- *Corresponding Author:
- Prachi Bansa
Department of Chemistry, Dayalbagh Educational Institute, Dayalbagh, Agra-282 110
E‑mail: prachichemdraw@gmail.com
| Date of Submission | 02 April 2015 |
| Date of Revision | 22 November 2014 |
| Date of Acceptance | 04 January 2014 |
| Indian J Pharm Sci 2015;77(2):208-217 |
Abstract
The present communication reports the comparison of in vivo antioxidant, antimelanoma and antimutagenic activities of Eruca sativa seed oil and its bio principles (allyl isothiocyanate, phenylethyl isothiocyanate and sulphoraphane) against B16F10 melanoma cells induced in C57BL/6 mice model. Among the various treatments considered for the study, isothiocyanates combination (allyl isothiocyanate, phenylethyl isothiocyanate and sulphoraphane; 1:1:1; 10 µM) exhibited optimum antioxidant activity, 51.95±1.14 µM glutathione per mg protein compared to seed oil 25.91±1.26 µM. Lipid peroxidation value was 9.97±1.72 µM malondialdehyde per mg wet weight for isothiocyanates combination against seed oil, 28.45±1.87 µM and rendered significant protection against oxidative stress induced by melanoma in liver tissue. Isothiocyanates combination significantly suppressed various parameters, such as tumor growth, isothiocyanates combination by 36.36% while the seed oil by 15.23%; tumor weight, isothiocyanates combination by 45.9% and seed oil by 19.6%; tumor volume, isothiocyanates combination by 41.7% while the seed oil by 32.3%, measured for antimelanoma activity at a concentration of 10 µM. Isothiocyanates combination has been found to be more cytotoxic bioagent against B16F10 melanoma cells induced in C57BL/6 mice compared to naturally occurring Eruca sativa seed oil.
Keywords
Antimelanoma, In vivo, oxidative stress, B16F10 melanoma cell line, C57BL/6 mouse
The proven efficacies of phytochemicals have created a revolutionary interest and awareness among the scientific community [1]. The secondary metabolites of the plants serve as a defence system against various infestations [2]. Thus, unlike compounds synthesized in the laboratory, secondary metabolites from plants are virtually guaranteed to have biological activity [3]. Plant-based chemopreventive research has been associated with the issue that whether whole plant extract or bioactive principle is more potent [4]. Several biologically active compounds in a plant work together (chemical partnership) to produce greater effect than that of a single chemical and also delay the development of resistance while isolated bioactive principles add to our therapeutic armamentarium. Search for plant-derived chemicals with pharmacological efficacies either in crude state or isolated state becomes an important area of sustained research [5].
The plant Eruca sativa ( cruciferae) originated in the Mediterranean region, is widely distributed all over the world, particularly in India [6,7], where the seeds are used for the production of a traditional spicy (taramira) oil. The role of Eruca sativa seed oil for antioxidant, antimicrobial activity [8] and inhibition of proliferation of melanoma [9] has been reported by us. In continuation, the present communication reports the comparison of In vivo antioxidant (glutathione and lipid peroxidation assay) and antimelanoma activity (body weight, tumor weight, tumor delay time, chromosomal assay, micronucleus assay) of its isolated bioactive principles (allyl isothiocyanate, phenylethyl isothiocyanate and sulphoraphane either given alone or in combination) and Eruca sativa seed oil against B16F10 melanoma cells induced in C57BL/6 mice.
Materials and Methods
C57BL/6 strain mice 7-8 weeks old, weighing 25±5 g were maintained in a ventilated animal house of Department of Research, Jawaharlal Nehru Cancer Hospital and Research Centre, Bhopal (India). All the mice were kept at controlled environmental condition (22±2°, 60±5% humidity) with 12 h light/dark cycle. They were provided with standard pallet diet and water ad libitum. B16F10 mouse melanoma cells were obtained from the National Centre for Cell Sciences, Pune. These cells were maintained in Dulbecco’s Modified Essential Medium supplemented with antibiotics, L-glutamine and fetal calf serum. All the chemicals and reagents used were of AR grade. Protocols of the animal experiments were approved by the institutional animal ethics committee constituted and approved by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Govt. of India.
Animal experimental design, treatment and monitoring
To investigate the effects of test sample against reference anticancer drug (doxorubicin), mice were randomized and divided into 15 groups of 6 animals each. Eruca sativa seed oil in DMSO, allyl isothiocyanate (AITC), phenylethyl isothiocyanate (PEITC), sulphoraphane (SUL) either alone or in combination were the test compounds used with corn oil as vehicle, saline was used as control. Volume equivalent to 10 μM of test compound has been used. Two doses, 10 and 30 μM of isothiocyanates have been considered for the present study on the basis of earlier reported study [10]. Group I was kept as normal control and Groups 2-15 were injected with B16F10 mice melanoma cells (4×105) subcutaneously in the dorsal flank on day zero. Mice were injected intraperitoneally with saline (group II), corn oil (group III), 2% dimethyl sulphoxide (group IV), 1 mg/kg of doxorubicin in 4 doses on the day 1, day 5, day 9 and day 13 of treatment (group V), allyl isothiocyanate 10 μM (group VI), allyl isothiocyanate 30 μM (group VII), phenylethyl isothiocyanate 10 μM (group VIII), phenylethyl isothiocyanate 30 μM (group IX), sulphoraphane 10 μM (group X), sulphoraphane 30 μM (group XI), AITC, PEITC and SUL combination 10 μM (group XII), AITC, PEITC and SUL combination 30 μM (group XIII), seed oil 10 μM (group XIV), seed oil 30 μM (group XV). The incorporation of (4×105) viable cells (highly proliferative and metastatic melanoma cells) in the dermis is likely to complete one mitotic cycle with in 24 h and develop significant tumor within 3-4 d. Therefore, this regimen was considered for screening of anticancer activity. Doxorubicin induces apoptosis by induction of DNA fragmentation and cell shrinkage in tumor cells and has been in use for more than 30 y in treating a variety of malignancies [11-13], therefore, it has been considered as a reference drug for the present study. B16F10 mice melanoma cells have been injected in subcutaneous layer of skin at the back of mice and single tumor was developed there only. Local tumor growth was determined by measuring blindly diameter with callipers every other day, starting with the day when tumor became palpable. B16F10 melanoma cells injected subcutaneously into mice when grew to average size of tumor volume 2000 mm3 in the control group. Tumor volume (mm3) was estimated by the formula, 4/3×π×(1/2×smaller diameter)2×(1/2×larger diameter) [14]. Tumor growth delay was determined according to the method of Corbett et al. 1997 [15] and was calculated as follows, tumor growth delay=T−C, where T represents median time (in days) required for the treatment group tumors to reach a volume of 100 mm3 and C represents median time (in days) required for the control group tumors to reach the same size. Body weights of all animals were measured every alternative day during treatment period to detect life threatening toxicity by test samples and reference drug. Mice in all groups were observed daily for survival and sacrificed on day 26 after the experimental schedule. The tumors were dissected, weighed and stored at −80° until analysis was completed. To examine the histopathology, tumors from each group of animals were removed and fixed in 10% formalin solution for 24 h. Tissues were then embedded into paraffin. A section (4 μm) was stained with haematoxylin and eosin and examined under a microscope [16].
Assay for reduced glutathione
The liver tissue was dissected, weighed and homogenized in saline (1.15% potassium chloride) to give a 10% homogenate (w/v). The crude homogenate was centrifuged at 2000 rpm for 15 min and supernatant was collected. Phosphate ethylene diamine tetraacetate buffer (0.9 ml) and 5, 5’-dithiobis-( 2-nitrobenzoic acid) solution (0.050 ml) was added to supernatant (0.050 ml) making the solution 1.0 ml. The reaction mixture was incubated at room temperature for 20 min and the optical density was measured at 410 nm. The GSH levels were monitored by the reduction of 5,5’-dithio-bis-2-nitrobenzoic acid (DTNB) to 5-thio-2-nitrobenzoate (TNB) [17,18].
Assay for lipid peroxidation
After euthanasia of mouse, the liver tissue was homogenised in 1.15% potassium chloride solution by homogeniser (1 g of tissue in 9 ml of 1.15% potassium chloride solution). Sodium dodecyl sulphate (8.1%) was added to 0.2 ml of sample in test tube and pH was adjusted to 3.5 with 5 N sodium hydroxide. To this, 1.5 ml of 0.8% aqueous solution of thiobarbituric acid was added and mixture made up of 4 ml with distilled water and heated at 95° for 60 min. After cooling under tap water, 1 ml of distilled water and 5 ml of mixture of n-butanol and pyridine (15:1) were added and shaken vigorously [19]. The solution was centrifuged at 3900 rpm for 10 min. Upper organic layer was removed and absorbance was measured at 532 nm using UV/Vis spectrophotometer.
Chromosomal aberrations test
Cytogenetic damage in the bone marrow cells was studied by chromosomal aberration analysis at the end of experiment. All the animals were injected 0.025% colchicine intraperitoneally and sacrificed 2 h later to arrest the cells in metaphase by cervical dislocation. The femurs were dissected and cleaned to remove adherent muscles. The bone marrow was flushed into centrifuge tubes through repeated aspirations with 2 ml medium using fine needle. After sampling of bone marrow from femurs of the animals, the cells were centrifuged at 1000 rpm for 10 min. The supernatant was discarded completely and pellet was suspended in hypotonic solution (5 ml, 0.56% potassium chloride). These tubes were kept in water bath at 37° for 20 min. After incubation, cells were recentrifuged at 1400 rpm for 5 min, the supernatant was discarded and the pellet was resuspended in freshly prepared chilled Cornoy‘s fixative solution (2 ml, methanol:glacial acetic acid mixture in 3:1 ratio) and again centrifuged at 1400 rpm for 10 min [20]. The pellet was resuspended in fresh fixative and the process was repeated 2-3 times. Cells kept for overnight fixation (4°) were centrifuged for 1400 rpm for 10 min again and the pellet was redispersed in fresh fixative (0.7-1 ml) depending on the amount of pellet. The cells were agitated and mixed thoroughly using Pasteur pipette and dropped on to the precleaned chilled slides from a distance of 30-40 cm. The slides were left in air to dry. The slides were dipped in 5% Giemsa solution for 10 min and rinsed in double distill water (DDW) and air-dried. A total of 100 well spread metaphase plates/animal were analyzed for different types of chromosomal damage including breaks, fragments, exchanges and multiple aberrations including pulverizations at a magnification of (100X×10X) for all treated groups. The slides prepared were used for the counting of Mitotic Index. Metaphase plates were prepared by the air drying method [21].
Assay for micronucleus test
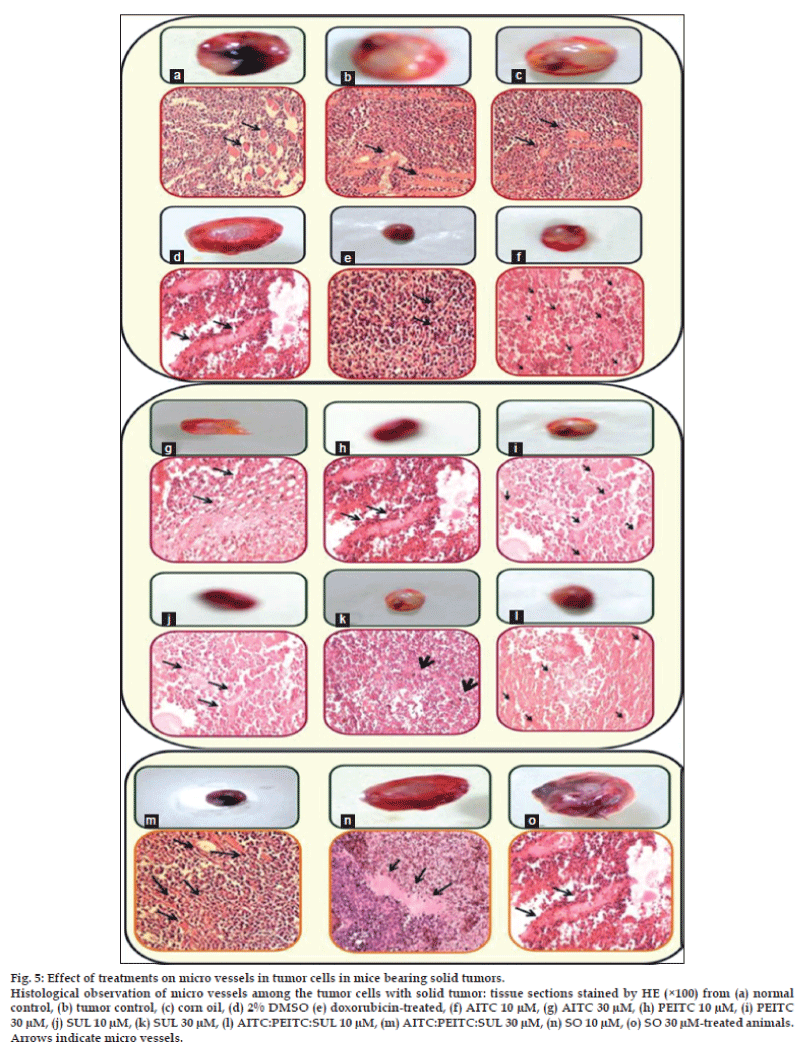
The bone marrow was flushed out using minimum essential medium, centrifuged and the pellet was resuspended in few drops of fetal bovine serum. Smears were prepared on preclean glass slides [22], stained with May-Grawnwald (5 min) and followed by Giemsa stain (5% giemsa solution for 10 min), rinsed in DDW and air-dried [23]. The number of polychromatic erythrocytes (PCEs) and normochromatic erythrocytes (NCEs) and the frequency of micronucleated PCEs and NCEs were recorded at a magnification of (100X×10X) for all treated groups. Tumor micro vessels and density evaluation has been carried out using inbuilt MOTIC software.
Statistical analysis
All experimental data were given as mean±SD. Statistical analysis was carried out using the one-way Analysis of Variance (ANOVA). Post Dunnett test was applied between control, reference drug and test samples using Graph Pad Prism software. Probability values were found to be less than 0.05.
Results and Discussion
In vivo antioxidant, antimelanoma and antimutagenic activities of Eruca sativa seed oil and its bioactive principles, allyl isothiocyanate, phenylethyl isothiocyanate and sulphoraphane either given alone or in combination given at the doses of 10 and 30 μM against B16F10 melanoma cells induced in C57BL/6 mice strain. However, no marked increase in all the parameters considered for the study was observed with increase in dose from 10 μM onwards. Therefore, 10 μM dose was considered optimum. No further increase in the dose was considered because of toxicity issues of Isothiocyanates.
In order to determine the content of bio-active isothiocyanates in seed oil, we exploited the volatility of isothiocyanates present in seed oil. The coupled Head Space sampling enriched by solid phase micro extraction (HS/SPME) was used. Among the several fibres tested, a polar polyacrylate fibre showed to afford the best sensitivity and linearity in GC-MS analysis. Identification of isothiocyanates was accomplished by comparison with NIST 05 MS-library (f-fit>700; r-fit>650) and was confirmed using authentic standards in all cases. Good quality chromatograms were obtained and reported in our earlier publication [8]. Various identifying parameters of isothiocyanates in Eruca sativa seed oil have been presented here also as follows (Table 1).
| Retention time (min) | Compound | Mol.Wt. | Characteristic ionsa (m/z) | Amount (µg/g) |
|---|---|---|---|---|
| 6.237 | Allyl isothiocyanate | 99.15 | 41, 72, 99 (bp) | 40.3 |
| 22.450 | 2-Phenylethyl isothiocyanate | 163.24 | 91 (bp), 105, 163 | 158.5 |
| 28.373 | Sulforaphane | 177.29 | 55, 72 (bp), 85, 115, 160 | 743.1 |
aThe base peak (100%) is indicated as bp, while the molecular ion (M+•), when visible in the spectrum, is given in the table
Table 1: Hs/spme/gc–ms analysis of volatile isothiocyanates in eruca sativa seed oil
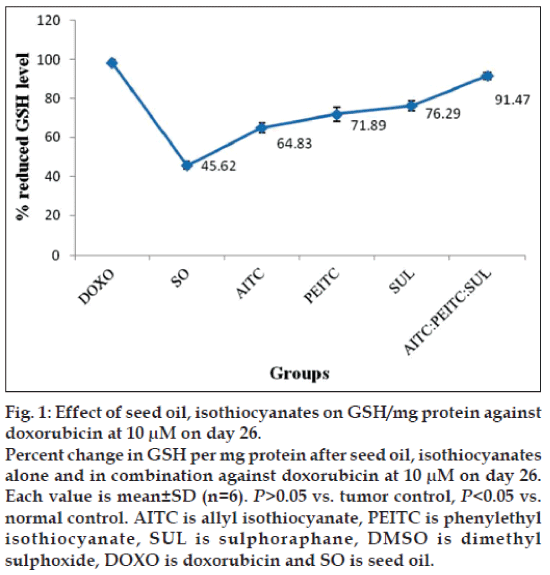
The down regulation level of reduced glutathione in liver of experimental mice was investigated to determine the antioxidative effect of test groups against the oxidative stress induced by melanoma cells. After induction of B16F10 mice melanoma cells, the weights and the level of GSH of liver tissues of experimental mice were recorded. The liver weight of isothiocyanates treated mice (1.20 g) was found almost near to the normal control group mice (1.26 g) compared to the tumor control mice (1.64 g). Decreased concentration of reduced GSH, 15.75 μM per mg protein in tumor control group has been observed compared to normal control 59.82 μM. The reduced GSH level of test samples at two concentrations were as follows: at 10 μM concentration AITC -36.82 μM; PEITC -40.83 μM; SUL –43.33 μM; AITC:PEITC:SUL -51.95 μM; SO –25.91 μM; while at 30 μM concentration AITC –42.57 μM; PEITC –44.12 μM; SUL –47.95 μM; AITC:PEITC:SUL –52.26 μM; SO –26.70 μM against the reference drug doxorubicin 56.79 μM. The increase in the concentration from 10-30 μM of isothiocyanates treatment alone or in combination did not result any noticeable change in antioxidant activity. Therefore, 10 μM has been considered optimum dose.
The percentage change on level of GSH per mg protein as a function of seed oil and isothiocyanates alone or in combination against doxorubicin at 10 μM on d 26 has been presented in fig. 1.
Although level of reduced GSH is higher in case of individual isothiocyanates, interestingly their combination at 10 μM concentration exhibited a further increase compared to seed oil. Subcutaneous induction of B16F10 melanoma showed a significant lowering of reduced glutathione in liver (characteristic of antioxidants) compared to normal and reduced the scavenging of reactive oxygen species. Among the groups studied, optimum value of reduced GSH per mg protein is found to be in the order, doxorubicin>isothiocyanates combination> isothiocyanates given alone>seed oil. Increased concentration of MDA (63.3 μM) per mg wet weight has also been observed compared to normal control 65±1.83 μM. Concentration of MDA/mg were as follows: at 10 μM concentration AITC –23.42±1.98 μM; PEITC –22.9±1.88 μM; SUL –23.5±1.85 μM; AITC:PEITC:SUL –9.77±1.72 μM; SO -28.45±1.65 μM; at 30 μM concentration AITC –19.87±1.79 μM; PEITC –19.95±1.94 μM; SUL –17.7±1.81 μM; AITC:PEITC:SUL -8.77±1.96 μM; SO –24.36±1.69 μM. Among the groups studied, optimum value of MDA per mg wet weight is found to be in the order: doxorubicin>isothiocyanates combination>isothiocyanates given alone>seed oil. Based on our observation, 10 μM of AITC:PEITC:SUL exhibited optimum antioxidant activity and rendered significant protection against oxidative stress induced by melanoma in liver tissues.
Injection of B16F10 melanoma cells subcutaneously into mice was followed by 26 d observation, monitoring the mean body weight, tumor weight and tumor growth delay of all the experimental groups (Table 2). The body weights of the control and treated mice were determined periodically to assess non-specific toxicity of isothiocyanates. The average body weights of the control and isothiocyanate alone or in combination treated mice did not differ significantly by one-way ANOVA suggesting that isothiocyanates administration did not cause weight loss. The mice in isothiocyanates-treated group appeared healthy and did not show any other sign of non-specific toxicity, such as food and water withdrawal and impaired movement. Concentration of 10 μM in each case was found optimum as no significant change in body weight with the increase in concentration to 30 μM. Average tumor weight in the tumor control group and test samples treated group were depicted, and can be ordered as: doxorubicin<isothiocyanates combination<isothiocyanates given alone<seed oil<Tumor control. Similar trend was also observed when tumor delay time in all the experimental groups has been studied. Overall, significant reduction in tumor weight and tumor delay time was found in isothiocyanates combination treated mice.
| Groups | Body weight±SD (g) | Tumor weight±SD (g) | Tumor delay time (days) |
|---|---|---|---|
| Normal control | 31.4±1.27 | - | - |
| Tumor control | 29.0±1.20 | 1.78±1.35 | 0 |
| Corn oil | 30.8±1.16 | 1.63±1.27 | 0 |
| 2% DMSO | 32.3±1.17 | 1.65±1.15 | 0 |
| DOXO | 26.8±1.25 | 1.12±1.03 | 6 |
| PEITC (10 µM) | 27.9±1.05 | 1.34±1.15 | 3 |
| PEITC (30 µM) | 27.4±1.22 | 1.30±1.26 | 4 |
| AITC (10 µM) | 31.1±1.15 | 1.31±1.12 | 3 |
| AITC (30 µM) | 28.3±1.22 | 1.27±1.23 | 4 |
| SUL (10 µM) | 27.5±1.25 | 1.36±1.18 | 3 |
| SUL (30 µM) | 28.3±1.13 | 1.31±1.28 | 4 |
| AITC: PEITC: | 29.4±1.07 | 1.22±1.06 | 5 |
| SUL (10 µM) | |||
| AITC: PEITC: | 28.4±1.20 | 1.20±1.09 | 5 |
| SUL (30 µM) | |||
| SO (10 µM) | 28.6±1.22 | 1. 52±1.38 | 1 |
| SO (30 µM) | 28.0±1.01 | 1. 47±1.23 | 2 |
Each value is mean±SD (n=6). P>0.05 vs. tumor control, P<0.05 vs. normal control. NC is normal control, TC is tumor control, AITC is allyl isothiocyanate, PEITC is phenylethyl isothiocyanate, SUL is sulphoraphane, DMSO is dimethyl sulphoxide, DOXO is doxorubicin and SO is seed oil
Table 2: Effect of isothiocyanates, seed oil and doxorubicin on normal and melanoma tumor bearing mice day 26
The above findings support the view that multiple active phytochemicals result into synergism in such a way that outcome may not be additive but multiplicative [24]. The uses of two or more bioagents have been recognized recently, providing enhanced therapeutic bioefficacy [25,26].
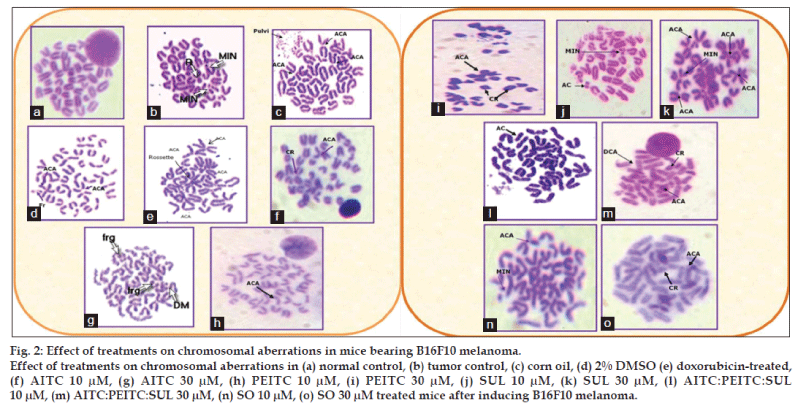
The effect of the test samples on percent chromosomal aberration was measured in terms of chromatid breaks, centric rings, acrocentric association, acentric fragments, intracalary deletion, minutes and total abnormal metaphases (fig. 2). Percent of aberrant metaphase in various groups were in the range as follows: cancer control (80%), doxorubicin-treated (72%), AITC (20-34%), PEITC (36-48%), SUL (40-60%), AITC:PEITC:SUL (12-20%), seed oil (46-52%) at 10 μM-30 μM concentrations, providing the following order: isothiocyanates combination<isothiocyanates alone<seed oil<doxorubicin<cancer control group (Table 3). Isothiocyanates combination is found to exhibit maximum reduction in all the chromosomal aberrations studied in bone marrow cells compared to tumor control and standard doxorubicin.
| Group | CB | CR | FR | ACA | ICD | AC | MIN |
|---|---|---|---|---|---|---|---|
| NC | 12.5±1.14 | 0±0.0 | 25.0±1.13 | 12.5±1.02 | 0.0±0.0 | 12.5±1.05 | 0.0±0.0 |
| TC | 12.6±1.85 | 8.14±1.85 | 17.1±2.09 | 38.5±2.14 | 11.8±1.98 | 9.62±1.65 | 2.96±1.74 |
| CO | 17.8±2.54 | 10.2±1.58 | 9.55±1.74 | 27.4±1.65 | 8.28±1.62 | 11.5±1.06 | 3.18±1.32 |
| 2% DMSO | 21.9±1.78 | 7.74±1.98 | 9.03±2.41 | 29.0±2.10 | 9.67±2.14 | 9.67±1.65 | 5.64±1.57 |
| DOXO | 25.1±1.85 | 7.44±1.65 | 8.37±1.84 | 31.6±1.78 | 9.30±0.98 | 10.2±1.56 | 5.11±1.64 |
| AITC (10 μM) | 12.6±0.94 | 5.26±0.68 | 7.36±0.84 | 16.8±0.98 | 6.31±0.75 | 10.5±0.72 | 5.26±0.86 |
| AITC (30 μM) | 14.1±1.28 | 5.46±1.21 | 7.03±1.12 | 19.5±1.06 | 8.59±1.15 | 10.9±1.25 | 4.68±1.09 |
| PEITC (10 μM) | 14.1±0.68 | 5.05±0.92 | 7.07±0.84 | 18.2±0.65 | 6.06±0.94 | 11.1±0.81 | 5.05±0.75 |
| PEITC (30 μM) | 15.3±1.75 | 5.64±1.56 | 8.06±1.34 | 19.4±1.32 | 8.87±1.45 | 12.1±1.64 | 4.83±1.68 |
| SUL (10 μM) | 14.7±1.78 | 3.92±1.58 | 7.84±1.46 | 19.6±1.34 | 7.84±1.52 | 12.7±1.64 | 4.9±1.74 |
| SUL (30 μM) | 15.3±2.45 | 5.83±2.34 | 8.02±2.25 | 18.2±2.12 | 8.75±2.32 | 13.1±2.41 | 4.37±2.48 |
| AITC:PEITC:SUL (10 μM) | 7.69±1.78 | 4.32±1.95 | 6.69±2.1 | 15.4±2.15 | 5.98±2.08 | 5.76±1.80 | 3.84±1.71 |
| AITC:PEITC:SUL (30 μM) | 16.7±1.74 | 5.55±2.14 | 11.1±2.45 | 19.4±3.21 | 5.55±2.35 | 11.1±2.08 | 5.55±1.87 |
| SO (10 μM) | 18.2±2.34 | 6.81±1.08 | 13.6±0.75 | 19.7±0.85 | 9.09±1.32 | 9.09±1.21 | 6.06±1.54 |
| SO (30 μM) | 20.0±1.24 | 6.66±1.48 | 16.7±1.09 | 23.3±1.36 | 11.7±0.95 | 11.7±1.75 | 6.66±2.48 |
Each value is mean±SD (n=6). P>0.05 vs. tumor control, P<0.05 vs. normal control. NC is normal control, TC is tumor control, AITC is allyl isothiocyanate, PEITC is phenylethyl isothiocyanate, SUL is sulphoraphane, DMSO is dimethyl sulphoxide, DOXO is doxorubicin and SO is seed oil. CB is chromatid breaks, CR is chromatid rings, FR is fragments, ACA is acrocentric association, ICD is intercalary deletion, AC is acentric association
Table 3: Effect of isothiocyanates, seed oil and doxorubicin on the bone marrow of c57bl/6 mice after induction of melanoma
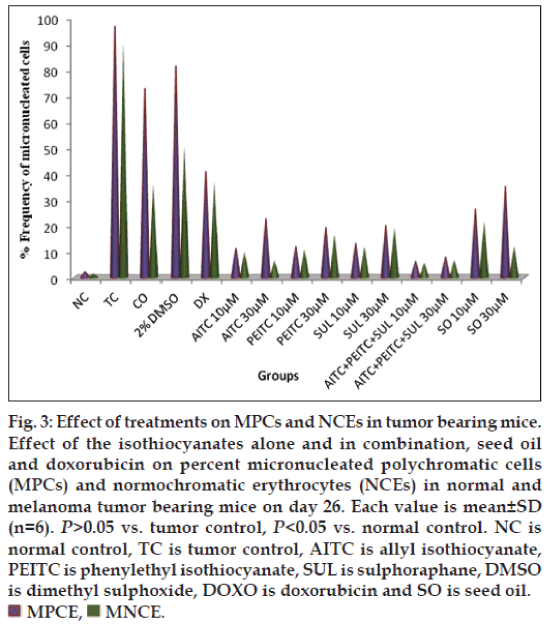
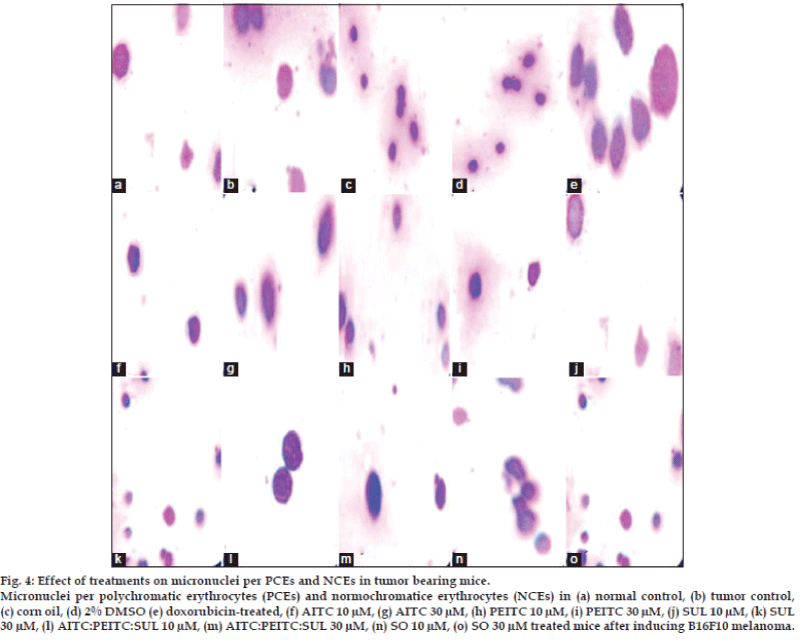
The effect of various treatments on melanoma-induced mice was determined in terms of micronucleated polychromatic erythrocytes (MPCEs) and normochromatic erythrocytes (NCEs) per 1000 cells. Percent of MPCE and NCE in various groups were in the range as follows: cancerous control (90-97%, doxorubicin-treated (37-40%), AITC (6-22%), PEITC (10-19%), SUL (13-20%), seed oil (12-34%), AITC:PEITC:SUL (5-8%) at 10-30 μM concentrations (fig. 3). Isothiocyanates combination treated mice were significantly (P>0.05) reduced the micronuclei in PCEs and NCEs comparable with tumor control and doxorubicin treated group. The Isothiocyanates combination reduced the frequency of micronuclei per polychromatic (PCEs) and normochromatic erythrocytes (NCEs) compared to standard drug and tumor control group (fig. 4).
Fig. 3: Effect of treatments on MPCs and NCEs in tumor bearing mice. Effect of the isothiocyanates alone and in combination, seed oil and doxorubicin on percent micronucleated polychromatic cells (MPCs) and normochromatic erythrocytes (NCEs) in normal and melanoma tumor bearing mice on day 26. Each value is mean±SD (n=6). P>0.05 vs. tumor control, P<0.05 vs. normal control. NC is normal control, TC is tumor control, AITC is allyl isothiocyanate, PEITC is phenylethyl isothiocyanate, SUL is sulphoraphane, DMSO is dimethyl sulphoxide, DOXO is doxorubicin and SO is seed oil.
The observation finds support from Musk and Johnson [27] who have demonstrated the role of Isothiocyanates as significant inducers of chromosomal damage protecting laboratory animals from the induction of tumors and recommended the presence of isothiocyanates in the human diet.
Accumulating evidences [28-31] demonstrate that tumor growth and lethality are dependent on angiogenesis. An observation of histological slides (fig. 5) exhibits the decrease in tumor growth in mice by the isothiocyanates treatment which may be attributed to decreased host angiogenesis. A marked and dense microvasculature was observed in the control tumors. Tumors treated with isothiocyanates combination (31.23±6.45%) and doxorubicin (27.6±6.67%) had significantly fewer microvessels compared with the AITC (45.27±8.68%), PEITC (47.8±8.34%), SUL (42.35±8.42%) and tumor control (62.6±8.7%). Angiogenesis inhibition observed with isothiocyanates combination treatment is indicative of drug accumulation in the tumor and decreased tumor microvessel density which is further associated to the suppression of angiogenic vascularization, inhibited tumor cell proliferation and increased tumor cell apoptosis.
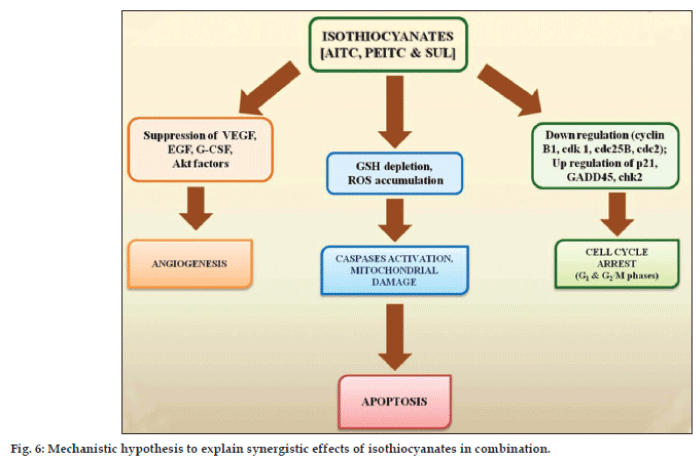
The mechanism of anticarcinogenic activity of isothiocyanates has not yet been fully elucidated. Isothiocyanates are reported to reduce activation of carcinogens and increase their detoxification finally exhibiting anticarcinogenic activity [32,33]. The mechanism of the enhancement in anticancer activity of isothiocyanates combination is not easy to predict owing to their complex pharmacological actions. However, the intrinsic properties of isothiocyanates in synergism play important role towards enhancement in target bioefficacy. Based on experimental findings and relevant available literature, a hypothesis is synthesized to explain observed synergistic action of isothiocyanates (fig. 6). Isothiocyanates act through apoptosis and exhibit anticancer activity by multiple pathways including oxidative stress [34,35], inhibition of cell cycle progression [36,37], angiogenesis [38] and MAPK signalling [39-41]. Isothiocyanates effectively disables the glutathione (GSH) antioxidant system and causes ROS accumulation preferentially in the transformed cells due to their active ROS output. Excessive ROS cause oxidative mitochondrial damage, cytochrome c release, inactivation of redox-sensitive molecules (GXP), and massive cell death. Isothiocyanates may induce cell cycle arrest (G1 and G2/M phases) in different phases in a cell line dependent manner. Inhibition of angiogenesis employed by ITCs to prevent cancer involves inactivation of Akt, suppression of VEGF and EGF expression, and G-CSF secretion. Isothiocyanates may also induce apoptosis through a caspase-3-dependent MAPK mechanism based on JNK and caspase-3-dependent mechanism. It induces rapid and transient induction of caspase-3 activity and stimulates proteolytic cleavage of poly-(ADP-ribose) polymerase, resulting into caspase activation and precedes DNA fragmentation.
The present piece of In vivo experiments highlight the effectiveness of isothiocyanates-combination among all the treatment studied and is found capable for reducing melanoma against reference drug doxorubicin. Overall, the results nicely complement each other depicting the safe and health promoting value of dietary consumption of isothiocyanates containing Eruca sativa seed oil. Our results also indicate that the isothiocyanates combination at 10 μM is a better cytotoxic bioagent against B16F10 melanoma cells induced in C57BL/6 mice compared to naturally occurring seed oil. It has enough potential for clinical applications and lends support to its use in traditional medicine. It is worth mentioning that neither life threatening toxicity nor a loss of body weight during the isothiocyanates combination treatment was observed compared to normal control. The finding may be considered significant in comparison with side effects (loss of body weight) normally observed in adjuvant therapy, highlighting the ability of isothiocyanates combination to inhibit melanoma growth with the view to develop new antitumor substances with low toxic potential.
Acknowledgements
The authors gratefully acknowledge Prof. V. G. Das, Director, Dayalbagh Educational Institute, Dayalbagh, Agra, for providing necessary research facilities. PB acknowledges University Grants Commission (New Delhi), for Research Fellowship under Basic Scientific Research (UGC-BSR) sanctioned to the Department.
References
- El Sohaimy SA. Functional Foods and Nutraceuticals-Modern Approach to Food Science. World ApplSci J 2012;20:691-708.
- War AR, Paulraj MG, Ahmad T, Buhroo AA, Hussain B,Ignacimuthu S, et al. Mechanisms of Plant Defense against Insect Herbivores. Plant Signal Behav 2012;7:1306-20.
- Duke SO. Natural pesticides from plants. In: J Janick and JE Simon, editor. Advances in new crops, Portland, OR: Timber Press; 1990. p. 511-7.
- Liu RH. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am J ClinNutr 2003;78:517-20S.
- Lin JH, Lu AY. Role of Pharmacokinetics and Metabolism in Drug Discovery and Development. Pharmacol Rev 1997;49:403-49.
- Zeven AC, de Wet JM. Dictionary of cultivated plants and their regions of diversity. 2nd ed. Wageningen: Centre for Agricultural Publishing and Documentation; 1982. p. 107.
- Warwick SI, Francis A. Guide to the wild germplasm of Brassica and allied crops. Part V. Life History and Geographical Data for wild species in the tribe Brassicaceae (Cruciferae). 2nd ed. Tech Bull. Ottawa Canada: Centre for Land and Biological Resources Research, Agriculture and Agri-Food; 1994. p. 61.
- Khoobchandani M, Ojeswi BK, Ganesh N, Srivastava MM, Gabbanini S, Matera R. Antimicrobial properties and analytical profile of traditional Eruca Sativa seed oil: Comparison with various aerial and root plant extracts. Food Chem 2010;120:217-24.
- Khoobchandani M, Ganesh N, Gabbanini S, Valgimigli L, Srivastava MM. Phytochemical Potential of Eruca Sativa for inhibition of melanoma tumor growth. Fitoterapia 2011;82:647-53.
- Srivastava SK, Xiao D, Lew KL, Hershberger P, Kokkinakis DM, Johnson CS, et al. Allylisothiocyanate, a constituent of cruciferous vegetables, inhibits growth of PC-3 human prostate cancer xenograftsin vivo. Carcinogenesis 2003;24:1665-70.
- Gewirtz DA. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. BiochemPharmacol 1999;57:727-41.
- Elmore LW, Rehder CW, Di X, McChesney PA, Jackson-Cook CK, Gewirtz DA, et al. Adriamycin-induced senescence in breast tumor cells involves functional p53 and telomere dysfunction. J BiolChem 2002;277:35509-15.
- Skladanowski A, Konopa J. Adriamycin and daunomycin induce programmed cell death (apoptosis) in tumour cells. BiochemPharmacol 1993;46:375-82.
- Feleszko W, Mlynarczuk I, Olszewska D, Jalili A, Grzela T, Lasek W, et al. Lovastatin potentiates antitumor activity of doxorubicin inmurine melanoma via an apoptosis-dependent mechanism. Int J Cancer 2002;100:111-8.
- Corbett T, Valeriote F, LoRusso P, Polin L, Panchapor C, Pugh S, et al. In vivo methods for screening and preclinical testing; use ofrodent solid tumors for drug discovery, In: Teicher B, editor. Anticancer drug development guide: Preclinical screening, clinical trials, and approval. Totowa NJ: Humana Press Inc; 1997. p. 75-99.
- Chen JX, Gao Y, Liu JW, Tian YX, Zhao J, Cui XY. Antitumor effects of human ribonuclease inhibitor gene transfected on B16 melanoma cells. Int J Biochem Cell Biol 2005;37:1219-31.
- Brown PL, Jeffries CD. Liver glutathione and glutathione reductase response of endotoxin-treated mice. Infect Immun 1975;11:8-13.
- Beutler E. In Red Cell Metabolism. In: Beultec E, editor. New York: Grunne and Stratton; 1984. p. 77-136.
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979;95:351-8.
- Maistro EL, Mota SF, Lima EB, Bernardes BM, Goulart FC. Genotoxicity and mutagenicity of Rosmarinusofficinalis (Labiatae) essential oil in mammalian cells in vivo. Genet Mol Res 2010;9:2113-22.
- Savage JR. Update on target theory as applied to chromosomalaberrations. Environ Mol Mutagen 1993;22:198-207.
- Schmid W. The micronucleus test. Mutat Res 1975;31:9-12.
- Krishna G, Hayashi M. In vivo rodent micronucleus assay: Protocol, conduct and data interpretation. Mutat Res 2000;455:155-60.
- Ojeswi BK, Khoobchandani M, Hazra DK, Srivastava MM. Green chemicals: Prospects and future in designing new drugs for cancer. Int J Wastewater Treat Green Chem 2009;1:17-22.
- Bloland PB, Ettling M, Meek S. Combination therapy for malaria in Africa: Hype or hope? Bull World Health Organ 2000;78:12.
- Braham M. What you need to know about combination therapy-it’s your life, live it. [NOOK Book]. Boston, USA: Cancer Group Institute Publication; 2011. p. 35.
- Musk SR, Johnson IT. The clastogenic effects of isothiocyanates. Mutat Res 1993;300:111-7.
- Folkman J. Angiogenesis research: From laboratory to clinic. Forum (Genova) 1999;9(Suppl 3):59-62.
- Saaristo A, Karpanen T, Alitalo K. Mechanisms of angiogenesis and their use in the inhibition of tumor growth and metastasis. Oncogene 2000;19:6122-9.
- Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol 2002;29:15-8.
- Chekenya M, Hjelstuen M, Enger PQ, Thorsen F, Jacob AL, Probst B, et al. The NG2 proteoglycan promotes angiogenesis dependent tumorgrowth in CNS by sequestering angiostatin. FASEB J 2002;16:586-8.
- Zhang Y, Talalay P. Anticarcinogenic activities of organic isothiocyanates-chemistry and mechanisms. Cancer Res 1994;54:1976-81s.
- Hecht SS. Chemoprevention by isothiocyanates. J Cell Biochem 1995;22:195-209.
- Trachootham D, Zhou Y, Zhang H, Demizu Y, Chen Z, Pelicano H, et al. Selective killing of oncogenically transformed cells through aROS-mediated mechanism by β-phenylethylisothiocyanate. Cancer Cell 2006;10:241-52.
- Xu K, Thornalley PJ. Involvement of GSH metabolism in the cytotoxicity of the phenethylisothiocyanate and its cysteine conjugate to human leukaemia cells in vitro. BiochemPharmacol 2001;61:165-77.
- Zhang YS, Tang L, Gonzalez V. Selected isothiocyanates rapidly induce growth inhibition of cancer cells. Mol Cancer Ther 2003;2:1045-52.
- Zhang RF, Loganathan S, Humphreys I, Srivastava SK. Benzyl isothiocyanate-induced DNA damage causes G2/M cell cycle arrest and apoptosis in human pancreatic cancer cells. J Nutr 2006;136:2728-34.
- Xiao D, Singh SV. Phenethylisothiocyanate inhibits angiogenesis invitro and ex vivo. Cancer Res 2007;67:2239-46.
- Xu CJ, Shen GX, Yuan XL, Kim JH, Gopalkrishnan A, Keum YS, et al. ERK and JNK signalling pathways are involved in the regulation of activator protein 1 and cell death elicited by three isothiocyanates in human prostate cancer PC-3 cells. Carcinogenesis 2006;27:437-45.
- Hu R, Kim BR, Chen C, Hebbar V, Kong ANT. The roles of JNK and apoptosis signalling pathways in PEITC-mediated responses in human HT-29 colon adenocarcinoma cells. Carcinogenesis 2003;24:1361-7.
- Satyan KS, Swamy N, Dizon DS, Singh R, Granai CO, Brard L. Phenethylisothiocyanate (PEITC) inhibits growth of ovarian cancer cells by inducing apoptosis: Role of caspase and MAPK activation. GynecolOncol 2006;103:261-70.