- *Corresponding Author:
- K. R. Kim
Department of Dental Hygiene, Kyungpook National University, Sangju, 37224, South Korea
E-mail: rim0804@knu.ac.kr
| Date of Submission | 29 August 2018 |
| Date of Revision | 18 October 2018 |
| Date of Acceptance | 08 March 2019 |
| Indian J Pharm Sci 2019;81(3):533-538 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The antiinflammatory effect of propolis collected from different geographic regions in South Korea was investigated in the present study. All propolis samples, from six different regions, dose-dependently inhibited the production of nitric oxide and prostaglandin E2 in RAW 264.7 macrophages stimulated with lipopolysaccharide. Propolis also suppressed the expression of inducible nitric oxide synthase and cyclooxygenase-2, stimulated by lipopolysaccharide. Additionally, propolis extracts inhibited ear edema induced by phorbol-12-myristate-13-acetate in mice. Overall, in both in vitro and in vivo studies, Korean propolis extracts showed significant antiinflammatory effects without any geographical variation. In conclusion, Korean propolis could be of the potential for drug development against inflammatory reactions.
Keywords
Inflammation, Korean propolis, lipopolysaccharide, macrophages, ear edema
Propolis, a natural substance produced by honey bees and is derived from plant resins. Propolis is made from flowers of sprout and other plant resinous extracts. Bees collect propolis and mix it with beeswax and β-glycosidase enzymes from their saliva [1]. It has been used in traditional medicines worldwide and reported to exhibit antiinflammatory, antibiotic, antioxidant, antitumor, and antifungal activities [2,3]. As an antiinflammatory agent, propolis has been reported to suppress carrageenan-induced paw edema [4] and improve immune function in aged mice [5]. Brazilian green propolis has also shown beneficial effects on immune responses [6].
The antiinflammatory effects of propolis included inhibition of the production of prostaglandin E2 (PGE2) and the expression of cyclooxygenase (COX)-2, the inducible isoform of COX [3,7,8]. It also reduced nitric oxide (NO), a potent inflammatory mediator produced by endothelial and inflammatory cells that can induce tissue damage [9]. Bufalo et al. reported the antiinflammatory potential of Brazilian propolis and caffeic acid, its major phenolic compound [10]. These two compounds suppressed NO levels and nuclear factor kappa-B activity in lipopolysaccharide (LPS)-stimulated RAW 264.7 cells. Subsequently, several studies reported antiinflammatory effects of phytochemical samples using in vitro and in vivo models.
Because the proportions of chemical compounds in propolis are different in each geographical region, its efficacy is also slightly different [11,12]. There are not many studies on the propolis produced in Korea. Therefore, the antiinflammatory effect of Korean propolis was examined. In this present study, propolis samples collected from six different regions (Uijeongbu, Ansan, Hongcheon, Iksan, Gwangju, and Sangju) of South Korea were investigated. The antiinflammatory effect of Korean propolis extracts (PE) was evaluated in LPS-stimulated RAW 264.7 macrophages and a phorbol-12-myristate-13-acetate (PMA)-induced ear edema animal model. Also, the antiinflammatory effects of different PE samples collected were compared.
Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), phosphate-buffered saline (PBS), and antibiotic-antimycotic mixture containing 100 U/ml penicillin and 100 μg/ml streptomycin were purchased from Gibco BRL (Grand Island, NY). LPS from Escherichia coli O111:B4 and dimethyl sulfoxide (DMSO) were obtained from Sigma-Aldrich (St. Louis, MO). PMA was purchased from Calbiochem-Novabiochem (Nottingham, United Kingdom). Antirabbit COX-2 polyclonal antibody (Cell Signaling Technology, Danvers, MA), antirabbit β-actin polyclonal antibody (Sigma-Aldrich), and antiiNOS monoclonal antibody and horseradish peroxidase (HRP)-conjugated secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA) were used in this study. All reagents used in this study were of analytical grade.
Korean propolis samples, collected from different areas of Korea, were gift samples received from Dong-A University. Table 1 show the region in Korea where each propolis sample was collected. In brief, crude propolis was extracted with ethanol for 24 h. Then, the suspension was separated, concentrated in a rotary evaporator and dissolved in ethanol. PE samples were stored at −20° until analysed.
| Propolis extract (PE) | Region | Province |
|---|---|---|
| PE1 | Uijeongbu | Gyeonggi |
| PE2 | Ansan | Gyeonggi |
| PE3 | Hongcheon | Gangwon |
| PE4 | Iksan | Jeonbuk |
| PE5 | Kwangju | Jeonnam |
| PE6 | Sangju | Gyeongbuk |
Table 1: Propolis extracts from six different regions of Korea
Five-week-old BALB/c mice were obtained from Raon Bio (Yongin, Korea). All mice were permitted free access to standard rodent chow and tap water ad libitum. Mice were housed under specific pathogenfree conditions with a 12 h light-dark cycle and a relative humidity of 55±5 % at 25±2°. Animal studies were conducted in accordance with the guidelines for the use and care of animals established by the Animal Ethics Committee of Kyungpook National University, Korea (Approval No. 2016-0062).
Mice were randomly divided into groups, each with 5-6 animals of similar body weights. The right ear of each mouse was topically treated with PE (2 mg/ear) in 50 μl vehicle (DMSO:acetone,15:85, v/v) for 30 min prior to the application of 5 nmol PMA. The left ears were treated with vehicle alone. Control mice received vehicle alone on both ears. After 4 h of PMA treatment, mice were sacrificed by cervical dislocation and the ears were punched out with a puncher (6 mm in diameter) and the ear punches were weighed. The vehicle-treated left ear was used as a blank and the difference in weight between the right and left ears was measured as the degree of antiinflammatory activity of propolis.
Murine RAW 264.7 macrophage cell line was purchased from the Korean Cell Line Bank (Seoul, Korean) and cultured in DMEM containing 2 mM l-glutamine, antibiotic-antimycotic mixture, and 10 % FBS in a humidified atmosphere of 5 % CO2 at 37°. Cells (1×104 cells/well) were plated into 96-well plates and incubated in DMEM with or without various concentrations of PE. After incubation for 24 h, cell viability was assessed using Cell Counting Kit-8 (Dojindo, Kumamoto, Japan) according to the manufacturer’s protocol. Absorbance at 450 nm was measured using a microplate reader (Bio-Rad Laboratories, Hercules, CA).
Nitrite accumulated in culture medium was measured as an indicator of NO production using the Griess reaction. RAW 264.7 cells (1×105 cells/well) were plated into 96-well plates and incubated overnight. Cells were washed with PBS and cultured with 1 μg/ml LPS in the presence or absence of PE at 37°. After 24 h incubation, 100 μl culture supernatant was mixed with 100 μl Griess reagent (equal volumes of 1 % (w/v) sulphanilamide in 5 % (v/v) phosphoric acid and 0.1 % (w/v) N-(1-naphthyl)ethylenediamine in distilled water) in a new 96-well plate and incubated at room temperature for 10 min. Absorbance at 540 nm was measured using a microplate reader. The amount of nitrite in each sample was quantified based on a sodium nitrite serial dilution standard curve.
RAW 264.7 cells (2.5×105 cells/well) were plated into a 24-well plate and incubated with 1 μg/ml LPS in the presence or absence of PE for 24 h. Conditioned medium of control and treated cells was collected and centrifuged at 2000×g for 10 min at 4° and stored at −70°. Amounts of PGE2 released from cells were quantified using an enzyme-linked immunosorbent assay kit (R&D Systems, Minneapolis, MN).
Cells (1×106 cells/well) were cultured in media containing 1 μg/ml LPS and/or PE for 24 h, and then were lysed in RIPA buffer containing 1 mM PMSF and protease inhibitor cocktail tablets (Roche, Mannheim, Germany). Cell lysates were centrifuged at 15 000×g for 20 min at 4°, and then supernatants were collected. Protein content was quantified using bicinchoninic acid assay reagents (Pierce, Rockford, IL). Equal amounts of protein (30 μg) were loaded onto a gel for 10 % sodium dodecyl sulphate-polyacrylamide gel electrophoresis, and then proteins were transferred to a polyvinylidene difluoride membrane (Millipore, Danvers, MA). The membrane was blocked with 5 % skim milk in Trisbuffered saline containing 0.1 % Tween-20 for 1 h at room temperature and then incubated with primary antibodies against iNOS, COX-2, or β-actin overnight at 4°. Blots were incubated with the appropriate HRP-conjugated secondary antibody for 1 h at room temperature, and target proteins were detected using an Amersham ECL western blotting detection kit (GE Healthcare, Piscataway, NJ).
Data were expressed as the means±standard error of the mean (SEM) of three independent experiments and analysed via one-way analysis of variance with multiple comparisons using SPSS statistical software version 21 (SPSS Inc., Chicago, IL). P values less than 0.05 were considered to indicate statistically significant differences.
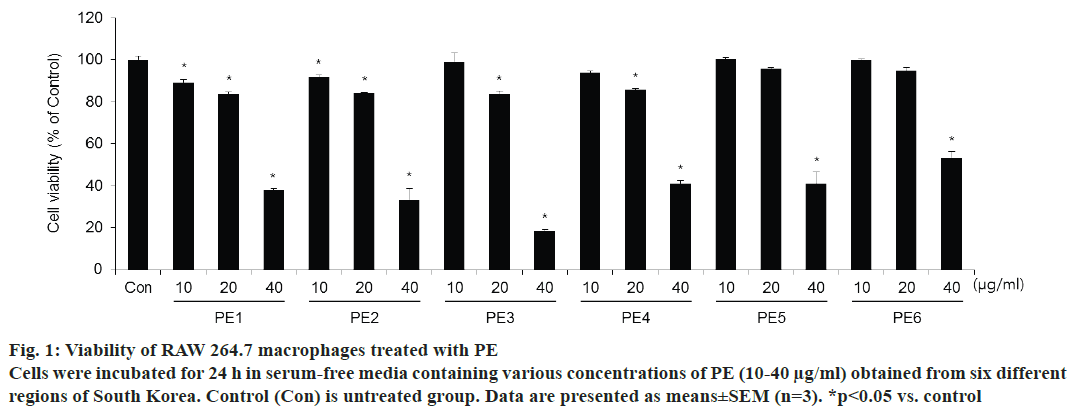
To test the effects of propolis samples that were collected from six different regions of Korea on cell viability, the CCK-8 assay was performed. Fig. 1 shows the results of cytotoxicity. PE samples from each region significantly reduced the survival of RAW 264.7 cells at 40 μg/ml. In the presence of PE at concentrations below 20 μg/ml, viability was found to be >85 % of that of the control cells. In order to use non-toxic doses that do not affect cell viability, 10 and 20 μg/ml of all region PE samples was used in subsequent experiments.
Figure 1: Viability of RAW 264.7 macrophages treated with PE
Cells were incubated for 24 h in serum-free media containing various concentrations of PE (10-40 μg/ml) obtained from six different
regions of South Korea. Control (Con) is untreated group. Data are presented as means±SEM (n=3). *p<0.05 vs. control
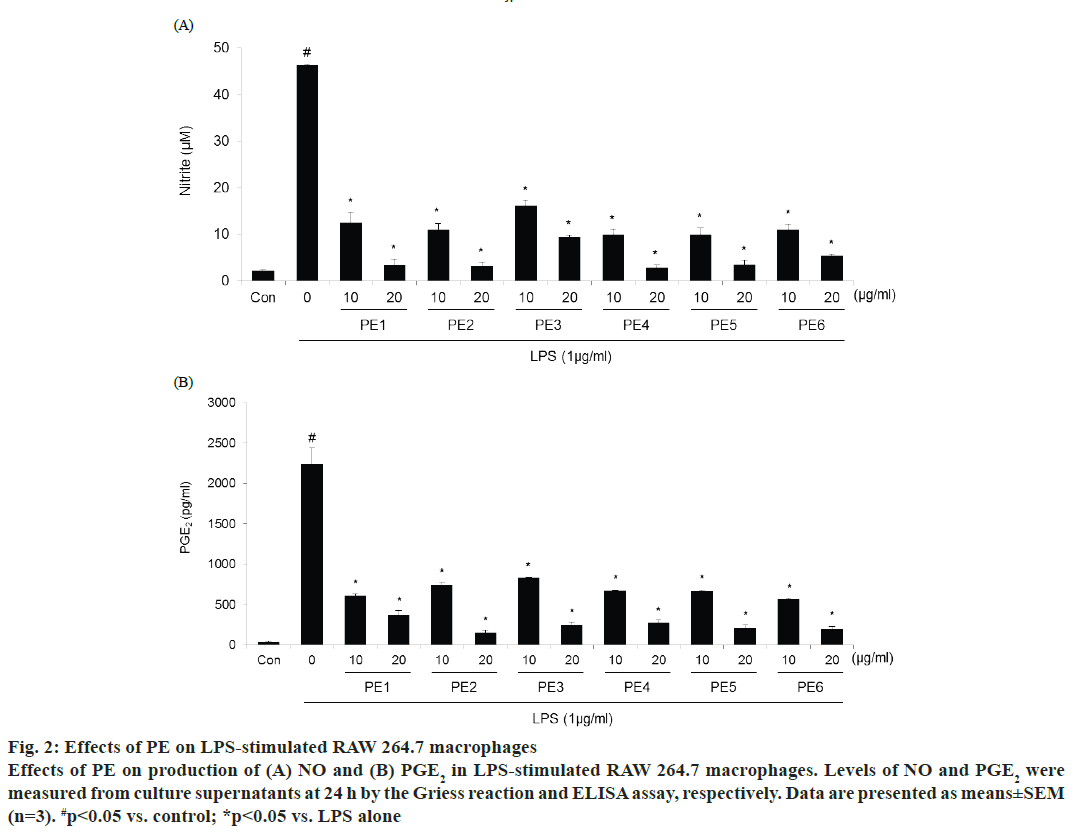
The effect of PE was studied on the production of inflammatory mediators in LPS-stimulated macrophages. The production of nitrite, which was used as an indicator for NO, and PGE2 was measured by the Griess reaction or ELISA assays, respectively. Cells were treated with or without 1 μg/ml LPS and two concentrations of PE for 24 h. LPS significantly increased the levels of nitrite and PGE2 compared with control (fig. 2). Korean propolis samples from each of the six different regions efficiently suppressed LPSinduced NO and PGE2 production in a dose-dependent manner. Indeed, over 80 % inhibition was shown at the highest concentration (20 μg/ml) for each of the PE samples.
Figure 2: Effects of PE on LPS-stimulated RAW 264.7 macrophages
Effects of PE on production of (A) NO and (B) PGE2 in LPS-stimulated RAW 264.7 macrophages. Levels of NO and PGE2 were
measured from culture supernatants at 24 h by the Griess reaction and ELISA assay, respectively. Data are presented as means±SEM
(n=3). #p< 0.05 vs. control; *p< 0.05 vs. LPS alone
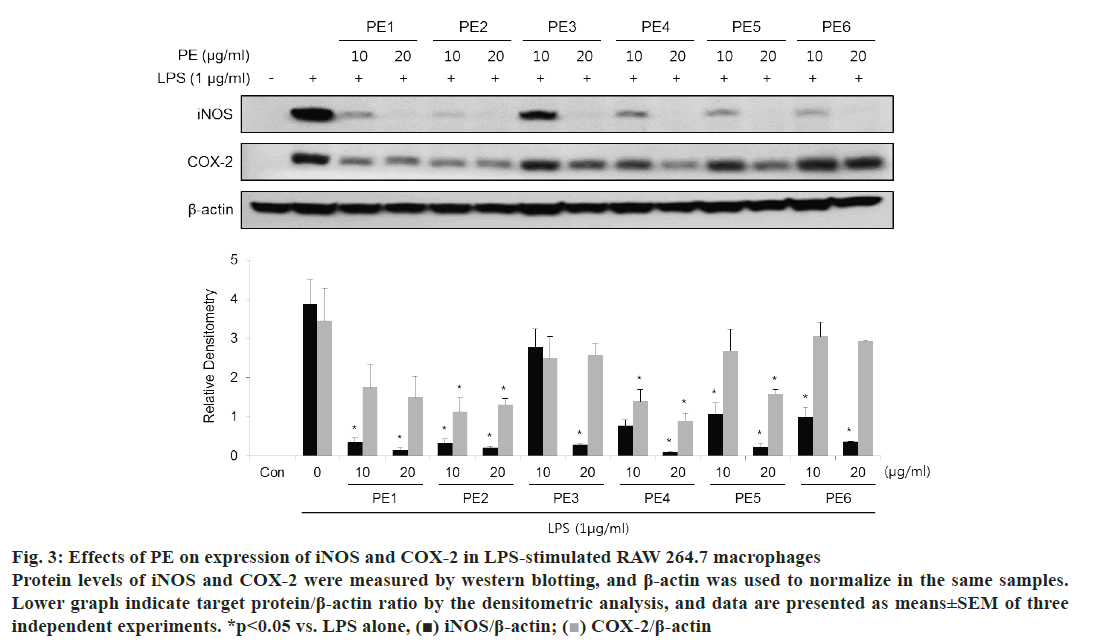
To assess the effect of PE on the protein expression levels of iNOS and COX-2, western blot analysis was performed. As shown in fig. 3, all Korean propolis samples attenuated the expression of iNOS and COX-2 that induced to LPS stimulation in a dose-dependent manner. Notably, 20 μg/ml PE samples from each region significantly inhibited the induction of iNOS.
Figure 3: Effects of PE on expression of iNOS and COX-2 in LPS-stimulated RAW 264.7 macrophages
Protein levels of iNOS and COX-2 were measured by western blotting, and β-actin was used to normalize in the same samples.
Lower graph indicate target protein/β-actin ratio by the densitometric analysis, and data are presented as means±SEM of three
independent experiments. *p< 0.05 vs. LPS alone, (■) iNOS/β-actin;  COX-2/β-actin
COX-2/β-actin
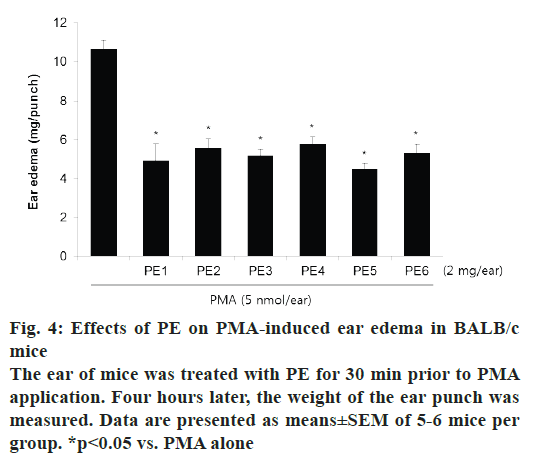
PMA-induced ear edema model was used as an acute inflammation animal model to evaluate the PE samples. Topical application of 5 nmol PMA on mouse ear induced prominent edema as an acute inflammatory response (fig. 4). Pretreatment with PE decreased PMA-induced ear edema by about 50 %. There was no significant differences in the inhibitory effects of PE collected from different regions.
Propolis, used worldwide as a traditional medicine for many years, is a resinous natural material that is collected from honeybees. Many studies have shown that propolis exhibited antimicrobial, antiinflammatory, and antitumor activities [13]. Its pharmacological properties, which are related chemical compositions, depended on the environmental condition of geographical regions from which the resin is collected. Wang et al. investigated the total polyphenol and flavonoid contents of PE samples obtained from 20 different geographic regions, and reported the correlation between their composition ratios and biological properties of each propolis [14]. In their results, Korean PE has a significant amount of total phenolic contents compared to Australia, Brazil and China, where propolis is widely used.
In the present study, the antiinflammatory activities of Korean PE were assessed in vitro and in vivo and confirmed the cytotoxicity of PE samples that were collected from six regions (Uijeongbu, Ansan, Hongcheon, Iksan, Gwangju, and Sangju) of South Korea. RAW 264.7 macrophages were over 85 % viable at concentration below 20 μg/ml for all PE samples. Consequently, non-toxic doses of PE samples collected from each region of Korea was used and NO production and iNOS expression were measured. NO, which is the enzymatic product of iNOS, mediates various biological functions, including vascular homeostasis, apoptosis, and immune responses [15,16]. As a regulator of NO production, iNOS has also been found to play an important role in inflammation [17]. Levels of NO and iNOS increase in inflamed tissues. Macrophages, the primary immune cell responding to inflammation, release NO upon LPS stimulation, and excessive NO production can be induced by upregulation of iNOS [18,19]. In RAW 264.7 macrophages, which are commonly used as an in vitro inflammation model, NO and iNOS levels were raised by LPS, which were dose-dependently reduced by PE samples, irrespective of the region from where the sample was collected. Similarly, PE samples from different regions produced similar inhibitory effects iNOS expression as were observed on NO production. These results suggest that the reduction of NO production in response to PE treatment is caused by iNOS down-regulation.
All PE samples inhibited PGE2 level and COX-2 expression, as determined by ELISA and western blot analysis, respectively. PGE2, a major metabolite of the COX-2 pathway, is known to be an important component of the acute and chronic inflammatory response. COX-2, compared to COX-1, is highly inducible by inflammatory stimuli, such as LPS [20]. The COX-2/PGE2 signaling has been studied in relation to cancer [21]. It was confirmed that PE treatment significantly reduced LPS-induced COX-2 over expression and consequently inhibited PGE2 release from LPS-stimulated RAW 264.7 macrophages. The inhibitory effects of PE on COX-2/PGE2 signaling are also similar to those on iNOS/NO levels.
To assess the acute inflammatory effect of PE, an in vivo study was conducted on PMA-induced ear edema in mice. PMA is known to activate various cell types that respond to acute inflammation, and its application to animal skin is widely used as an animal model to evaluate drug effects on acute inflammation [22]. Topical application of PMA for 4 h significantly induced ear edema in mice, and pretreatment with all PE samples suppressed this swelling. The degree of the inhibitory activity of all PE samples was in the range of 45.9- 57.8 %, and there was almost no difference between samples of PE collected from different regions.
The biological effects of propolis, that was collected in Korea, have not been extensively studied. The antiinflammatory effects of Korean propolis was investigated in the present study, and found that this effect was not significantly different for samples collected in different regions of South Korea. Various propolis-containing products can be easily purchased at pharmacies, but these are mainly from other countries. The findings of this investigation could serve as basic data for the development of antiinflammatory agents and products using Korean propolis as an ingredient.
Acknowledgements:
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (2017R1C1B1008203). The authors thank Professor Ahn of Dong-A University for providing the propolis samples used in this study.
Conflicts of interest:
The authors declare that there is no conflict of interest.
References
- Park YK, Ikegaki M, Abreu JAdS, Alcici NMF. Estudo da Preparação dos Extratos de Própolis e suas Aplicações. Ciênc Tecnol Aliment 1998;18(3):313-8.
- Banskota AH, Tezuka Y, Kadota S. Recent progress in pharmacological research of propolis. Phytother Res 2001;15:561-71.
- Burdock GA. Review of the biological properties and toxicity of bee propolis (propolis). Food Chem Toxicol 1998;36:347-63.
- Tan-No K, Nakajima T, Shoji T, Nakagawasai O, Niijima F, Ishikawa M, et al. Anti-inflammatory effect of propolis through inhibition of nitric oxide production on carrageenin-induced mouse paw edema. Biol Pharm Bull 2006;29:96-9.
- Gao W, Wu J, Wei J, Pu L, Guo C, Yang J, et al. Brazilian green propolis improves immune function in aged mice. J Clin Biochem Nutr 2014;55:7-10.
- Chirumbolo S. Anti-inflammatory property of propolis. J Clin Biochem Nutr 2015;56:163-4.
- Marcucci MC. Propolis: chemical composition, biological properties and therapeutic activity. Apidologie 1995;26:16.
- Krol W, Scheller S, Czuba Z, Matsuno T, Zydowicz G, Shani J, et al. Inhibition of neutrophils' chemiluminescence by ethanol extract of propolis (EEP) and its phenolic components. J Ethnopharmacol 1996;55:19-25.
- Lope ER, Chapadeiro E, Raso P, Tafuri WL. Bogliolo - Patologia. 4th ed. Belo Horizonte: Guanabara Koogan; 1987. p. 45.
- Bufalo MC, Ferreira I, Costa G, Francisco V, Liberal J, Cruz MT, et al. Propolis and its constituent caffeic acid suppress LPS-stimulated pro-inflammatory response by blocking NF-kappaB and MAPK activation in macrophages. J Ethnopharmacol 2013;149:84-92.
- Bankova V, Christov R, Kujumgiev A, Marcucci MC, Popov S. Chemical composition and antibacterial activity of Brazilian propolis. Z Naturforsch C 1995;50:167-72.
- Choi SJ, Shimomura K, Kumazawa S, Ahn MR. Antioxidant Properties and Phenolic Composition of Propolis from Diverse Geographic Regions in Korea. Food Sci Technol Res 2013;19:211-22.
- Sforcin JM, Bankova V. Propolis: is there a potential for the development of new drugs? J Ethnopharmacol 2011;133:253-60.
- Wang X, Sankarapandian K, Cheng Y, Woo SO, Kwon HW, Perumalsamy H, et al. Relationship between total phenolic contents and biological properties of propolis from 20 different regions in South Korea. BMC Complement Altern Med 2016;16:65.
- Green SJ, Mellouk S, Hoffman SL, Meltzer MS, Nacy CA. Cellular mechanisms of nonspecific immunity to intracellular infection: cytokine-induced synthesis of toxic nitrogen oxides from L-arginine by macrophages and hepatocytes. Immunol Lett 1990;25(1-3):15-9.
- Pereira AC, Paulo M, Araujo AV, Rodrigues GJ, Bendhack LM. Nitric oxide synthesis and biological functions of nitric oxide released from ruthenium compounds. Braz J Med Biol Res 2011;44(9):947-57.
- Stichtenoth DO, Frolich JC. Nitric oxide and inflammatory joint diseases. Br J Rheumatol 1998;37:246-57.
- Kobayashi Y. The regulatory role of nitric oxide in proinflammatory cytokine expression during the induction and resolution of inflammation. J Leukoc Biol 2010;88(6):1157-62.
- Zhang L, Wang CC. Inflammatory response of macrophages in infection. Hepatobiliary Pancreat Dis Int 2014;13:138-52.
- Williams CS, Mann M, DuBois RN. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene 1999;18:7908-16.
- Greenhough A, Smartt HJ, Moore AE, Roberts HR, Williams AC, Paraskeva C, et al. The COX-2/PGE2 pathway: key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis 2009;30:377-86.
- Wershil BK, Murakami T, Galli SJ. Mast cell-dependent amplification of an immunologically nonspecific inflammatory response. Mast cells are required for the full expression of cutaneous acute inflammation induced by phorbol 12-myristate 13-acetate. J Immunol 1988;140:2356-60.