- *Corresponding Author:
- Ana Barjaktarevic
Department of Pharmacy,
Center for Molecular Medicine and Stem Cell Research,
Faculty of Medical Sciences,
University of Kragujevac,
Kragujevac 34000,
Serbia
E-mail: ana.radovanovickg@gmail.com
| Date of Received | 29 January 2020 |
| Date of Revision | 07 April 2021 |
| Date of Acceptance | 05 December 2021 |
| Indian J Pharm Sci 2021;83(6):1280-1287 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Salvia verticillata L. is a well-known plant from the family Lamiaceae. The purpose of this research was to analyze the cytotoxic, antioxidant and antimicrobial activities of chloroform and petroleum ether root extracts of Salvia verticillata. The total content of phenolic compounds and flavonoids were determined in both of the extracts. The antioxidant activity was estimated in vitro using different assays. The antimicrobial potential was tested against different test strains using micro-well dilution assay. The cytotoxic activity was evaluated on epithelial, human breast cancer cell line MDA-MB-231 and human colorectal carcinoma cell line HCT 116 by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay. The extracts showed similar antioxidant activity. The values for the antimicrobial activity differed depending on the microbial strain as well on the solvent used. There were significant differences in cytotoxic effect on investigated cell lines depending of the concentration. Half maximal inhibitory concentration value for chloroform extract was 77.16 μg/ml for MDA-MB-231 cell line and 105.08 μg/ml for the HCT 116 cell line. Half maximal inhibitory concentration value for petroleum ether extract was 30.90 μg/ml for MDA- MB-231 cell line and 44.28 μg/ml for the HCT 116 cell line.
Keywords
Salvia verticillata, antioxidant activity, antimicrobial activity, cytotoxicity
Salvia verticillata L. (S. verticillata) (purple rain) from the family Lamiaceae is a medicinal plant, with natural habitat in Europe and Asia [1]. It has been also native to Serbia. In traditional medicine, S. verticillata extracts have been used for reducing blood sugar, cardiovascular diseases, abdominal pain, cold or as an antiseptic [1,2]. Phytochemical analysis of different plant parts has shown the presence of phenolic acids, flavonoids, volatile oils, diterpenoids and triterpenoids in S. verticillata [3].
According to the previously published data, S. verticillata could be considered as a natural antioxidant, though values for antioxidant activity differ depending on the investigated part of the plant as well on the used assay [1]. Methanol and aqueous extract of S. verticillata showed strong antioxidant effects [3-7]. Roots of this plant are stronger antioxidants than leaves (by 2,2-Diphenyl-1-picrylhydrazyl (DPPH) test), while radical scavenging activity measured by 2,2'-Azino- bis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS) proved leaf extract to be superior to root [8].
Different Salvia species showed considerable cytotoxic effects. Some isolated compounds from the roots of selected Salvia species (diterpenoids) also showed anticancer activity [9]. Özcelik et al. evaluated cytotoxicity of chloroform and methanol extract of S. verticillata (expressed as maximum non- toxic concentrations) [10]. The cytotoxic activity of the essential oil of this plant was also evaluated [11]. Extracts of S. verticillata aerial parts showed cytotoxic activity on human cancer cell lines [7]. The anticancer potential of S. verticillata root extracts has not been reported yet.
Although antimicrobial activity of many Salvia species was previously reported, the literature data about antimicrobial activity of S. verticillata are limited. The essential oil of this plant exhibited antibacterial activity against different strains, but it showed weak antifungal properties [12]. Ethanol extract of S. verticillata aerial parts proved to have antimicrobial activity [4,6,13].
The purpose of this research was to analyze the cytotoxic, antioxidant and antimicrobial activities of two different root extracts of S. verticillata (chloroform and petroleum ether).
Materials and Methods
Plant materials and preparation of extracts:
The plant material was gathered from the area of Stara Planina (Serbia), on spring. We used the fresh roots of S. verticillata. Taxonomic and botanical identification was confirmed by the Institute for Ecology and Biology in Kragujevac, Serbia [14]. The dried and powdered plant materials were extracted under reflux in the boiling chloroform and petroleum ether. The extract was filtered and the solvent was completely removed using the rotary evaporator (RV05 basic, IKA, Germany) under the low pressure to obtain the dry extract.
Determination of Total Phenolic Content (TPC):
The TPC of phenolic compounds in the extracts was determined using the Folin-Ciocalteu reagent, measuring the absorbance at 750 nm. TPC value was expressed as Gallic Acid Equivalents (GAE) (Micrograms (µg) of GAE/mg Dry Weight (dw)) of three replicates [15,16].
Determination of Total Flavonoid Content (TFC):
Aluminum chloride colorimetric method was used for the determination of TFC of flavonoids. The absorbance of yellow complexes between aluminum chloride and carbonyl and hydroxyl groups of the flavonoids was measured at 510 nm. The results were expressed as Rutin Equivalents (µg RE/mg extract) of three replicates [17].
Antioxidant activity assays:
ABTS test: TheABTS test was used for the determination of the ABTS radical scavenging activity of investigated extracts, measuring the absorbance at 734 nm. The results were expressed as Trolox Equivalents (µg TE/ mg dry extract) of three replicates [15].
DPPH test: The DPPH test was used for the determination of the DPPH radical scavenging activity of S. verticillata root extracts, measuring the absorbance at 515 nm. The results were expressed as Trolox equivalents (µg TE/mg dw) of three replicates [15].
Cupric Reducing Antioxidant Capacity (CUPRAC) test: CUPRAC was measured according to the procedure previously described, measuring the absorbance at 450 nm. The results were expressed as Trolox Equivalents (µg TE/mg dry extract) [16].
Ferric Reducing Antioxidant Power (FRAP) assay: The method described by Dimitrijevic et al. was used for estimating the ferric reducing power of the extracts, measuring the absorbance at 595 nm. This method is based on the electron transfer between the antioxidant molecules and Fe3+ ions. The results were expressed as Fe (II) Equivalents (µg Fe/mg dw) of three replicates [15].
Total Reducing Power (TRP) assay: The method described by Dimitrijevic et al. was used for estimating the reducing power of S. verticillata extracts, measuring the absorbance at 700 nm. The results were expressed as Ascorbic Acid Equivalents (µg AAE/mg dry extract) of three replicates [15].
Antioxidant Potency Composite Index (ACI): The method described by Seeram et al. was used for the calculation of ACI [18].
Antimicrobial activity assay:
A series of diluted plant extracts with concentrations ranging from 0.04 to 100.0 mg/ml were analyzed. Antimicrobial activity of the extracts was tested against 9 bacterial strains (Pseudomonas aeruginosa (P. aeruginosa), Bacillus cereus (B. cereus), Staphylococcus aureus (S. aureus), Enterococcus faecalis (E. faecalis), Klebsiella pneumoniae (K. pneumoniae), Salmonella enteritidis (S. enteritidis), Proteus mirabilis (P. mirabilis), Escherichia coli (E. coli), Enterobacter aerogenes (E. aerogenes)) and 1 fungal strain (Candida albicans (C. albicans)).
Microorganisms were grown overnight and microbial suspensions were prepared in a sterile normal saline solution at a concentration of 106 Colony Forming Units (CFU)/ml. Their turbidity was adjusted to 0.5 McFarland. Antimicrobial activity was determined using micro- well dilution assay (Clinical and Laboratory Standards Institute (CLSI) 2009, with some modifications) in 96 multi-well microtiter plates. All the antibacterial tests were performed in Müller–Hinton broth and the antifungal test was performed on Sabouraud dextrose agar. Dimethylsulfoxide (DMSO) (100 %) was used as a negative control with concentrations ranging from 0.02 to 50.0 mg/ml, while doxycycline and nystatin in concentrations ranging from 0.01 to 20.0 µg/ml were used as a positive control. To indicate microbial growth, 20 µl 0.5 % triphenyl tetrazolium chloride solution was added to each well.
The antimicrobial evaluation was determined using Minimum Inhibitory Concentration (MIC) and Minimal Bactericidal Concentration (MBC)/Minimal Fungicidal Concentration (MFC) (in mg/ml). MIC is the lowest concentration of the extract that inhibited the visible growth of a microorganism. MBC/MFC is concentration of the extract that killed 99.9 % of microbial cells. All measurements were performed in triplicate.
Cytotoxic activity assay:
A series of plant extracts with concentrations ranging from 0.1 to 100.0 μg/ml were analyzed. Cytotoxic effect of S. verticillata extracts was evaluated by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) colorimetric assay [19]. Experimental procedure for cytotoxic activity is described precisely by Denizot et al. The cytotoxicity of the investigated extracts was assessed on two different human cancer cell lines: Human Breast Cancer Cell Line (MDA-MB-231) and Human Colorectal Carcinoma Cell Line (HCT 116). The same tumor cell lines, which were not treated, were used as the negative control, while vinblastine was used as a positive control. The multiplate reader was used for measuring the optical density at 590 nm. The viability of cells was determined following the equations:
% Viability=100´(Absorbance mean value of treated cells/Absorbance mean value of negative control)
% Cytotoxicity=100-% Viability
All measurements have been performed in triplicate.
Statistical analysis:
The results were reported as mean±Standard Deviation (SD) and analyzed using Statistical Package for the Social Sciences (SPSS) version 19.0 program. Significant differences between extracts were analyzed by One-way Analysis of Variance (ANOVA), Student's t-test and appropriate nonparametric test. Statistical significance was set at p<0.05 and p<0.01. The equation of dose response curves was used for Half Maximal Inhibitory Concentration (IC50 ) calculations (50 % of inhibited growth).
Results and Discussion
Antioxidant activity of investigated extracts, TPC and TFC values are presented in Table 1. Considering the differences in methodology of antioxidant assays currently available, it is highly recommended to use multiple assays approach in the research for the most reliable results. Therefore, we decided to test the antioxidant activity of chloroform and petroleum ether extracts by five different assays (Table 1).
| S. verticillata extracts | ABTS µg TE/mg | DPPH µg TE/mg | CUPRAC µg TE/mg |
FRAP µg Fe/mg | TRP µg AAE/mg | TPC µg GAE/mg | TFC µg RE/mg |
|---|---|---|---|---|---|---|---|
| Chloroform | 33.26±1.44 | 13.63±0.33 | 69.60±0.36 | 50.22±0.65 | 0.12±0.011 | 36.92±0.75 | 406.50±2.87 |
| Petroleum ether | 44.11±0.59 | 11.63±0.17 | 38.58±0.59 | 30.12±0.45 | 0.10±0.017 | 31.63±1.26 | 829.00±11.27 |
Note: Values are expressed in mean±SD of triplicate measurement (n=3)
Table 1: Antioxidant Effect, TPC And TFC Values of S. verticillata Extracts
Studies on species S. verticillata confirmed the high content of polyphenols and this could be one of the reasons for the exhibited antioxidant activity [20]. We found a small difference in TPC in chloroform and petroleum ether extract, in favor of the chloroform extract (Table 1). However, petroleum ether extract contained a higher level of flavonoids in comparison to chloroform extract (Table 1). TPC in our research was lower than in previously conducted studies [21].
Tosun et al. demonstrated that S. verticillata contained the highest TPC value (167.1 mg GAE/g dw) among eight investigated Salvia species from Turkish flora [5]. For example, methanolic extract of S. verticillata aerial part contained 175.6 mg GAE/g dw of TPC [6]. Our focus was on less polar extracts (chloroform and petroleum ether) and results of the content of total polyphenolic compounds may be due to the resulting polarity of solvents we used and the fact that they did not sufficiently extract main antioxidant-contributing compounds.
Higher value of TPC contributes to higher antioxidant activity of the plant extracts [5,20]. That is also the case in our research, higher TPC in chloroform extract may be correlated to the stronger antioxidant activity of chloroform extract. However, we observed an inverse correlation between total flavonoids and the antioxidant activity of the extracts.
Petroleum ether of S. verticillata had more than twice higher TFC than chloroform extract, but showing at the same time lower antioxidant activity, with exception of the results of ABTS assay. In general, total phenolic and flavonoid contributed in antioxidant activity (as chain breakers, free radical scavengers and electron donors). Negative correlation between flavonoids and the antioxidant activity of the extracts is not uncommon in literature. Kainama et al. even reported such case for methanolic extract, which as polar solvent should extract more phenolic compounds [22]. The possible reason for such result can be higher antioxidant activity of present compounds beside flavonoids like xanthones, benzophenones, coumarins, stilbenes and depsidones [22,23]. Also, not only phenolic compounds contribute to antioxidant activity but also previous studies have indicated contribution of triterpenoids in antioxidant activity and it is possible that activity of our non-polar extracts result from triterpenoids content [24]. Hence, in our study total flavonoids contributed lower antioxidant activity of the extracts.
Both of the extracts exhibited similar radical scavenging activity and TRP, also (Table 1). On the other hand, the chloroform extract exhibited higher values in the reducing power tests (FRAP, CUPRAC) than petroleum ether extract (Table 1). The antioxidant potential of root extracts of Salvia genus is considerable, but it depends on the species [8]. The previous research supports that S. verticillata could be considered as a natural antioxidant [1,4-7]. The most investigated were methanol and aqueous extracts of S. verticillata aerial part which showed high antioxidant activity measured by different antioxidant assays (ABTS, DPPH, FRAP and CUPRAC) [4,6,7]. On the other hand, methanol and aqueous extract of S. verticillata aerial parts showed higher antioxidant activity measured by ABTS and DPPH assay than both of our extracts [4,6,7]. In the study of Erbil and Di?rak, aqueous and methanol, S. verticillata extracts exhibited high values in the reducing power assays (FRAP and CUPRAC), much higher than in our study [4].
Chloroform extract of S. verticillata shows a higher ACI than petroleum ether extract of S. verticillata (Table 2).
| S. verticillata?xtracts | ABTS | DPPH | CUPRAC | FRAP | TRP | Average |
|---|---|---|---|---|---|---|
| Chloroform | 75.40 | 100 | 100 | 100 | 100 | 95.08 |
| Petroleum ether | 100 | 85.33 | 55.43 | 59.98 | 83.33 | 76.81 |
Table 2: ACI Values For S. verticillata Extracts
The increase in TPC cause the increased ACI but there is no significant correlation. However, there is a negative correlation between TFC and ACI of investigated extracts (r2=0.998 and r=0.9989), which is shown in fig. 1. This is another confirmation that flavonoids in S. verticillata extracts contributed lower antioxidant activity of the extracts.
Antimicrobial activity of investigated S. verticillata extracts is shown in Table 3. S. enteritidis, E. coli, E. aerogenes, E. faecalis and S. aureus were more sensitive to petroleum ether extract with MIC value 6.25 mg/ml. On the other hand, B. cereus was more sensitive to chloroform extract of S. verticillata with the same MIC value.
|
Microorganisms |
ATCC |
S. verticilata extracts MIC/MBC in mg/ml | Doxycyclin/Nystatin MIC/MBC in µg/ml | |
|---|---|---|---|---|
| Chloroform | Petroleum ether | |||
| Gram-negative bacteria | ||||
| E. coli | 25922 | 12.50/50.0 | 6.25/25.0 | 15.61/15.61 |
| P. aeruginosa | 9027 | 25.0/50.0 | 25.0/50.0 | 15.61/15.61 |
| S. enteritidis | 13076 | 25.0/50.0 | 6.25/25.0 | 0.90/1.90 |
| P. mirabilis | 12453 | 25.0/50.0 | 25.0/50.0 | 7.81/15.61 |
| K. pneumoniae | 10031 | 25.0/50.0 | 25.0/50.0 | 15.61/15.61 |
| E. aerogenes | 13048 | 25.0/50.0 | 6.25/25.0 | 7.81/15.61 |
| Gram-positive bacteria | ||||
| B. cereus | 11778 | 6.25/25.0 | 25.0/25.0 | 0.90/15.61 |
| S. aureus | 25923 | 25.0/25.0 | 6.25/25.0 | 7.81/15.61 |
| E. faecalis | 19433 | 12.5/25.0 | 6.25/25.0 | 0.90/1.90 |
| Fungi | ||||
| C. albicans | 14053 | 12.50/50.0 | 12.50/12.50 | 7.81/15.61 |
Table 3: Antimicrobial Effects of S. verticillata Extracts
The literature data indicate the antimicrobial effect of extracts and essential oil of S. verticillata. Our extracts showed weak antimicrobial activity and these results correspond more or less with the previously published results for methanolic extract of S. verticillata aerial parts [1,4,6,13].
Katani? Stankovi? et al. showed notable antibacterial activity of methanol extract of S. verticillata aerial parts towards B. cereus (?IC 1,25 mg/ml) and moderate antibacterial activity towards Bacillus mycoides (B. mycoides), Micrococcus lysodeikticus (M. lysodeikticus) and Azotobacter chroococcum (A. chroococcum) (MIC 10 mg/ ml). On the other hand, E. faecalis, E. coli, K. pneumonia, P. aeruginosa demonstrated much weaker sensitivity with MIC values higher than 20 mg/ml. The antifungal activity of S. verticillata was less notable, except for Penicillium canescens (P.canescens)(?IC5mg/ml)andC.albicans(?IC10mg/ ml) [6]. Erbil and Di?rak also investigated antimicrobial activities of different methanolic extracts of root and aerial parts of S. verticillata. It was demonstrated that the most sensitive bacteria were Bacillus subtilis and E. aerogenes, but none of extracts exhibited antimicrobial activity against E. coli, C. albicans and Saccharomyces cerevisiae [4].
The essential oil of S. verticillata aerial parts also showed moderate to high antibacterial effects against different strains (S. aureus, K. pneumoniae and E. coli), but the oil had no antifungal potential against C. albicans [12].
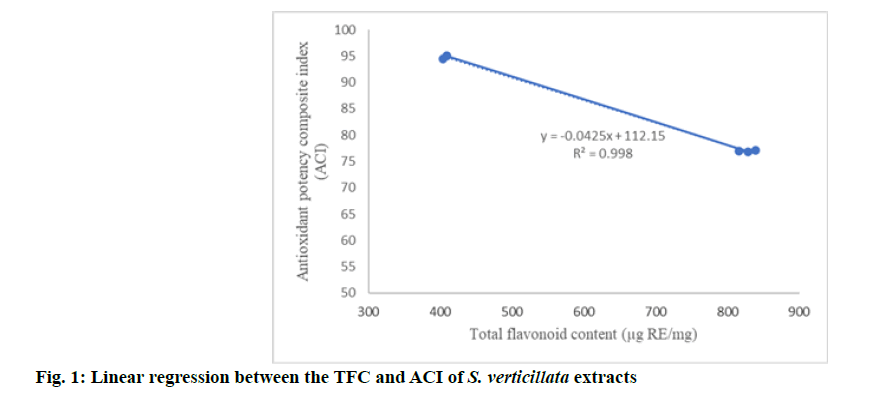
Cytotoxic effect of S. verticillata chloroform and petroleum ether root extracts in 6 different concentrations was analyzed on two different human cancer cell lines. The results were presented in fig. 1 and fig. 2. There are significant differences in cytotoxic effect on investigated cell lines treated by chloroform extract, depending of the concentration (F=4490, p=0.015 and F=89.632, p<0.01 respectively). IC50 value for chloroform extract was 77.16 μg/ml for MDA- MB-231 cell line and 105.08 μg/ml for HCT 116 cell line. There are also significant differences in cytotoxic potential on investigated cell lines treated by petroleum ether extract, depending of the concentration (F=6.825, p=0.003 and F=89.738, p<0.01 respectively). IC50 value for petroleum ether extract of was 30.90 μg/ml for MDA-MB-231 cell line and 44.28 μg/ml for HCT 116 cell line (fig. 3).
Figure 3: Cytotoxic effects of S. verticillata extracts on HCT 116 cell line Note: *p=0.01 compare to 50 μg/ml; **p<0.01 compare to 100 μg/ml; (•) indicates p<0.01 compare to 25 μg/ml; (••) p<0.01 compare
to 100 μg/ml;  p<0.01 compare to 25 μg/ml;
p<0.01 compare to 25 μg/ml;  p<0.01 compare to 100 μg/ml;
p<0.01 compare to 100 μg/ml; p<0.01 compare to 100 μg/ml;
p<0.01 compare to 100 μg/ml; 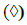 p<0.01 compare to 100 μg/ml;
p<0.01 compare to 100 μg/ml;  Chloroform extract;
Chloroform extract; Petroleum ether extract
Petroleum ether extract
Our attempt to evaluate the cytotoxicity of S. verticillata was based on local experiences in traditional medicine in Serbia. Traditional extract of S. verticillata is used in the form of ethanol extract. Actually, traditional extract is obtained by domestic brandy, but in order to make reproducible experiment we decided to use dilute ethanol, because it is most similar to domestic brandy. Therefore, as pilot experiment we tested ethanol extract of S. verticillata roots. However, the absence of the cytotoxic effect of ethanol extract of S. verticillata roots on both cell lines led us to continue the research considering the use of nonpolar solvents which have potential to extract more cytotoxic compounds. Hence, our cytotoxic evaluation of S. verticillata root extracts progressed by using chloroform and petroleum ether as solvents in obtaining the investigated plant extracts. Extracts applied in the highest concentration (100 μg/ ml) exhibited the maximal inhibitory potential on cell growth. According to IC50 value, chloroform extract was more active against MDA-MB-231 cell line than against HCT 116 cell line. Similar values of IC50 for both tested cell lines were observed after being treated by petroleum ether extract. The American National Cancer Institute recommends further purification of crude extract to develop a new anticancer drug if IC50 value of extract is less than 30 μg/ml [25]. Taking this criterion into account, we think that petroleum ether extract of S. verticillata in our research could be potential candidate for anticancer agent.
Zengin et al. investigated dichloromethane, methanol and water extracts of S. verticillata aerial parts in terms of cytotoxicity against two human cancer cell lines (human alveolar lung epithelial carcinoma (A549) and human breast adenocarcinoma (MCF-7)). These extracts showed moderate cytotoxic activity which was time and dose dependent [7]. Özçelik et al. investigated cytotoxicity of chloroform and methanol extracts of S. verticillata aerial parts on Vero (African green monkey kidney) and Madin-Darby Bovine Kidney (MDBK) cell lines, but their results were not expressed as IC50 value, but as maximum non-toxic concentrations [10].
Consequently, the comparison is not possible. The essential oil of investigated plant also exhibited a cytotoxic activity on different cell lines: Human colon adenocarcinoma cell line (Caco-2), Human colorectal adenocarcinoma cell line (HT-29), Human ductal breast carcinoma ductal cell line (T-47D) and the Swiss mouse embryo fibroblast (NIH-3T3) [5,11].
Our results correspond to the general data from previous studies on different Salvia species that reported potential antitumor activity-Salvia officinalis, Schisandra chinensis and Salvia miltiorrhiza showed cytotoxic activity against HCT-116, MCF-7 and MDA- MB-231 cell lines [26-32]. Salvia blepharochlaena and Salvia euphratica also showed moderate cytotoxicity against MCF-7 and A549 cells. The documented cytotoxic effects of Salvia plants are related to their chemical composition. It is reported that diterpenoids, sesquiterpenoids and triterpenoids from Salvia species have notable cytotoxic effects [7].
The literature states a positive linear correlation between the cytotoxic potential of herbal extracts and their total phenols/flavonoids [21]. Our investigation showed that petroleum ether extract of S. verticillata with considerable higher TFC exhibits cytotoxicity with lower IC50 values than chloroform extract against investigated cell lines. Therefore, we assume that TFC in tested S. verticillata extracts significantly contributed to their cytotoxic activity.
Chloroform and petroleum ether extracts of S. verticillata L. root showed considerable antioxidant effects and they were similar regarding the TPC, while petroleum ether extract showed twice more content of flavonoids. According to the obtained IC50 values, chloroform extract of S. verticillata was more active against MDA- MB-231 cell line while both of the extracts showed similar activity against HCT 116 cell line. Regarding the results of this study, S. verticillata L. is a good natural antioxidant and contains possible cytotoxic compounds. Since we have scarce knowledge about S. verticillata L., expansion of this research may include elucidation of molecular structures and if possible, isolation of individual compounds contributing to these biological effects of S. verticillata L.
Acknowledgements:
This work was supported by Projects of the Ministry of Education, Science and Technological Development of Serbia No. OI 172051, 172047, 175069.
Conflict of interests:
The authors declared no conflict of interest.
References
- Nasermoadeli S, Rowshan V, Abotalebi A, Nasermoadeli L, Charkhchian MM. Comparison of Salvia verticillata essential oil components in wild and cultivated population. Ann Biol Res 2013;4(5):252-5.
- Tabanca N, Demirci B, Aytaç Z, Bas?Er Kh. The chemical composition of Salvia verticillata L. subsp. verticillata from Turkey. Nat Volatiles Essent Oils 2017;4(1):18-28.
- Nickavar B, Rezaee J, Nickavar A. Effect-directed analysis for the antioxidant compound in Salvia verticillata. Iran J Pharm Res 2016;15(1):241.
- Erbil NU, Digrak ME. Total phenolic and flavonoid contents,antimicrobial and antioxidant properties of Salvia verticillata L. var. amasiaca and Salvia microstegia Boiss & Bal from Turkish Flora. J Microbiol Antimicrob Agents 2015;1(1):23-9.
- Tosun M, Ercisli S, Sengul M, Ozer H, Polat T, Ozturk E. Antioxidant properties and total phenolic content of eight Salvia species from Turkey. Biol Res 2009;42(2):175-81.
- Stankovi? JS, Sre?kovi? N, Miši? D, Gaši? U, Imbimbo P, Monti DM, et al. Bioactivity, biocompatibility and phytochemical assessment of lilac sage, Salvia verticillata L. (Lamiaceae)-A plant rich in rosmarinic acid. Ind Crops Prod 2020;143:111932.
- Zengin G, Llorent-Martínez EJ, Fernández-de Córdova ML, Bahadori MB, Mocan A, Locatelli M, et al. Chemical composition and biological activities of extracts from three Salvia species: S. blepharochlaena, S. euphratica var. leiocalycina, and S. verticillata subsp. amasiaca. Ind Crops Prod 2018;111:11-21.
- Matkowski A, Zieli?ska S, Oszmia?ski J, Lamer-Zarawska E. Antioxidant activity of extracts from leaves and roots of Salvia miltiorrhiza Bunge, S. przewalskii Maxim., and S. verticillata L. Bioresour Technol 2008;99(16):7892-6.
- Firuzi O, Miri R, Asadollahi M, Eslami S, Jassbi AR. Cytotoxic, antioxidant and antimicrobial activities and phenolic contents of eleven Salvia species from Iran. Iran J Pharm Res 2013;12(4):801-10.
- Özçelik B, Orhan ?E, Kan Y. Determination of antiviral activity and cytotoxicity of selected sage (Salvia L.) species. J Pharm Sci 2011;36:155-60.
- Khosravi DN, Ostad SN, Maafi N, Pedram S, Ajani Y, Hadjiakhoondi A, et al. Cytotoxic activity of the essential oil of Salvia verticillata L. Res J Pharmacogn 2014;1:27-33.
- Yousefzadi M, Sonboli A, Karimi F, Ebrahimi SN, Asghari B, Zeinali A. Antimicrobial activity of some Salvia species essential oils from Iran. Z Naturforsch C 2007;62(7-8):514-8.
- Sreckovic N, Katanic J, Ninkovic V, Mihailovic V. Antimicrobial activity and phenolic composition of the Salvia verticillata L. plant extract. In: “XXIII Savetovanje O Biotehnologiji”. Zbornik Radova, Cacak: University of Kragujevac Faculty of Agronomy; 2018. p. 493.
- Josifovic M. Lamiaceae family, In: Flora of Serbia. 4th ed. Belgrade: SANU; 1972.
- Dimitrijevic M, Jovanovic VS, Cvetkovic J, Mihajilov- Krstev T, Stojanovic G, Mitic V. Screening of antioxidant, antimicrobial and antiradical activities of twelve selected Serbian wild mushrooms. Anal Methods 2015;7(10):4181-91.
- Rajurkar NS, Hande SM. Estimation of phytochemical content and antioxidant activity of some selected traditional Indian medicinal plants. Indian J Pharm Sci 2011;73(2):146.
- Baba SA, Malik SA. Determination of total phenolic and flavonoid content, antimicrobial and antioxidant activity of a root extract of Arisaema jacquemontii Blume. J Taibah Univ Sci 2015;9(4):449-54.
- Seeram NP, Aviram M, Zhang Y, Henning SM, Feng L, Dreher M, et al. Comparison of antioxidant potency of commonly consumed polyphenol-rich beverages in the United States. J Agric Food Chem 2008;56(4):1415-22.
- Denizot F, Lang R. Rapid colorimetric assay for cell growth and survival: Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods 1986;89(2):271-7.
- Tepe B, Eminagaoglu O, Akpulat HA, Aydin E. Antioxidant potentials and rosmarinic acid levels of the methanolic extracts of Salvia verticillata (L.) subsp. verticillata and S. verticillata (L.) subsp. amasiaca (Freyn & Bornm.) Bornm. Food Chem 2007;100(3):985-9.
- Tian S, Shi Y, Zhou X, Ge L, Upur H. Total polyphenolic (flavonoids) content and antioxidant capacity of different Ziziphora clinopodioides Lam. extracts. Pharmacogn Mag 2011;7(25):65-8.
- Kainama H, Fatmawati S, Santoso M, Papilaya PM, Ersam T. The relationship of free radical scavenging and total phenolic and flavonoid contents of Garcinia lasoar PAM. Pharm Chem J 2020;53(12):1151-7.
- Ersam T, Fatmawati S, Fauzia DN. New prenylated stilbenes and antioxidant activities of Cajanus cajan (L.) millsp. (Pigeon pea). Indones J Chem 2016;16(2):151-5.
- Zou YH, Liu WT, Zhang JX, Xiang DC. Triterpenoids from the bark of Dysoxylum hainanense and their anti-inflammatory and radical scavenging activity. Fitoterapia 2017;121:159-63.
- Abu-Dahab R, Afifi F, Kasabri V, Majdalawi L, Naffa R. Comparison of the antiproliferative activity of crude ethanol extracts of nine Salvia species grown in Jordan against breast cancer cell line models. Pharmacogn Mag 2012;8(32):319-24.
- Fiore G, Nencini C, Cavallo F, Capasso A, Bader A, Giorgi G, et al. In vitro antiproliferative effect of six Salvia species on human tumor cell lines. Phytother Res 2006;20(8):701-3.
- Ghorbani A, Esmaeilizadeh M. Pharmacological properties of Salvia officinalis and its components. J Tradit Complement Med 2017;7(4):433-40.
- Kamatou GP, Makunga NP, Ramogola WP, Viljoen AM. South African Salvia species: A review of biological activities and phytochemistry. J Ethnopharmacol 2008;119(3):664-72.
- Talib WH, Mahasneh AM. Antiproliferative activity of plant extracts used against cancer in traditional medicine. Sci Pharm 2010;78(1):33-46.
- Valiyari S, Baradaran B, Abdolalizadeh J, Bandehagh A, Azadmehr A, Hajiaghaee R. Inhibitory and cytotoxic activities of Salvia officinalis L. extract on human lymphoma and leukemia cells by induction of apoptosis. Adv Pharm Bull 2013;3(1):51-5.
- Zhao Q, Huo XC, Sun FD, Dong RQ. Polyphenol-rich extract of Salvia chinensis exhibits anticancer activity in different cancer cell lines, and induces cell cycle arrest at the G0/G1- phase, apoptosis and loss of mitochondrial membrane potential in pancreatic cancer cells. Mol Med Rep 2015;12(4):4843-50.
- Zhang X, Luo W, Zhao W, Lu J, Chen X. Isocryptotanshinone induced apoptosis and activated MAPK signaling in human breas
 indicates p=0.028 compare to 100 μg/ml;
indicates p=0.028 compare to 100 μg/ml;  Chloroform extract;
Chloroform extract; Petroleum ether extract
Petroleum ether extract