extract on DPPH radicals.
In hydroxyl radical scavenging assay, the potential of the plant extract to scavenge the radicals was found to be highest at the concentration of 2000 μg/ml. The highest percentage inhibition was obtained as 70.24 %±0.90 % and the IC50 value of the plant extract was observed to be 767.118 μg/ml. Fig. 2 shows the increasing percentage inhibition of hydroxyl radicals with increasing concentration of the plant extract.
Similarly, the nitric oxide radical scavenging potential of the plant extract was found to be 67.37 %±1.20 % at the highest concentration of 2000 μg/ml. The IC50 value of the plant extract obtained for the assay was found to be 783.351 μg/ml. The increasing percentage inhibition of nitric oxide radicals with an increase in the concentration of the plant extract is depicted in fig. 3.
In the case of superoxide radical scavenging ability of the plant extract also, the maximum inhibition was observed at the highest concentration as 76.00 %±1.07 %. The IC50 value of the plant extract obtained for this assay was 919.973 μg/ml. Fig. 4 show the increasing percentage inhibition of superoxide radicals with increasing concentration of the plant extract.
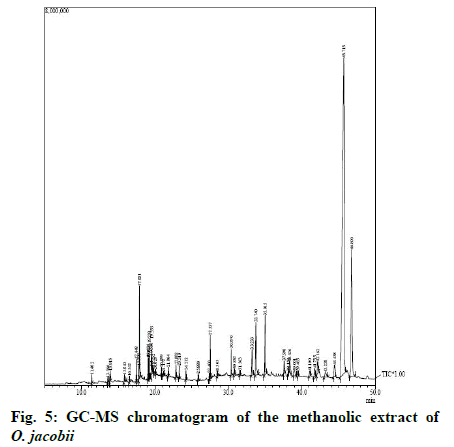
The chemical composition of the methanolic extract of O. jacobii analysed by GC-MS revealed the presence of 27 phytocomponents belonging to various classes of compounds. GC-MS chromatogram of the methanolic extract of the plant is shown in fig. 5. The results are summarized in Table 1.
| RT (min) | Name of the compound | Molecular formula | Class of the compound | Peak area (%) |
|---|---|---|---|---|
| 11.402 | Tetradecane | C14H30 | Alkane | 0.632 |
| 13.566 | Octadecanoic acid | C18H36O2 | Fatty acid | 0.359 |
| 16.542 | Neophytadiene | C20H38 | Sesquiterpenoid | 0.118 |
| 17.448 | Hexadecanoic acid, methyl ester | C17H34O2 | Fatty acid, methyl ester | 0.443 |
| 17.881 | n-Hexadecanoic acid | C16H32O2 | Fatty acid | 2.841 |
| 19.074 | Linoleic acid, methyl ester | C19H34O2 | Fatty acid, methyl ester | 0.368 |
| 19.133 | 8,11,14-docosatrienoic acid, methyl ester | C23H40O2 | Fatty acid, methyl ester | 0.341 |
| 19.239 | Phytol | C20H40O | Diterpene alcohol | 0.802 |
| 19.5 | Linoleic acid | C18H32O2 | Fatty acid | 0.311 |
| 19.555 | 7-Tetradecenal, (z) | C14H26O | Fatty aldehyde | 0.439 |
| 20.029 | Nonadecane | C19H40 | Alkane | 0.085 |
| 20.899 | Heneicosane | C21H44 | Alkane | 0.159 |
| 21.804 | Hexacosane | C26H54 | Alkane | 0.935 |
| 25.88 | Nonacosane | C29H60 | Alkane | 0.56 |
| 27.537 | Squalene | C30H50 | Triterpenoid | 1.314 |
| 30.39 | Solanesol | C45H74O | Terpene alcohol | 1.09 |
| 30.832 | 14-Oxatricyclo [9..2.1.0(1,10)]tetradecane, 2,6,6,10,11-pentamethyl- | C18H30O | Alkane | 0.307 |
| 31.563 | Vitamin E | C29H50O2 | Phenol | 0.206 |
| 33.233 | Campesterol | C28H48O | Sterol | 1.768 |
| 33.74 | Stigmasterol | C29H48O | Sterol | 3.88 |
| 35.015 | gamma-Sitosterol | C29H50O | Sterol | 4.124 |
| 37.598 | Lupenyl acetate | C32H52O2 | Triterpene | 2.089 |
| 38.158 | gamma-Sitostenone | C29H48O | Sterol | 0.137 |
| 39.001 | Incanin | C17H22O5 | Sesquiterpenoid lactone | 0.649 |
| 41.1 | Humulane-1,6-dien-3-ol | C15H26O | Sesquiterpenoid | 58.193 |
| 43.238 | Lupan-3-ol, acetate | C32H54O2 | Triterpenoid | 16.495 |
| 44.438 | 3- Epipapyriferic acid | C33H54O7 | Triterpene | 1.355 |
Note: RT: Retention time
Table 1: phytocompounds identified from the methanolic extract of O. Jacobii using GC-MS analysis
The major components in the plant extract were terpenoids (80.213 %) which included sesquiterpenoids like neophytadiene (0.118 %), incanin (0.649 %), humulane-1,6-dien-3-ol (58.193 %) and triterpenoids like squalene (1.314 %), lupenyl acetate (2.089 %), lupan-3-ol acetate (16.495 %) and 3-epipapyriferic acid (1.355 %). 1.892 % of terpene alcohols were also found in the extract that included phytol (0.802 %) and solanesol (1.09 %). The extract contained four types of sterols namely, campesterol (1.768 %), stigmasterol (3.880 %), gamma sitosterol (4.124 %) and gammasitostenone (0.137 %). Vitamin E (0.206 %) was the phenolic compound found in the extract. Bioactive fatty acids like octadecanoic acid (0.359 %), n-hexadecanoic acid (2.841 %), linoleic acid (0.311 %) were also obtained from the GC-MS analysis. In addition to these, several alkane compounds, fatty acid esters and fatty aldehydes were also revealed in the analysis.
Thus the GC-MS analysis revealed the presence of eight different classes of compounds like terpenoids (80.213 %), sterols (9.909 %), fatty acids (3.511 %), alkanes (2.678 %), terpene alcohols (1.892 %), fatty acid esters (1.152 %), fatty aldehydes (0.439 %) and phenols (0.206 %).
Therapeutic potential of medicinal plants can be attributed to the presence of various classes of secondary metabolites. These are bioactive phytoconstituents performing specific physiological activities on our body like antioxidant, anticancer and antimicrobial[23]. So, phytochemical screening is very important to identify the phytoconstituents that are responsible for such pharmacological activities[24]. Several reports are suggesting that the phytocomponents like phenols, flavonoids, terpenoids, sterols, tannins and coumarins have antioxidant properties[25]. Recent studies reveal that the protective effects of the plant extracts against oxidative stress are due to the synergistic action of their phytoconstituents[8]. In the present study secondary metabolites like phenols, flavonoids, sterols, terpenoids, tannins, alkaloids, anthraquinones, coumarins, glycosides, cardiac glycosides, carbohydrates, proteins and fats were present and thus the free radical scavenging potential exhibited by the plant extract might be the contribution of these compounds.
Free radical assays are the most commonly used methods to determine the antioxidant potential of plant extracts[26]. In all these assays, the ability of the extract or the phytochemicals to donate hydrogen atoms or electrons and to stabilize the free radicals is considered as an index for antioxidant capacity[27]. Antioxidant efficacy of the testing material depends upon the chemical and physical parameters available within the experimenting system and thus a single test model would not be enough to conclude the antioxidant activity of a sample[28]. Thus four different assays were done to evaluate the potential of the plant extract to scavenge free radicals like DPPH, hydroxyl, nitric oxide and superoxide radicals.
DPPH radical scavenging assay is one of the simplest and easiest in vitro assays to determine the antioxidant potential of the plant extract[29]. DPPH is a stable purple coloured free radical that converts to yellow coloured compound due to the reduction reaction by antioxidants present in the sample[27]. The hydrogen donating ability of the sample is related to the greater discoloration of the free radical that results in lower absorbance[30]. The present study suggests that the plant extract has significant scavenging effects on the DPPH radicals with considerable percentage inhibition.
In hydroxyl radical scavenging activity, the free radicals are generated by Fenton reaction. They attack 2-deoxy-2-ribose and break them into fragments which on heating with TBA at low pH form a red colour[31]. So less red colour indicates higher antioxidant potential. Hydroxyl radicals form the most dangerous ROS since cells lack an enzymatic mechanism to remove them. They attack all biological molecules like lipids, proteins and cell membranes and cause cell death[4]. Thus the inhibition of hydroxyl radicals by the sample suggests that the test material is a good antioxidant agent that can be considered for more trials.
Nitric oxide radicals are potent ROS that cause severe tissue damages as well as cardiovascular diseases[8]. The principle behind nitric oxide radical scavenging assay is the production of nitric oxide by sodium nitroprusside in aqueous solution at physiological pH. The produced nitric oxide reacts with oxygen and forms nitrite ions whose quantity can be estimated using Griess reagent[32]. Thus the antioxidant potential of the sample depends on the ability to compete with oxygen to inhibit nitrite ion formation. In the present study, the plant extract has exhibited a significant percentage of nitric oxide radical inhibition.
Superoxides, though they are weak oxidants, produce highly dangerous free radicals like hydroxyls and singlet oxygen[33]. Thus their presence within the cells is very dangerous as they can generate other additional free radicals. The sample tested has shown a significant percentage of inhibition of the radicals and thus could be a considerable antioxidant agent.
In all the assays done, the percentage inhibition and IC50 values obtained for the plant extract is significant, though the results are lower than that obtained for the standards taken. This is explainable since the standards used are in their pure form and the plant extract is in its crude state that has to be processed further for obtaining the specific compounds responsible for the bioactivity[34]. Though in crude form, the study undertaken proves that the plant has the potential antioxidant ability to scavenge dangerous ROS by inhibiting significant percentage of free radicals.
For further evaluation, the plant extract was analysed by GC-MS, which is an ideal technique for the qualitative and quantitative estimation of volatile components[35]. The results showed that the major phytocomponents were terpenoids, followed by sterols and many compounds obtained from the analysis were highly bioactive. The most important and the major phytocomponents recognized was humulane-1,6-dien- 3-ol (58.193 %), which is found to be a sesquiterpenoid with anti-inflammatory, antibacterial, antiviral and antitumour activities[36]. A triterpene compound known as 3-epipapyriferic acid identified from the plant extract is a compound with several reported pharmacological effects. Reports on the use of the compound in the studies on neurodegenerative diseases, ageing, cardiovascular diseases, liver diseases and gastrointestinal diseases suggest that the compound is highly bioactive[37].
The triterpene compound, squalene was reported to possess several properties such as antioxidant, anticarcinogenic, anti-allergic, immune stimulant, cholesterol-lowering and skin protection[38]. Another compound identified was incanin, a sesquiterpenoid lactone, with potential antioxidant and antiinflammatory properties[39]. Phytosterols are important compounds that have gained attention as antioxidants. They inhibit the production of ROS and prevent angiogenesis[8]. The analysis of the plant extract revealed the presence of bioactive phytosterols like campesterol, stigmasterol, gamma-sitosterol and gamma-sitostenone and these might contribute to the antioxidant potential of the plant. Fatty acid n-hexadecanoic acid and its ester with reported antioxidant property[40] were also identified in the analysis. Two terpene alcohols with great pharmacological properties, namely solanesol and phytol were also revealed by GC-MS. Solanesol exhibits antimicrobial, antitumour, anti-inflammatory and anti-ulcer activities and acts as an intermediate for the synthesis for other medicinally significant compounds like coenzyme Q10 and vitamin K2[41]. Phytol has antinociceptive and antioxidant properties and is also useful in the production of vitamins E and K[35]. The phenolic compound obtained in the analysis was vitamin E, which has great antioxidant potential. Generally, phenols are well-recognized compounds for radical scavenging and chain-breaking reactions and thus protecting cells against ROS[42]. Thus the bioactivity potential of all the components could be contributing towards the antioxidant capacity of the plant extract.
The present study on the methanolic extract of O. jacobii suggests that the plant is rich in highly bioactive secondary metabolites that play a major role in different pharmacological activities. The in vitro assays on antioxidant potential suggests that the plant could be a potent antioxidant source for reducing oxidative stress and thereby could prevent many diseases caused by the free radicals. GC-MS analysis shows that the extract has many antioxidant constituents well known for radical scavenging activities. The presence of compounds like squalene, stigmasterol, phytol and vitamin E substantiates the radical scavenging effects of the plant. So the antioxidant efficacy of the plant might be the synergistic contribution of these compounds. More in vivo trials will help to explore more bioactive potentials of the plant as a medicine. Thus the study, suggests that the plant O. jacobii could be a very strong candidate for drug development.
Acknowledgements:
S. N. Preethamol acknowledges the Forest Department of Kerala, India, for providing the permission for the collection of the plant specimens.
Conflict of interests:
The authors declare that they have no conflict of interest.
References
- Al-Jaber NA, Awaad AS, Moses JE. Review on some antioxidant plants growing in Arab world. J Saudi Chem Soc 2011;15(4):293-307.
- Kasote DM, Katyare SS, Hegde MV, Bae H. Significance of antioxidant potential of plants and its relevance to therapeutic applications. Int J Biol Sci 2015;11(8):982-91.
[Crossref] [Google Scholar] [PubMed]
- Sumathi S, Padma PR, Gathampari S, Vidhya S. Free radical scavenging activity of different parts of Withania somnifera. Anc Sci Life 2007;26(3):30-4.
[Google Scholar] [PubMed]
- Sharma P, Jha AB, Dubey RS, Pessarakli M. Reactive oxygen species, oxidative damage and antioxidative defense mechanism in plants under stressful conditions. J Bot 2012;2012:1-26.
- Safaei-Ghomi J, Ebrahimabadi AH, Djafari-Bidgoli Z, Batooli H. GC/MS analysis and invitro antioxidant activity of essential oil and methanol extracts of Thymus caramanicus Jalas and its main constituent carvacrol. Food Chem 2009;115(4):1524-8.
- Hemalatha R, Nivetha P, Mohanapriya C, Sharmila G, Muthukumaran C, Gopinath M. Phytochemical composition, GC-MS analysis, in vitro antioxidant and antibacterial potential of clove flower bud (Eugenia caryophyllus) methanolic extract. J Food Sci Technol 2016;53(2):1189-98.
[Crossref] [Google Scholar] [PubMed]
- Bajalan I, Zand M, Goodarzi M, Darabi M. Antioxidant activity and total phenolic and flavonoid content of the extract and chemical composition of the essential oil of Eremostachys laciniata collected from Zagros. Asian Pac J Trop Biomed 2017;7(2):144-6.
- Oyebode OA, Erukainure OL, Ibeji CU, Koorbanally NA, Islam MS. Phytochemical constituents, antioxidant and antidiabetic activities of different extracts of the leaves, stem and root barks of Alstonia boonei: An in vitro and in silico study. Bot Lett 2019;166(4):444-56.
- Hareesh VS, Salish MJ, Wu L, Joseph G, Sabu M. Ophiorrhiza jacobii (Rubiaceae) sp. Nov. from Western Ghats, India. Nord J Bot 2018;36(1):1-4.
- Hareesh VS, Sabu M. The genus Ophiorrhiza (Rubiaceae) in Andaman and Nicobar Islands, India with a new species. Phytotaxa 2018;383(3):259-72.
- Rajan R, Varghese SC, Kurup R, Gopalakrishnan R, Venkataraman R, Satheeshkumar K, et al. Search for camptothecin-yielding Ophiorrhiza species from southern Western Ghats in India: A HPTLC-densitometry study. Ind Crops Prod 2013;43:472-6.
- Preethamol SN, Thoppil JE. Evaluation of cytotoxicity and apoptotic potential of Ophiorrhiza pectinata Arn. A potent anticancer agent. Asian J Pharm Clin Res 2019;12:97-102.
- Kumar GS, Jayaveera KN, Kumar CK, Sanjay UP, Swamy BM, Kumar DV. Antimicrobial effects of Indian medicinal plants against acne-inducing bacteria. Trop J Pharm Res 2007;6(2):717-23.
- Trease GE, Evans WC. Pharmacognosy. 15th ed. London: Saunders Publishers; 2002.
- Siddiqui AA, Ali M. Practical Pharmaceutical Chemistry. New Delhi: CBS Publishers and Distributors; 1997. p. 126-31.
- Edeoga HO, Okwu DE, Mbaebie BO. Phytochemical constituents of some Nigerian medicinal plants. Afr J Biotechnol 2005;4(7):685-8.
- Sofowora A. Medicinal plants and traditional medicine in Africa. Nigeria: Spectrum Book Ltd; 1993. p. 195-238.
- Evans WC, Evans D. Trease and Evans' Pharmacognosy. 15th ed. New York: Elsevier Health Sciences; 2002. p. 21-4.
- Chang ST, Wu JH, Wang SY, Kang PL, Yang NS, Shyur LF. Antioxidant activity of extracts from Acacia confusa bark and heartwood. J Agric Food Chem 2001;49(7):3420-4.
[Crossref] [Google Scholar] [PubMed]
- Kunchandy E, Rao MN. Oxygen radical scavenging activity of curcumin. Int J Pharm 1990;58(3):237-40.
- Kumaran A, Karunakaran RJ. Nitric oxide radical scavenging active components from Phyllanthus emblica L. Plant Foods Hum Nutr 2006;61(1):1-5.
[Crossref] [Google Scholar] [PubMed]
- Valentao P, Fernandes E, Carvalho F, Andrade PB, Seabra RM, Bastos ML. Antioxidative properties of cardoon (Cynara cardunculus L.) infusion against superoxide radical, hydroxyl radical and hypochlorous acid. J Agric Food Chem 2002;50(17):4989-93.
[Crossref] [Google Scholar] [PubMed]
- Krishnaiah D, Devi T, Bono A, Sarbatly R. Studies on phytochemical constituents of six Malaysian medicinal plants. J Med Plants Res 2009;3(2):67-72.
- Mungole AJ, Awati R, Chaturvedi A, Zanwar P. Preliminary phytochemical screening of Ipomoea obscura (L): A hepatoprotective medicinal plant. Int J Pharmtech Res 2010;2(4):2307-12.
- Tayade AB, Dhar P, Sharma M, Chauhan RS, Chaurasia OP, Srivastava RB. Antioxidant capacities, phenolic contents and GC/MS analysis of Rhodiola imbricata Edgew. Root extracts from Trans?Himalaya. J Food Sci 2013;78(3):C402-10.
[Crossref] [Google Scholar] [PubMed]
- Pracheta SV, Paliwal R, Sharma S. Preliminary phytochemical screening and in vitro antioxidant potential of hydro-ethanolic extract of Euphorbia neriifolia Linn. Int J Pharmtech Res 2011;3(1):124-32.
- Hajji M, Jarraya R, Lassoued I, Masmoudi O, Damak M, Nasri M. GC/MS and LC/MS analysis and antioxidant and antimicrobial activities of various solvent extracts from Mirabilis jalapa tubers. Process Biochem 2010;45(9):1486-93.
- Badarinath AV, Rao KM, Chetty CM, Ramkanth ST, Rajan TV, Gnanaprakash K. A review on in vitro antioxidant methods: Comparisions, correlations and considerations. Int J Pharmtech Res 2010;2(2):1276-85.
- Alam MN, Bristi NJ, Rafiquzzaman M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm J 2013;21(2):143-52.
[Crossref] [Google Scholar] [PubMed]
- Dasgupta S, Khatarani A. Evaluation of antioxidant properties of fruit extracts of Cactus (Opuntia ficus-indica) grown in Saurashtra region of Gujarat. Int J Res Educ Sci Methods 2021;9(6):3230-5.
- Vazhayil BK, Sundaram RS, Patel D, Alex AR, Gomathi S, Roy PI, et al. In vitro antioxidant potential of standardized ethanol extract of Clerodendron serratum Linn. leaves. Asian J Chem 2016;28(6):1288-92.
- Okon EE, Chibuzor EF, Christian OE, Nsikan UM, Francis AM. In vitro antioxidant and nitric oxide scavenging activities of Piper guineense seeds. Glob J Med Plant Res Indig Med 2013;2(7):475-84.
- Patel RM, Patel NJ. In vitro antioxidant activity of coumarin compounds by DPPH, Super oxide and nitric oxide free radical scavenging methods. J Adv Pharm Educ Res 2011;1:52-68.
- Peteros NP, Uy MM. Antioxidant and cytotoxic activities and phytochemical screening of four Philippine medicinal plants. J Med Plant Res 2010;4(5):407-14.
- Arora N, Pandey-Rai S. GC-MS analysis of the essential oil of Celastrus paniculatus Willd. seeds and antioxidant, anti-inflammatory study of its various solvent extracts. Ind Crops Prod 2014;61:345-51.
- Jiao SG, Zhang RF, Li JJ, Wuken SN, Zhang HX, Tu PF, et al. Phytochemical and pharmacological progress on humulane-type sesquiterpenoids. Zhongguo Zhong Yao Za Zhi 2018;43(22):4380-90.
[Crossref] [Google Scholar] [PubMed]
- Cao J, Zhang X, Qu F, Guo Z, Zhao Y. Dammarane triterpenoids for pharmaceutical use: A patent review (2005–2014). Expert Opin Ther Pat 2015;25(7):805-17.
[Crossref] [Google Scholar] [PubMed]
- Reddy LH, Couvreur P. Squalene: A natural triterpene for use in disease management and therapy. Adv Drug Deliv Rev 2009;61(15):1412-26.
[Crossref] [Google Scholar] [PubMed]
- Chadwick M, Trewin H, Gawthrop F, Wagstaff C. Sesquiterpenoids lactones: Benefits to plants and people. Int J Mol Sci 2013;14(6):12780-805.
[Crossref] [Google Scholar] [PubMed]
- Kumar PP, Kumaravel S, Lalitha C. Screening of antioxidant activity, total phenolics and GC-MS study of Vitex negundo. Afr J Biomed Res 2010;4(7):191-5.
- Yan N, Liu Y, Zhang H, Du Y, Liu X, Zhang Z. Solanesol biosynthesis in plants. Molecules 2017;22(4):510.
[Crossref] [Google Scholar] [PubMed]
- Ndhlala AR, Moyo M, Van Staden J. Natural antioxidants: Fascinating or mythical biomolecules? Molecules 2010;15(10):6905-30.
[Crossref] [Google Scholar] [PubMed]
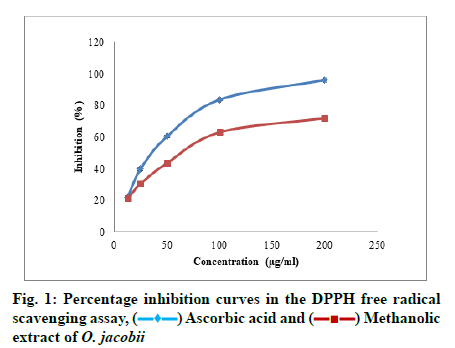
 Ascorbic acid and
Ascorbic acid and  Methanolic extract of O. jacobii
Methanolic extract of O. jacobii