- *Corresponding Author:
- S. Sharma
Department of Plant Breeding and Genetics, Punjab Agricultural University, Ludhiana-141 004, India
E-mail: sunita_sharma@pau.edu
| Date of Submission | 03 March 2017 |
| Date of Revision | 04 October 2017 |
| Date of Acceptance | 20 May 2018 |
| Indian J Pharm Sci 2018;80(4):739-744 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Heterocyclic compounds are known to exhibit a variety of biological activities because of their resemblance to a number of naturally occurring metabolites. Various 2-phenyl-1H-indoles and benzimidazole derivatives have been tested for total antioxidant capacity by phosphomolybednum reduction method and 2,2-diphenyl-1-picrylhydrazide free radical scavenging activity. The compounds with electron donating substituents were found to be better antioxidants with IIIa to be most effective. The best radical scavenger was IVb. Antimicrobial activity was screened in vitro against Pseudomonas sp., Enterobacter sp. (Gram-negative) and Bacillus sp. (Gram-positive). It was found that Gram-negative bacteria were more susceptible than Gram-positive bacteria. Indoles exhibited better antibacterial activity compared to benzimidazoles.
Keywords
Total antioxidant capacity, radical scavenging, indoles, benzimidazoles, antimicrobial
Heterocyclic chemistry forms an integral part of synthetic chemistry and has been a source of a number of bioactive compounds. Its utility lies in the capability of heterocyclic compounds to mimic the structure of peptides and to bind reversibly to proteins [1-4]. To a synthetic chemist, it provides an opportunity to synthesize a library of compounds based on a core scaffold and to screen the derivatives for various biological activities. Various biologically active synthetic compounds have five membered nitrogen containing ring as the core pharmacophore [5]. Free radicals are well-known oxidants because of their tendency to accept the electrons from the potential donors [6]. Reactive oxygen species are a product of normal cellular metabolism [7]. They have an important role in regulating physiological functioning of the cell [8]. To counteract them, antioxidants have the ability to donate electrons to free radicals [9]. Antioxidants, even at low concentration, significantly delay or prevent the oxidation of easy oxidizable substrates [10]. It is reported that the compounds bearing O–H bonds behave as antioxidants but along with them, amines having N–H bond functions as the antioxidant, which have attracted much research attention because cyclic amines have always been the central structure in many currently used drugs [11]. Indoles and benzimidazoles are very popular nitrogen containing heterocycles. Indoles have bicyclic structure having benzene ring fused to pyrrole and are known to possess anticancer [12], antifungal [13], anticonvulsant [14] and antiviral [15] activities. Indoles of commercial importance include drugs such as indomethacin (antiinflammatory, an antipyretic and an analgesic) and indoxole (an antiinflammatory and antipyretic drug). Indoles also present in naturally occurring compounds like dimethyltryptamine (hallucinogen), tryptophan (amino acid) and melatonin [16] (antioxidant as free radical scavenger). Indoles are found in edible substances like brown algae Sargassum thunbergii [17] and in the form of alkaloids in peaches [18] and that of Melodinus cochinchinensis [19]. Another nitrogen containing heterocycle is benzimidazole, which has an imidazole ring fused to benzene. The biological significance of benzimidazoles is due to its close relationship to the structure of purines. A number of other benzimidazole derivatives possessed various biological activities like antimicrobial [20], antiviral [21], antifungal [22], antimalarial [23], antitumor [24], anticancer [25], antihypertensive [26], antiinflammatory [27] and antioxidant [28]. The present study was focused on evaluating substituted indole and benzimidazole derivatives (Table 1) for total antioxidant capacity (TAC), free radical scavenging and antimicrobial activity.
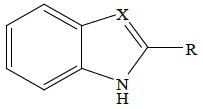 |
||
|---|---|---|
| Compound No. | X | R |
| 2-Phenyl-1H-indoles | ||
| Ia | CH | 4-tolyl |
| IIa | CH | 4-chlorophenyl |
| IIIa | CH | 4-bromophenyl |
| IVa | CH | 3-nitrophenyl |
| Va | CH | 4-nitrophenyl |
| Benzimidazoles | ||
| Ib | N | ethyl |
| IIb | N | propyl |
| IIIb | N | pentyl |
| IVb | N | phenoxymethyl |
| Vb | N | 4-chlorobenzyl |
Table 1: Structures of test compounds (Ia-Va, Ib-Vb)
2-Phenyl-1H-indoles (Ia-Va) were synthesized by reacting equimolar amounts of phenylhydrazine with different substituted acetophenones to form respective phenylhydrazones. The phenylhydrazones were further condensed in presence of polyphosphoric acid to afford the indoles [29]. Substituted carboxylic acids were refluxed with orthophenylenediammine in equimolar amounts in presence of 4 N HCl. On completion of reaction, the mixture was basified with dilute NaOH to afford benzimidazole derivatives (Ib-Vb). The compounds (Ia-Va, Ib-Vb) were screened for antioxidant activity to determine TAC and free radical scavenging activity (RSA) using DMSO as the control and ascorbic acid (AA) as a standard.
TAC was determined by phosphomolybdenum complex formation method [30]. The assay is based on the reduction of Mo (VI) to Mo (V) by the test compounds and subsequent formation of a green phosphate/Mo (V) complex at acidic pH. Three millilitres of test compound was combined with 1 ml of reagent solution (0.6 M sulfuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate). In case of blank, 3 ml of DMSO was used in place of test compounds. The tubes containing the reaction solution were capped and incubated in a boiling water bath at 95° for 90 min. After cooling to room temperature, the absorbance of the solution was measured at 695 nm using a spectrophotometer. The standard curve of AA was prepared using different concentrations of AA. The antioxidant capacity of each compound was expressed as AA equivalent using the following linear Eqn. established using AA as the standard, (A=0.016C–0.03; R²=0.990) where A is the absorbance at 695 nm and C is the concentration as AA equivalent (μg/mg).
The RSA of the compounds was tested by their ability to bleach the stable radical 2,2-diphenyl-1-picrylhydrazyl (DPPH), as reported previously [31]. Because of its odd electronic structure, DPPH gave a strong absorption band at 517 nm in the visible region. DPPH solution (0.1 mM) was prepared by dissolving 4 mg in minimum quantity of DMSO and making up the final volume of the solution to 250 ml. Different concentrations (2, 1 and 0.5 mg/ml) of test compounds (40 μl) and AA (40 μl), dissolved in DMSO, were added to 3.96 ml of the DPPH solution. The absorbance at 517 nm was determined after 30 min at room temperature, and the scavenging activities calculated as percent of the radical reduction. Each experiment was performed in triplicate. DMSO was used as a control solution and AA as the reference compound. The RSA was obtained as follows, RSA (%) = ODcontrol– ODsample/ODcontrol×100, where, ODcontrol and ODsample is the absorbance of blank (DMSO) and test compounds, respectively at 517 nm. The IC50 values were further calculated.
The effect of derivatives of indoles and benzimidazoles on the growth of Pseudomonas sp., Enterobacter sp. and Bacillus sp. was assessed by bacterial sensitivityfilter paper disc method [32]. Bacterial cultures were procured from Pulses Microbiology Laboratory, Department of Plant Breeding and Genetics, Punjab Agricultural University, Ludhiana. The plates were prepared by pouring 15-20 ml of the sterilized nutrient agar media [33] (HiMedia) on sterilized Petri plates (Tarsons). Plates were then allowed to solidify and stored for 2 d to ensure sterility. Freshly inoculated broth cultures (3-4 h old) of the test organism were then spread on the required medium plates. Sterile filter paper discs of 7 mm diameter moistened with test compound solution in DMSO were carefully placed on the medium under aseptic conditions inoculated with the respective bacterial suspension. Sterilized filter paper discs dipped in DMSO, served as control. Plates were incubated at 28±1° and the diameter of growth inhibition zone (mm) was measured after 24 h. The growth of the organism on medium containing the test compound was also compared with the growth on the plates containing microorganism without test compound as control.
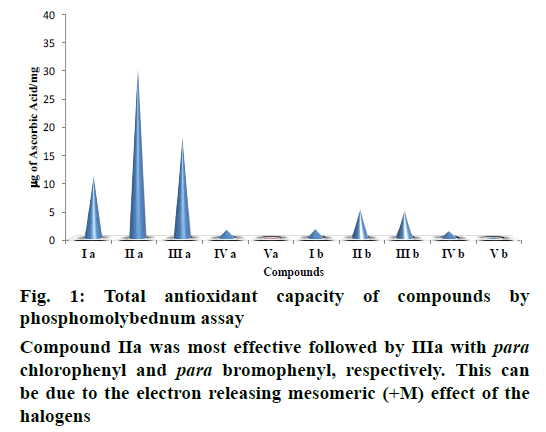
The heterocyclic compounds were assayed for their antioxidant studies as total antioxidants and free radical scavengers. TAC was assessed by reduction of Mo (VI) to Mo (V) as AA equivalents (Figure 1). Compound IIa was most effective followed by IIIa with para chlorophenyl and para bromophenyl, respectively. This can be due to the electron releasing mesomeric (+M) effect of the halogens. 2-Phenyl- 1H-indoles were found to show better antioxidant property than benzimidazole derivatives, which may be attributed to the presence of two electron withdrawing nitrogen atoms in the benzimidazole nucleus. Additional nitrogen atom may be responsible for prevention of acting as an oxidant. In case of indoles, further substitution was found to affect the antioxidant potential. Compound IIa was most effective followed by IIIa with para chlorophenyl and para bromophenyl, respectively. This can be due to the electron releasing mesomeric (+M) effect of the halogens. It was followed by compound Ia with para tolyl substitution due to hyperconjugation effect, which is lesser than mesomeric effect. The compounds with nitrophenyl group were less effective with para isomer (Va) being least effective, which may attributed to the fact that the group at para position is more involved in resonance than the meta position (IVa). In case of benzimidazole derivatives, inductive effect (+I) was very prominent. Compounds IIb and IIIb with propyl and pentyl chains exhibited highest antioxidant capacity with values being comparable among them, which may be due to the fact that inductive effect is distance oriented and is effective only up to γ carbon atom. Compound Ib with ethyl chain has lesser inductive effect and so follows the above two compounds. The other two compounds were less effective due to electron withdrawing substituents (phenoxymethyl and para chlorophenylmethyl).
The RSA was scanned using DPPH as substrate. This assay is based on the stabilization of radical reactant. The presence of electron donating substituent increase the stabilization of radical hence making the reactant a better radical scavenger. The radical scavenging efficiency has been calculated in percent and further IC50 values (mg/ml) are presented in Table 2. Out of 2-phenyl-1H-indoles, compounds with electron donating resonance effect were found to be more effective with the trend Ia>IIa>IIIa. This trend may be due to combination of resonance and inductive effect. The compounds with nitro substitution had meta isomer being slightly more effective than para isomer. In case of benzimidazoles (Ib-Vb), the compounds with aromatic substitution were more effective than aliphatic compounds. Most effective was the compound with phenoxymethyl substitution (IVb). Out of aliphatic substituted compounds, the efficiency decreased with increase in chain length.
| Compound No. | % RSA | IC50 (mg/ml) |
||
|---|---|---|---|---|
| 2.0 mg/ml | 1.0 mg/ml | 0.5 mg/ml | ||
| Ia | 15.0 | 14.0 | 12.5 | 3.35 |
| IIa | 13.5 | 12.0 | 10.5 | 3.88 |
| IIIa | 14.5 | 13.5 | 11.0 | 4.30 |
| IVa | 14.0 | 12.5 | 10.0 | 5.57 |
| Va | 14.0 | 13.0 | 11.5 | 5.57 |
| Ib | 13.0 | 12.0 | 10.5 | 4.53 |
| IIb | 14.0 | 13.0 | 12.0 | 6.26 |
| IIIb | 12.0 | 11.0 | 10.0 | 2.98 |
| IVb | 14.0 | 13.0 | 12.5 | 2.81 |
| Vb | 13.5 | 12.0 | 10.5 | 5.01 |
| Ascorbic acid | 99.8 | 99.0 | 98.9 | 0.012 |
RSA: Radical scavenging activity
Table 2: Radical scavanging activity of compounds
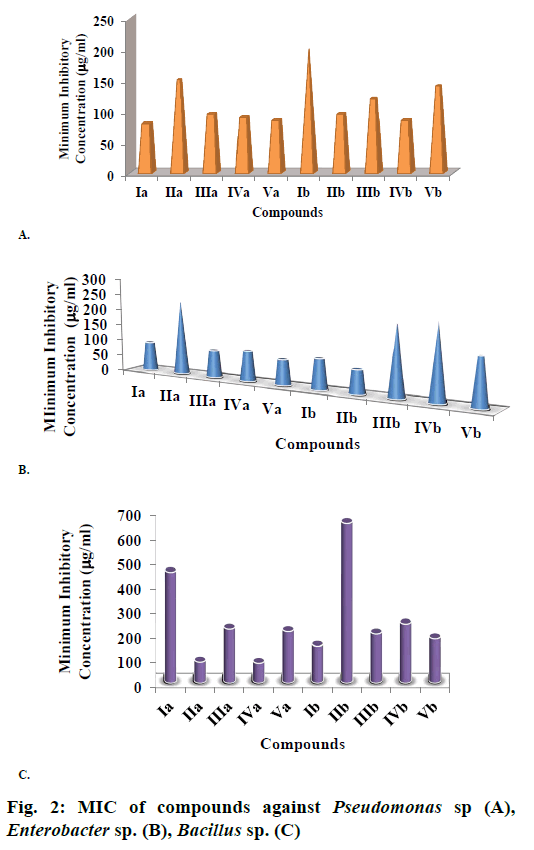
The perusal of data presented in Table 3 revealed that the test compounds were moderately effective against this bacterium. Six out of ten compounds were active at the concentration of 100 μg/ml with inhibition range of 7.5 to 8.5 mm. Most effective compound at all test concentrations was Ia with para methylphenyl substitution on indole ring. At higher concentrations (2000 μg/ml), all the 2-phenyl-1H-indoles had the inhibition zones more than 13.0 mm while those of benzimidazoles is above 11.5 mm. Out of the halogen substituted indoles, the bromo substituted (IIIa) was more effective at all test concentrations. Amongst the nitro substituted indoles, the para isomer was more promising than the meta isomer at the concentration less than 1000 μg/ml while the two were equally efficient at 2000 μg/ml. Out of benzimidazoles, most effective compound was IIb with 2-propyl substitution. The compounds were further studied for minimum inhibitory concentration (MIC). The data revealed that about six compounds had MIC less than 100 μg/ml (Figure 2A). Only indole IIa had MIC of more than 100 ppm. Compounds did not show any specific pattern against this bacterium. None of the compound was as effective as ampicillin at all the tested concentrations.
| Compound No. | Enterobacter sp. | Bacillus sp. | Pseudomonas sp. | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Concentrations (mg/ml) | |||||||||||||||
| 2000 | 1000 | 500 | 250 | 100 | 2000 | 1000 | 500 | 250 | 100 | 2000 | 1000 | 500 | 250 | 100 | |
| Ia | 14.0 | 13.0 | 11.0 | 9.5 | 8.0 | 13.0 | 12.0 | 9.0 | 0 | 0 | 15.0 | 14.0 | 12.5 | 11.5 | 8.5 |
| IIa | 12.0 | 10.0 | 9.5 | 7.5 | 0 | 14.0 | 12.0 | 10.5 | 9.5 | 8.0 | 13.5 | 12.0 | 10.5 | 8.0 | 0 |
| IIIa | 14.0 | 13.0 | 11.5 | 10.0 | 8.5 | 13.0 | 10.5 | 9.5 | 7.5 | 0 | 14.5 | 13.5 | 11.0 | 9.5 | 7.5 |
| IVa | 13.5 | 12.0 | 10.5 | 9.5 | 8.0 | 14.5 | 13.5 | 12.0 | 11.0 | 8.5 | 14.0 | 12.5 | 10.0 | 9.0 | 7.5 |
| Va | 14.5 | 13.5 | 12.0 | 10.5 | 9.0 | 13.0 | 11.5 | 10.0 | 8.5 | 0 | 14.0 | 13.0 | 11.5 | 9.5 | 8.0 |
| Ib | 13.5 | 12.0 | 10.5 | 9.5 | 8.0 | 12.5 | 11.5 | 10.5 | 9.5 | 0 | 13.0 | 12.0 | 10.5 | 9.5 | 0 |
| IIb | 14.5 | 13.5 | 12.5 | 11.0 | 9.5 | 10.0 | 8.5 | 0 | 0 | 0 | 14.0 | 13.0 | 12.0 | 10.5 | 7.5 |
| IIIb | 12.5 | 10.5 | 9.5 | 8.0 | 0 | 12.0 | 11.0 | 10.0 | 8.5 | 0 | 12.0 | 11.0 | 10.0 | 8.5 | 0 |
| IVb | 11.5 | 10.0 | 8.5 | 7.5 | 0 | 10.5 | 9.0 | 8.0 | 7.5 | 0 | 14.0 | 13.0 | 12.5 | 9.0 | 8.0 |
| Vb | 13.5 | 11.5 | 10.0 | 8.5 | 0 | 11.5 | 11.0 | 10.5 | 9.0 | 0 | 13.5 | 12.0 | 10.5 | 9.0 | 0 |
| Ampicillin | 25.0 | 22.0 | 19.0 | 17.5 | 15.0 | 27.0 | 23.5 | 19.5 | 17.0 | 15.5 | 20 | 18 | 16.5 | 15 | 14 |
Table 3: Inhibition zones of compounds against Enterobacter sp.
Screening of compounds against Enterobacter sp. revealed that four 2-phenyl-1H-indoles were active at 100 μg/ml with inhibition diameter in range of 8-9.5 mm (Table 3). The compounds Va and IIb were found to be of comparable efficacy at all the test concentrations. Most effective indole was Va with para nitro substitution on phenyl ring followed by IIIa and Ia. The only meta substituted compound (IVa) was found to be less effective than the other compounds except para chloro substituted (IIa), which was the least effective of indoles at all the test concentrations. Only two benzimidazoles (Ib and IIb) registered higher percent inhibition at all the test concentration amongst this class. The study was continued for analysing the MIC values against this bacterium, which revealed that compound IIb with 2-propyl substitution was most effective with the lowest MIC of 75 μg/ml (Figure 2B).
Bactericidal studies of tested compounds revealed that these were only moderately effective against Bacillus sp. (Table 3). Only two 2-phenyl-1H-indoles were found to be effective at 100 μg/ml. Compounds IVa was most effective at all concentrations followed by IIa. Out of halogenated indoles, chloro derivative was far better than the bromo derivative at all concentrations. The meta nitro group was most promising against this bacterium. The para methyl substitution was least effective amongst the indoles and had no inhibition below 500 μg/ml. None of the benzimidazoles were found to active at 100 μg/ml. The most effective amongst them at all concentration was Ib. The compounds were further tested for MIC values (Figure 2C). None of the compounds were found to be as active as standard ampicillin.
It may be concluded that the indoles and benzimidazoles possessed antioxidant activity. Indoles showed better antioxidant capacity and the effects were enhanced by the presence of electron donating substituents. The compounds with aromatic system with better radical stabilization had better RSA. The compounds were found to show moderate antimicrobial activity. The compounds were more effective on controlling Gramnegative bacteria in comparison to Gram-positive bacterium but none of them was as active as standard ampicillin at all tested concentrations.
Acknowledgements
The authors are thankful to CSIR, New Delhi for financial assistance.
Conflicts of interest
There are no conflicts of interest.
References
- Dolle RE, Nelson KH. Comprehensive survey of combinatorial library synthesis 1998. J Comb Chem 1999;1:235-82.
- Franzen RG. Recent advances in the preparation of heterocycles on solid support: A review of the literature. J Comb Chem 2000;2:195-214.
- Dolle RE. Comprehensive survey of combinatorial library synthesis 2000. J Comb Chem 2001;3:477-517.
- Hanessian S, McNaughton-Smith G, Lombart HG, Lubell WD. Design and synthesis of conformationally constrained amino acids as versatile scaffolds and peptide mimetics. Tetrahedron 1997;53:12789-854.
- Radwanski ER, Last RL. Tryptophan biosynthesis and metabolism: Biochemical and molecular genetics. Plant Cell 1995;7:921-34.
- Gilgun-Sherki Y, Melamed E, Offen D. Oxidative stress induced-neurodegenerative diseases: the need for antioxidants that penetrate the blood brain barrier. Neuropharmacology 2001;40(8):959-75.
- Horakova L, Stolc S. Antioxidant and pharmacodynamic effects of pyridoindole stobadine. Gen Pharmacol 1998;30(5):627-38.
- Valko M, Rhodes C J, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact 2006;60(1):1-40.
- Ratnam DV, Ankola DD, Bhardwaj V, Sahana DK, Kumar MN. Role of antioxidants in prophylaxis and therapy: a pharmaceutical perspective. J Control Release 2006;113(3):189-207.
- Shih MH, Ke FY. Syntheses and evaluation of antioxidant activity of sydnonyl substituted thiazolidinone and thiazoline derivative. Bioorg Med Chem 2004;12(17):4633-43.
- Bendary E, Francis RR, Ali HMG, Sarwat MI, El Hady S. Antioxidant and structure-activity relationships (SARs) of some phenolic and anilines compounds. AOAS 2013;58(2):173-81.
- Singh P, Kaur M, Verma P. Design, synthesis and anticancer activities of hybrids of indole and barbituric acids—Identification of highly promising leads. Bioorg Med Chem Lett 2009;19:3054-58.
- Bertinetti B, Scandiani M, Cabrera G. Analogs of antifungal indoles isolated from Aporpium caryae with activity against sudden-death syndrome of soybean. Am J Plant Sci 2011;2:245-54.
- Kumar A, Kumar D, Akram M, Kaur H. Synthesis and evaluation of some newer indole derivatives as anticonvulsant agents. IJPBA 2011;2(2):744-50.
- Leneva IA, Russell RJ, Boriskin YS, Hay AJ. Characteristics of arbidol-resistant mutants of influenza virus: implications for the mechanism of anti-influenza action of arbidol. Antiviral Res 2009; 81(2):132-40.
- Pojarová M, Kaufmann D, Gastpar R, Nishino T, Reszka P, Bednarski PJ, et al. [(2-Phenylindol- 3-yl)methylene] propanedinitriles inhibit the growth of breast cancer cells by cell cycle arrest in G2/M phase and apoptosis. Bioorg Med Chem 2007;15(23):7368-79.
- Kang MC, Ding Y, Kim EA, Choi YK, de Araujo T, Heo SJ, et al. Indole derivatives isolated from brown alga Sargassum thunbergii inhibit adipogenesis through ampk activation in 3T3-L1 preadipocytes. Mar Drugs 2017;15(4):E119.
- Miller AN, Walsh CS, Cohen J D. Measurement of indole-3-acetic acid in peach fruits (Prunus persica l. batsch cv redhaven) during development. Plant Physiol 1987;81:491-94.
- Shao S, Zhang H, Yuan CM, Zhang Y, Cao MM, Zhang HY, et al. Cytotoxic indole alkaloids from the fruits of Melodinus cochinchinensis. Phytochemistry 2015;116:367-73.
- Desai KG, Desai KR. Green route for the heterocyclization of 2-mercaptobenzimidazole into beta lactam segment derivatives containing -CONH- bridge with benzimidazole. Screening in vitro antimicrobial activity with various microorganisms. Bioorg Med Chem 2006;14:8271-79.
- Liu S, Nelson CA, Xiao L, Lu L, Seth PP, Davis DR, et al. Measuring antiviral activity of benzimidazole molecules that alter IRES RNA structure with an infectious hepatitis C virus chimer expressing renilla luciferase. Antiviral Res 2011;89:54-63.
- Fang B, Zhou CH, Rao XC. Synthesis and biological activities of novel amine-derived bis-azoles as potential anti-bacterial and anti-fungal agents. Eur J Med Chem 2010;45:4388-98.
- del Olmo E, Barboza B, Chiaradia LD, Moreno A, Carrero-Lérida J, González-Pacanowska D, et al. Antimalarial activity of imidazo [2, 1- a] isoindol-5-olderivatives and related compounds. Eur J Med Chem 2011;46:5379-86.
- Ramla MM, Omar MA, El-Khamry AM, El-Diwani HI. Synthesis and antitumor activity of 1-substituted-2-methyl-5-nitrobenzimidazoles. Bioorg Med Chem 2006;14:7324-32.
- Starcevic K, Kralj M, Ester K, Sabol I, Grce M, Pavelic K, et al. Synthesis, anti-viral and anti-tumor activity of 2-substituted-5-amidino-benzimidazoles. Bioorg Med Chem 2007;15:4419-26.
- Kubo K, Oda K, Kaneko T, Satoh H, Nohara A. Synthesis of [[2-(4- fluoroalkoxy-2-pyridyl)methyl]sulfinyl]-1H-benzimidazoles as antiulcer agents. Chem Pharm Bull 1990;38(10):2853-58.
- Achar KC, Hosamani KM, Seetharamareddy HR. In-vivo analgesic and anti-inflammatory activities of newly synthesized benzimidazole derivatives. Eur J Med Chem 2010;45:2048-54.
- Anisimova VA, Spasov AA, Kosolapov VA, Tolpygin IE, Kucheryavenko AF, Sysoeva VA, et al. Synthesis and pharmacological activity of 3-(2,2,2-trichloro-1-hydroxyethyl)imidazo[1,2-a]benzimidazole dihydrochlorides. Pharm Chem J 2009;43:491-94.
- Arora G, Sharma S, Joshi S. Synthesis of substituted 2-phenyl-1H-indoles and their fungicidal activity. Asian J Chem 2017;29(8):1651-54.
- Prieto P, Pineda M, Aguilar M. Spectrophotometric Quantitation of Antioxidant Capacity through the Formation of a Phosphomolybdenum Complex: Specific Application to the Determination of Vitamin E. Anal Biochem 1999;269:337-41.
- Blois M S. Antioxidant determinations by the use of a stable free radical. Nature 1958;181:1199-200.
- Bauer AW, Kirby WM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol 1966;45(4):493-6.
- Chen K, Wang XM, Chen F, Bai J. In vitro antimicrobial and free radical scavenging activities of the total flavonoid in petal and stamen of Crocus sativus. Indian J Pharm Sci 2017;79(3):482-7.