- Corresponding Author:
- Alka Mukne
Department of Pharmacognosy, Bombay College of Pharmacy, Kalina, Santacruz (E), Mumbai-400 098, India
E-mail: a_mukne@yahoo.com
| Date of Submission | 12 November 2013 |
| Date of Revision | 10 April 2014 |
| Date of Acceptance | 21 April 2014 |
| Indian J Pharm Sci 2014;76(3): 256-261 |
Abstract
Tuberculosis is one of the major public health problems faced globally. Resistance of Mycobacterium tuberculosis to antitubercular agents has called for an urgent need to investigate newer drugs to combat tuberculosis. Garlic (Allium sativum) is an edible plant which has generated a lot of curiosity throughout human history as a medicinal plant. Garlic contains sulfur compounds like allicin, ajoene, allylmethyltrisulfide, diallyltrisulfide, diallyldisulphide and others which exhibit various biological properties like antimicrobial, anticancer, antioxidant, immunomodulatory, antiinflammatory, hypoglycemic, and cardiovascular effects. According to various traditional systems of medicine, garlic is one of the established remedies for tuberculosis. The objective of the current study was to investigate in vitro antimycobacterial activity as well as anti-bacterial activity of various extracts rich in specific phytoconstituents from garlic. Preparation of garlic extracts was done based on the chemistry of the constituents and their stability. The estimation of in vitro antimycobacterial activity of different garlic extracts was done using Resazurin microtire plate assay technique whereas activity of garlic oil was evaluated by colony count method. The antibacterial activity of extracts and oil was estimated by zone of inhibition method. Extracts of garlic rich in allicin and ajoene showed appreciable antimycobacterial activity as compared to standard drugs. Garlic oil demonstrated significant antibacterial activity, particularly against methicillin-resistant Staphylococcus aureus.
Keywords
Ajoene, allicin, antimycobacterial, garlic oil, resazurin microtitre assay
Tuberculosis is a disease caused by Mycobacterium tuberculosis, which has been known to the mankind since a very long time. The Greco-Roman and Egyptian civilizations have mentioned of tuberculosis and evidences of tuberculous spondylitis can also be found in ancient Indian scriptures. The first known description being written in Sanskrit sometime between 1500 and 700 BC [1].
The current treatment module for tuberculosis takes a very long time to complete; thus there is a need to improve current treatment by shortening the total duration of treatment and/or by providing for more widely spaced intermittent treatment. Another major drawback of the currently approved drugs is the side effects, especially hepatotoxicity, which in some cases forces an untimely treatment termination. The emergence of multi drug resistant (MDR) strains of M. tuberculosis (defined as resistance against isoniazid and rifampin) is now common in number of patients because of uncontrolled application of antitubercular drugs. At present, a more drug resistant form of tuberculosis, called the extensively drug resistant tuberculosis (XDR-TB) has also been reported. XDR-TB is defined as an infection caused by strains that are resistant to the current front-line drugs (isoniazid and rifampin) in addition to fluoroquinolones and at least one of the three injectable second-line drugs (capreomycin, kanamycin, and amikacin). Because of rise in the MDR and XDR-TB strains, there is this urgent need to discover alternate drug molecules that are effective against all forms of infections [2].
Methicillin-resistant Staphylococcus aureus (MRSA) were first reported in the early 1960’s and are now regarded as a major hospital acquired pathogen worldwide. MRSA represents a challenge for all healthcare institutions. These strains are not only resistant to multiple antibiotics (including penicillins and cephalosporins), but also act as a reservoir for multiple drug resistant genes. Thus, they pose a major challenge in treatment and eradication [3].
Garlic (Allium sativum) is a natural plant, which is being used as a food as well as folk medicine across the globe since centuries. Garlic has been reported as a plant with widespread biological properties including antimicrobial, anticancer, antioxidant, immunomodulatory, antiinflammatory, hypoglycemic, and cardiovascular effects [4]. It is one of the established remedies for treatment of tuberculosis, according to Ayurvedic and Greek systems of medicine [5]. The in vitro activity of garlic against Mycobacterium tuberculosis has been reported way back in 1946 [6].
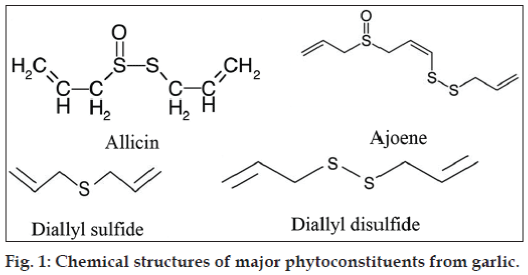
Many of the health benefits associated with garlic consumption have been attributed to the thiosulfinates, the most abundant class of organosulfur compounds, found in freshly chopped or crushed garlic. Oilmacerated garlic product is common as a health food in Europe but rare in the United States and Japan. Oil-macerated heated garlic products contained mainly vinyldithiins, ajoene and small amount of sulfides [7,8]. Condensation of two molecules of allylsulfenic acid produced one molecule allicin, a major sulfurcontaining intermediate, which was isolated and identified as an antibacterial substance [9].
Allicin is a labile compound, easily transformed to a number of stable lipid-soluble allylsulfides such as ajoene [8]. Ajoene (4,5,9-trithiadodeca-1,6,11-triene- 9-oxide) is one of the major natural compounds derived from garlic through the conversion of alliin into allicin, by an alliinase-induced cleavage. Allicin, in presence of a polar molecule such as a lower alcohol or even water, forms ajoene [10]. Ajoene has the advantage of a greater chemical stability than allicin [11].
Garlic oil is obtained by steam distillation and shows good antibacterial properties [12,13]. Steam distillation of garlic bulbs yields garlic oil that consists of diallylsulphides (57% of oil), allyl methyl sulphides (37% of oil) and dimethyl mono to hexasulfides; (6% of oil). The essential oil content of fresh garlic bulbs is between 0.09 to 0.35% [14]. The chemical structures of major phytoconstituents from garlic are presented in fig. 1.
The current study involves preparation and investigation of various garlic extracts and evaluation of their antimycobacterial as well as antibacterial activity. The ajoene-rich extract, allicin-rich extract and garlic oil were investigated for antimycobacterial activity whereas allicin-rich extract and garlic oil were also evaluated for antibacterial activity.
Garlic bulb samples were procured from the local market and identity of the sample was verified from Department of Botany, St. Xavier’s College, Mumbai. Isoniazid, ethambutol and rifampicin were obtained as gift samples from Lupin Ltd., Pune. Mycobacterium smegmatis and Mycobacterium phlei were purchased from NCIM, Pune. Mycobacterium tuberculosis H37Rv was obtained from National Institute for Research in Tuberculosis, Chennai. Staphylococcus aureus, Bacillus subtilis and Escherichia coli were obtained from M. K. Rangnekar Laboratories, Mumbai. MRSA was obtained from Hinduja hospital, Mumbai. All reagents and solvents used were of analytical grade.
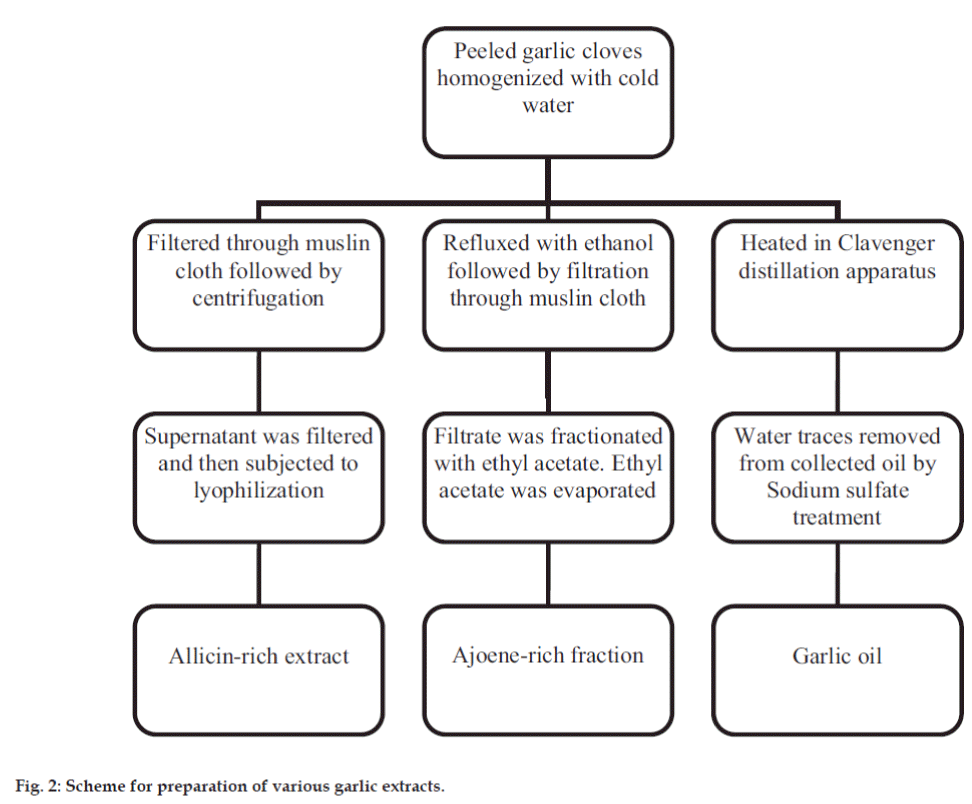
Peeled garlic cloves were weighed and minced in a homogeniser with cold sterile water. The homogenised mixture was treated differently to obtain various extracts. It was filtered through muslin cloth and the filtrate was centrifuged at 3000-4000 rpm. The obtained supernatant was filtered through sterile Whatmann filter paper #1 and lyophilised before storage. This dried extract was the allicin-rich extract. The ajoene-rich extract was prepared by method as per Yoshida et al. 1987 [15]. In a separate extraction process, homogenised mixture was refluxed with ethanol for 2 h. The garlic paste was then filtered using muslin cloth. Ethyl acetate was added to the filtrate and the mixture was shaken in a separating funnel. The upper ethyl acetate layer was collected and ethyl acetate was evaporated at room temperature to obtain a semi-solid extract. This extract was the ajoene-rich fraction. The homogenized mixture was transferred to a round bottom flask to which Clevenger distillation apparatus was attached. Heating was done for 2-3 h until no more oil was extracted out. Garlic oil was collected and stored at 4 °. Traces of water, if any, were removed using sodium sulfate. The preparation of extracts is summarized in fig. 2.
Evaluation of antimycobacterial activity was done using resazurin microtitre plate assay (REMA) technique [16]. Sterile drug solutions and extracts were prepared by dissolving in sterile water and then filtering through 0.22 μm nylon filter. Inoculum containing 3×107 CFU/ml of bacteria was used for the study and was prepared by carrying out 1:10 dilution of bacterial suspension having turbidity comparable to McFarland’s No. 1. Sterile Middlebrook 7H9-S broth and drug solution/extracts were dispensed into the respective wells of the 96-well microtitre plate followed by 100 μl of inoculum. Serial dilutions of the drug solution and extract were done. Necessary sterile and growth controls were also maintained. Plates were incubated at 37° for 3 d for Mycobacterium phlei and Mycobacterium smegmatis whereas for 7 d in case of Mycobacterium tuberculosis. After required incubation period, 30 μl of 0.01% of sterile resazurin sodium solution was added to each well. Colour change of the indicator solution was observed after 12-16 h. Colour change from blue to pink/colourless indicated growth [17]. The minimum inhibitory concentration (MIC) range for standard drugs as well as extracts was determined. The lowest drug concentration that prevented colour change from blue to pink was taken as the upper limit for MIC range; the highest drug concentration that showed change in colour from blue to pink was considered the lower limit. The study was performed in triplicate and the average of 3 readings was considered.
Antimycobacterial activity of garlic oil was evaluated by colony count method [18]. Sterile drug solutions and extracts were prepared by dissolving in sterile water and then filtering through 0.22 μm nylon filter. Inoculum containing 1.5×107 CFU/ml of bacteria was used for the study and was prepared by carrying out 1:10 dilution of bacterial suspension having turbidity comparable to McFarland’s No. 0.5. One hundred microlitres of the suspension of the test microorganism was inoculated and spread using sterile cotton on the surface of Middlebrook 7H11 agar plates to get uniform growth [19]. Paper disks of 6 mm size were cut from Whatman paper No. 1. Under aseptic conditions, empty sterilized discs were impregnated with 30 μl of varying concentrations of rifampicin solution (3 mg/ml) and garlic oil (10, 50 and 80 mg/ml). These disks are placed on the seeded agar plates. Appropriate growth, sterility and solvent controls were also maintained. The plates were left for 30 min at room temperature to allow the diffusion of oil and then incubated at 37° for 14-21 d. The antimycobacterial activity was evaluated by colony counting method. The study was performed in triplicate and the average of 3 readings was considered.
Antibacterial activity of garlic oil and allicin-rich extract was evaluated against Staphylococcus aureus, Escherichia coli, Bacillus subtilis and MRSA by zone of inhibition method using amoxicillin as a standard drug [12,13]. The study was performed in triplicate and the average of 3 readings was considered.
The MIC values of allicin-rich extract as well as ajoene-rich extract in comparison with MIC values of standard drugs are summarized in Table 1. The results of the antimycobacterial activity study of garlic oil in comparison to rifampicin are summarized in the Table 2. As evident from the results of REMA, allicin-rich extract as well as ajoene-rich extract show appreciable antimycobacterial activity as compared to standard drugs. Allicin-rich extract exhibits better antimycobacterial activity than INH and ETH whereas ajoene-rich extract has similar MIC range as compared to INH and ETH. In addition, garlic oil also showed anti-mycobacterial activity but at the concentrations that were significantly higher than standard drug. A concentration of 80 mg/ml of garlic oil almost completely inhibited the growth of M. tuberculosis H37Rv (almost 97% reduction in colony count) as against 0.03 mg/ml of rifampicin which showed similar inhibition of growth of M. tuberculosis H37Rv. Lower concentrations of garlic oil i.e. 50 mg/ ml and 10 mg/ml resulted in reduction in growth inhibition (74% and 48 % decrease in colony count, respectively). Above results indicate that allicin-rich extract, and ajoenerich extract showed comparable/even better in vitro antimycobacterial activity as compared to standard drugs (rifampicin, isoniazid and ethambutol). These compounds hold the distinct potential to be developed as effective mainline drugs in the treatment of tuberculosis or as adjuncts to the existing antitubercular agents.
| Sample | Minimum inhibitory concentration range (µg/ml) | ||
|---|---|---|---|
| M. phlei | M. smegmatis | M. tuberculosis H37Rv | |
| Allicin rich | 15.625-31.25 | 7.812-15.625 | 0.97-1.95 |
| extract | |||
| Ajoene- rich | 39.06-78.12 | 39.06-78.12 | 19.53-39.06 |
| extract | |||
| INH | 31.25-62.5 | 3.906-7.812 | 15.62-31.25 |
| RIF | 0.781-1.562 | 0.781-1.562 | ≤0.1 |
| ETH | 62.5-125 | 125-250 | 31.25-62.5 |
INH: Isoniazid, RIF: rifampicin and ETH: ethambutol
Table 1: Antimycobacterial activity results of allicin-.rich extract and ajoene-rich extract.
| Sample | Concentration | Average | % decrease in |
|---|---|---|---|
| (mg/ml) | colony count | colony count | |
| Garlic oil | 80.00 | 2.33±0.58 | 97.40 |
| Garlic oil | 50.00 | 23.00±6.03 | 46.84 |
| Garlic oil | 10.00 | 47.67±8.62 | 73.61 |
| Rifampicin | 0.03 | 2.00±1.00 | 97.77 |
| Growth control | - | 89.67±3.51 | - |
Average colony count is reported as: count±standard deviation for n=3 observations. % decrease in colony count is in comparison with growth control
Table 2: Antimycobacterial activity results of garlic oil
The zone of inhibition values for garlic extract and garlic oil were compared to amoxicillin. The data is summarized in Table 3. In case of antibacterial activity studies, allicin-rich extract showed comparable diameter of zone of growth inhibition against E. coli, S. aureus and B. subtilis, at concentrations lower than that of the standard drug amoxicillin. Though garlic oil showed inhibition against E. coli, S. aureus and B. subtilis, the concentrations of garlic oil showing inhibition of growth were considerably higher than that of allicinrich extract and amoxicillin.
| Sample | Concentration (mg/ml) | Diameter of Zone of growth inhibition (mm) for | |||
|---|---|---|---|---|---|
| S. aureus | E. coli | B. subtilis | MRSA | ||
| Allicin-rich extract | 1.00 | 32.33±2.51 | 29.67±3.51 | 30.67±2.08 | 31.33±1.53 |
| Allicin-rich extract | 0.50 | 25.67±3.06 | 21.00±1.73 | 24.66±1.53 | 25.00±2.52 |
| Garlic oil | 50.00 | 22.00±2.65 | 18.67±3.05 | 18±3.00 | 16.67±2.31 |
| Garlic oil | 10.00 | 16.00±4.00 | 18.33±2.08 | 13.67±0.58 | 8.67±1.53 |
| Amoxycillin | 3.00 | 33.00±3.61 | 17.00±2.00 | 34.33±2.08 | - |
Average zone of inhibition values for garlic extract, garlic oil and amoxicillin are reported as: zone of inhibition±standard deviation for n=3 observations. MRSA: Methicillin-resistant staphyllococcus aureus
Table 3: Antibacterial activity results of garlic oil
A significant finding of the study has been the growth inhibitory activity of both allicin-rich extract and garlic oil against MRSA. Developing effective chemotherapeutic agents against MRSA is a challenge that drug discovery scientists are grappling with today and allicin and other organosulfur compounds from garlic can throw up promising leads against the dreaded bug.
Acknowledgements
The research work reported was funded by All India Council for Technical Education (AICTE). The authors are also thankful to Lupin Ltd. for providing gift samples of drugs.
References
- De Backer AI, Mortelé KJ, De Keulenaer BL, Parizel PM. Tuberculosis: Epidemiology, manifestations, and the value of medical imaging in diagnosis. JBR-BTR 2006;89:243-50.
- Gupta VK, Shukla C, Bisht GR, Saikia D, Kumar S, Thakur RL. Detection of anti-tuberculosis activity in some folklore plants by radiometric BACTEC assay. LettApplMicrobiol 2004;52:33-40.
- Batabyal B, Kundu GK, Biswas S. Methicillin-Resistant Staphylococcus Aureus: A Brief Review. Int Res J BiolSci 2012;1:65-71.
- Reuter HD, Koch HP, Lawson LD. Therapeutic effects and applications of garlic and its preparations. In: Koch HP, Lawson LD, editors. Garlic: The Science and Therapeutic Application of Allium sativum L. and Related Species. Baltimore, MD, USA: Williams and Wilkins; 1996. p.135-512.
- Hannan A, IkramUllah M, Usman M, Hussain S, Absar M, Javed K. Anti-mycobacterial activity of Garlic (Allium Sativum) against multi-drug resistant and non-multi-drug resistant Mycobacterium Tuberculosis. Pak J Pharm Sci 2011;24:81-5.
- Rao RR, Rao SS, Natarajan S, Venkataraman PR. Inhibition of Mycobacterium tuberculosis by garlic extract. Nature 1946;157:441.
- Block E, Ahmad S. (E, Z)-Ajoene a potent antithrombotic agent from garlic. J Am ChemSoc 1984;106:95-6.
- Block E, Ahmad S, Catalfamo JL, Jain MK, Apitz-Castro R. Antithrombotic organosulfur compounds from garlic: structural, mechanistic and synthetic studies. J Am ChemSoc 1986;108:7045-55.
- Naznin MT, Akagawa M, Okukawa K, Maeda T, Morita N. Characterization of E- and Z-ajoene obtained from different varieties of garlics. Food Chem 2008;106:1113-9.
- Tatarintsev AV, Turgiev AS, inventors, Davidson JB, assignee. Contraceptive method using ajoene. United States Patent US 5,863,954. 1999 Jan 26.
- Hassan HT. Ajoene (natural garlic compound): A new anti-leukaemia agent for AML therapy. Leuk Res 2004;28:667-71.
- EL-Mahmood MA. Efficacy of crude extracts of garlic (Allium sativum Linn.) against nosocomial Escherichia coli, Staphylococcus aureus, Streptococcus pneumoniea and Pseudomonas aeruginosa. J Med Plants Res 2009;3:179-85.
- Ross ZM, O’Gara EA, Hill DJ, Sleightolme HV, Maslin DJ. Antimicrobial properties of garlic oil against human enteric bacteria: Evaluation of methodologies and comparisonswith garlic oil Sulfides and garlic powder. Appl Environ Microbiol 2009;67:475-80.
- Tripathi K. A Review –Garlic, the Spice of Life-(Part –I). Asian J Res Chem 2009;2:8-13.
- Yoshida S, Kasuga S, Hayashi N, Ushiroguchi T, Matsuura H, Nakagawa S. Antifungal Activity of Ajoene Derived from Garlic. Appl Environ Microbiol 1987;53:615-7.
- Martin A, Morcillo N, Lemus D, Montoro E, da Silva Telles MA, Simboli N, et al. Multicenter study of MTT and resazurin assays for testing susceptibility to first-line anti-tuberculosis drugs. Int J Tuberc Lung Dis 2003;9:901-6.
- Karuppusamy S, Rajasekaran KM. High throughput antibacterial screening of plant extracts by resazurin redox with special reference to medicinal plants of western ghats. Glob J Pharmacol 2009;3:63-8.
- Dibua UE, Odo GE, Udengwu S, Esimone CO. Cytotoxicity and antitubercular activity of Allium sativum and Lantana camara against mycobacterial isolates from people living with HIV/AIDS. Internet J Infect Dis 2010;8. Available from: http://ispub.com/IJID/8/1/12922 [Last accessed on 2013 Sept 26].
- Moghaddam AM, Shayegh J, Mikaili P, Sharaf JD. Antimicrobial activity of essential oil extract of Ocimumbasilicum L. leaves on a variety of pathogenic bacteria. J Med Plants Res 2011;5:3453-6.