- *Corresponding Author:
- G. V. Subbaraju
Laila Impex R & D Centre, Unit-I, Phase-III, Jawahar Autonagar, Vijayawada-520 007, India
E-mail: subbarajugottumukkala@hotmail.com
| Date of Submission | 29 March 2004 |
| Date of Revision | 21 April 2005 |
| Date of Acceptance | 24 February 2006 |
| Indian J Pharm Sci, 2006, 68 (1): 111-114 |
Abstract
Bioassay-guided fractionation, based on antiinflammatory activity of the methanolic extractives of Teramnus labialis led to the isolation and characterization of vitexin, bergenin, daidzin and 3-O-methyl-D- chiro -inositol as active constituents. Vitexin exhibited a dose-dependent inhibitory activity on 5-lipoxygenase enzyme. The isolated constituents were also screened for their antioxidant activity by nitroblue tetrazolium (NBT) riboflavin photo reduction method. Vitexin exhibited moderate antioxidant activity. This is the first reported occurrence of vitexin, bergenin, daidzin and 3-O-methyl-D- chiro -inositol in T. labialis.
Teramnus labialis Spreng (Family: Fabaceae) is a herb, commonly known as mashaparni (Sanskrit) and mashavan (Hindi), and a well-known medicinal plant in the Ayurvedic system of medicine. It has been reported to be useful in treating rheumatism, tuberculosis, nerve disorders, paralysis and catarrhs [1-3]. Phytochemical investigation on the seeds of T. labialis yielded a water-soluble gallactomannan [4]. Bioassay-guided fractionation, based on anti-hyperglycaemic activity of aqueous alcoholic extract of T. labialis, yielded fraxidin as the major active constituent [5]. In view of the reported use of T. labialis in rheumatism, we have evaluated the antiinflammatory activity of T. labialis, and we report in this paper the isolation and characterization of the active constituents.
The aerial parts of T. labialis were collected from the Tirumala Hills of Chittoor district, Andhra Pradesh, and were authenticated at the Department of Botany, S. V. University, Tirupati. The aerial parts were shade-dried and powdered. The powdered material was extracted, successively, with hexane, ethyl acetate and methanol. The extracts were concentrated under reduced pressure.
The extracts were subjected to antiinflammatory activity by carrageenin-induced rat paw oedema model of winter et al. [6]. Wistar rats of either sex weighing between 180 and 220 g were procured from NIN, Hyderabad. The rats were divided into five groups, each group consisting of six animals. One group served as negative control (received 1% Tween-80, 10 ml/kg); second group served as positive control (received 25 mg/kg, diclofenac sodium suspended in 1% Tween-80); third, fourth and fifth groups received 250 mg/kg of hexane, ethyl acetate and methanol extracts suspended in 1% Tween-80 respectively, by oral route.
All experimental protocols have been approved by the Institutional Animal Ethics Committee prior to the conduct of the experiments. Oedema was produced by injecting carrageenin solution 0.1 ml (1% w/v) to subplantar region of the left hind paw of rats of all groups. Drug treatment was given 1 h prior to the carrageenin injection. The paw volume was measured by a plethysmometer at zero and three hours after carrageenin injection. The difference between the initial and the final paw volume gave the oedema volume. The results obtained as mean increase in paw volume and percentage inhibition of oedema are presented in Table 1.
| Group | Treatment | Dose (mg/kg, p.o.) | Mean oedema volume ±SE (ml) | Percent inhibition of oedema |
|---|---|---|---|---|
| 1 | Control | 10 ml/kg | 0.63 ± 0.02 | |
| 2 | Diclofenac sodium | 25 | 0.15 ± 0.01 | 76.19 |
| 3 | Hexane extract | 250 | 0.61 ± 0.03 | 3.17 |
| 4 | Ethyl acetate extract | 250 | 0.59 ± 0.04 | 6.34 |
| 5 | Methanol extract | 250 | 0.45 ± 0.03 | 28.57* |
Number of animals in each group 6. *P< 0.01, when compared to control
Table 1: Effect of Different Extracts of T. Labialis on Carrageenin-Induced Rat Paw Oedema.
The methanol extract, which showed potent antiinflammatory activity, was further fractionated to isolate the active constituents. The methanol extractives (200 g) were chromatographed over silica gel column and eluted with chloroform and mixture of chloroform and methanol with increasing polarity. The chloroform-methanol (93:7) eluates afforded compound-A (170 mg), mp: 139-140°, [α]D -20.0° (c, 0.2, methanol); chloroform-methanol (90:10) eluates yielded compound-B (130 mg), mp: 232-233°; chloroform-methanol (85:15) eluates yielded compound-C (48 mg), mp: 275-276°; and chloroform-methanol (70:30) eluates yielded compound-D (5 g), mp: 180-182°.
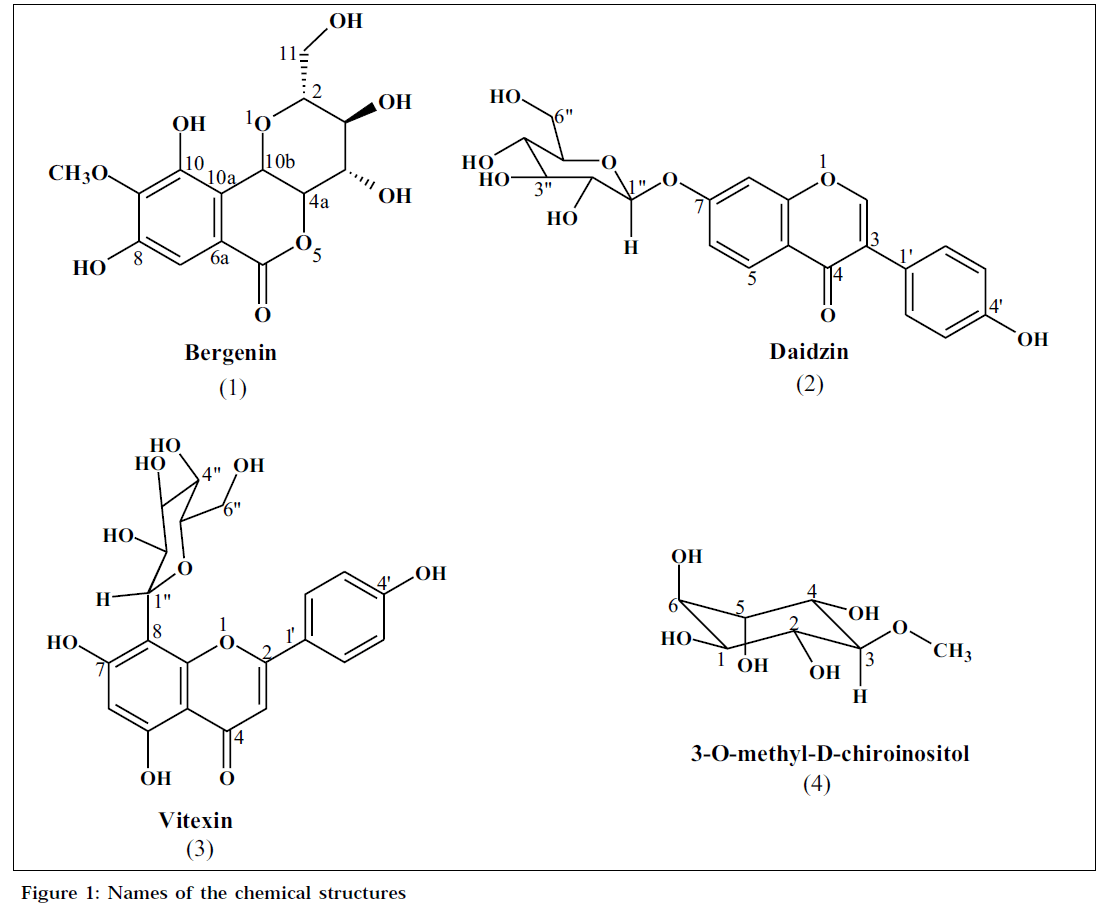
Compound-A, was obtained as colourless crystals from aqueous methanol, mp: 139-140°, analysed for C14H16O9 [LC-MS: m/z 327, (M-H)]. The IR spectrum showed bands at 3390 brs (hydroxyl), 1702 (carbonyl), 1612, 1528 and 1464 cm-1 (aromatic). The 1H NMR [500 MHz, d6-DMSO] spectrum showed singlet at δ 6.97 (1H), methoxyl group (δ 3.75, 3H, s) and a series of signals between δ 3.19 and 3.98 (6 H), characteristic of a sugar moiety. The absence of usual O-glycosidic anomeric proton signal in the region 5.00-5.20 ppm and the presence of signal at δ 4.95 (1H, d, J=10.4 Hz) indicated the presence of a C-glycoside. The 1H NMR spectrum also contained two phenolic hydroxyl protons δ 9.75 (1H, s) and 8.43 (1H, s). The 13C NMR [125 MHz, d6-DMSO] spectrum showed three oxygenated aromatic carbons (δ 140.6, 148.1 and 151.0), one aromatic carbon (δ 109.5), a methoxyl (δ 59.8), two quaternary carbons (δ 116.0 and 118.1), a lactone carbonyl (δ 163.4), and six signals at δ 61.0, 70.7, 72.1, 73.7, 79.8 and 81.7, which are attributable to a sugar moiety. The HMBC spectrum of the glycoside showed correlations between the aromatic proton (δ 6.97, s, H-7) and the lactone carbonyl (δ 163.4, C-6); sugar proton (δ 4.95, H-10b) and quaternary carbons (δ 116.0, C-10a and 118.1, C-6a). The above physical and spectral data of compound-A have been found to be corroborative with those reported for bergenin [7](1).
Compound-B was obtained as a white crystalline solid from aqueous methanol, mp: 232-233°, analysed for C21H21O9 [LC-MS: m/z 439, (M+Na)). The IR spectrum showed bands at 3372 brs (hydroxyl), 1623 (carbonyl), 1514 and 1445 cm-1 (aromatic). The 1H NMR [500 MHz, d6-DMSO] spectral data contained six aromatic protons constituted by a singlet at δ 8.36 (1H), and an ABX spin system, characteristic of a 1,2,4-trisubstitued phenyl unit [δ 8.04 (1H, d, J=8.8 Hz), 7.14 (1H, dd, J=2.2, 8.8 Hz) and 7.22 (1H, d, J=2.1 Hz)] and an AA’BB’ spin system [δ 7.40 (2H, d, J=8.6 Hz) and 6.81 (2H, d, J=8.6 Hz)] attributable to a para-disubstituted phenyl unit. A perusal of the above data indicated the presence of an isoflavonoid skeleton [8]. The spectrum also showed a group of signals between δ 3.10 and 3.70, in addition to an anomeric proton signal at δ 5.10 (1H, d, J=7.6 Hz), suggestive of a sugar unit. The 13C NMR [125 MHz, d6- DMSO] spectrum showed six quaternary carbons (δ 118.5, 122.0, 123.7, 157.0, 157.2 and 161.0), three aromatic carbons (δ 103.4, 115.6 and 127.0), a β-olefinic carbon (δ 153.3) and a carbonyl carbon resonating at δ 174.8. The signals at δ 60.7, 69.7, 73.2, 76.5, 77.2 and 100.0 are attributable to a sugar moiety. The 13C NMR chemical shifts of the sugar moiety matched well with those recorded for a glucose unit [9]. The presence of a glucose residue at C-7 of the isoflavonoid was supported by the HMBC correlations between C-7 and H-1”. The above physical and spectral data have been found to be identical with those reported for daidzin [10,11] (2).
The compound-C was obtained as a yellow solid, mp: 275- 276°, analysed for C21H20O10 [LC-MS: m/z 431, (M-H)]. The IR spectrum of the compound showed bands at 3381 (hydroxyl), 1652 (carbonyl), 1568, 1501 cm-1 (aromatic). The 1H NMR [400 MHz, d6-DMSO] spectrum showed a chelated hydroxyl proton (δ 13.18, 1H, s), an aromatic singlet (δ 6.28, 1H, s), a downfield signal (δ 6.79, 1H, s), suggestive of a flavonoid [12] and an AA’BB’ spin system [δ 8.03, (2H, d, J=8.5 Hz) and 6.09 (2H, d, J=8.5 Hz)], attributable to a para-disubstituted phenyl unit. In addition, the 1H NMR spectrum showed a series of signals between δ 3.19 and 3.98 (6H), characteristic of a sugar unit. The absence of usual O-glycosidic anomeric proton signal and the presence of signal at δ 4.69 (1H, d, J=9.88 Hz) indicated the presence of a C-glycoside. The above physical and spectral data of compound-C are in agreement with those reported for vitexin [13] (3).
Compound-D, was obtained as a white crystalline solid from methanol, mp: 180-182°, [α]D +51.0° (c, 0.75, H2O), analysed for C7H14O6 [LC-MS: m/z 193 (M-H)]. The IR spectrum showed bands at 3403 (hydroxyl) and 1072 cm-1 (ether). The 1H NMR [500 MHz, D2-O] spectrum showed the presence of a methoxyl group (δ 3.53, 3H, s) and six oxygenated methine protons [δ 3.27 (1H, t, J=9.7 Hz), 3.58 (1H, t, J=9.8 Hz), 3.69 (1H, dd, J=2.8, 9.8 Hz), 3.75 (1H, dd, J=2.8, 9.8 Hz) and δ 3.94 (2H, m). The 13C NMR [125 MHz, D2-O] spectrum showed the presence of a methoxyl group (δ 60.1) and six oxygenated methine carbons resonating at δ 70.2, 70.9, 71.8, 72.0, 72.5 and 83.1. These signals indicated the presence of an inositol skeleton [14]. The HMBC spectrum showed correlations between methoxyl group (δ 3.53, s) and an oxygenated carbon (δ 83.1, C-3); proton signal (δ 3.27, t, J=9.7 Hz, H- 3) and methoxyl carbon (δ 60.1).These correlations and coupling constants indicated the presence of an equatorial methoxyl group in the inositol [14]. The above physical and spectral data of the compound-D have been found to corroborate well with those reported for 3-O-methyl-D-chiro inositol [14,15](4) (Fig. 1).
The compounds, vitexin, bergenin, daidzin and 3-O-methyl- D-chiro-inositol were screened for 5-lipoxygenase inhibitory activity using colorimetric method of Gay et al. [16]. The assay mixture contained 50 mM phosphate buffer (pH 6.3), 5-lipoxygenase, various concentrations of test substances and linoleic acid (80 mM) in a total volume of 0.5 ml. After 5 min incubation of above reaction mixture, 0.5 ml ferric-xylenol orange reagent (in perchloric acid) was added and OD was measured after 2 min at 585 nm using a spectrophotometer. Controls were run along with test in a similar manner except using vehicle instead of test substance solution. Percent inhibition was calculated by comparing absorbance of test with that of control. Vitexin exhibited a dose-dependent inhibitory activity on 5-lipoxygenase enzyme (percent inhibition:dose in μM, 12.16:500; 22.2:1000; 36.49:2000; 64.48:3000). Daidzin, bergenin and 3-O-methyl-D-chiro inositol did not exhibit any activity even at 1000 μM dose.
Vitexin, bergenin, daidzin and 3-O-methyl-D-chiro-inositol were also screened for their antioxidant activity by the nitroblue tetrazolium (NBT) riboflavin photo-reduction method [17]. The reaction mixture comprises of EDTA (6 μM) containing 3 μg NaCN, riboflavin (2 μM), NBT (50 μM), various concentrations of the test substances and phosphate buffer (58 mM, pH 7.8) in a final volume of 3 ml. The tubes were uniformly illuminated with an incandescent lamp for 15 min, and the optical density was measured at 560 nm before and after illumination. The percentage inhibition of superoxide generation was calculated by comparing the absorbance values of the control and compound treated tubes. The IC50 values are obtained from the plot drawn, concentration vs percent inhibition. The results of antioxidant studies have been presented in Table 2.
| Compound | Superoxide-radical scavenging IC50 (µg) |
|---|---|
| Vitexin | 62 |
| Daidzin | >100 |
| Bergenin | 100 |
| Butylatedhydroxytoluene (BHT) | 90 |
Table 2: Antioxidant Activity of Chemical Constituents of T. Labialis
The present investigation reveals that the methanol extract of T. labialis possess strong antiinflammatory activity. The isolation and characterization of vitexin, bergenin and daidzin, the known antiinflammatory compounds, from the methanolic extractives of T. labialis, substantiates the traditional use of T. labialis in treating rheumatism. It showed further that the vitexin may be exhibiting antiinflammatory activity by inhibiting 5- lipoxygenase pathway. This is the first report on identification of vitexin, bergenin, daidzin and 3-O-methyl- D-chiro-inositol in the extracts of T. labialis.
Acknowledgements
The authors thank Sri G. Ganga Raju, Chairman, Laila Impex; and Smt. P. Sulochana, Correspondent, Sri Padmavathi School of Pharmacy, for encouragement; Dr. B. Lakshmana Raju for NMR spectral data; and Dr. K. Madhava Chetty, Department of Botany, S. V. University, Tirupati, for authentication of plant species.
References
- Chopra, R.N., Nayar, S.L. and Chopra, I.C., In; Glossary of Indian Medicinal Plants, 1st Edn., National Institute of Science Communication, CSIR, New Delhi, 1956, 241.
- Nadkarni, A.K., In: Indian MateriaMedica, 3rd Edn., Popular Prkashan, Mumbai, 1976, 1198.
- Anonymous, In; The Wealth of India: A dictionary of Indian Raw Materials and Industrial Products, CSIR, New Delhi, 1948, 157.
- Alam, N. and Gupta, P.C., Carbohydr. Res., 1986, 153, 334.
- Fort, D.M., Rao, K., Jolad, S.D., Luo, J., Carlson, T.J. and King, S.R.,
- Phytomedicine, 2000, 6, 465.
- Winter, C.A., Risely, E.A. and Nuss, G.W., Proc. Soc. Exp. Biol.Med., 1962, 111, 544.
- AtchutaRamaiah, P., Ramachandra Row, L., Sivakumar Reddy, D., Anjaneyulu, A.S.R., Ward, R. S. and Andrew Peltor., J. Chem. Soc.Perkin Trans 1., 1979, 2313.
- Hosny, M. and Rosazza, J.P.N., J. Nat. Prod., 1999, 62, 853.
- Al-Abed, Y., Sabri, S., Zarga, M.A., Shah, Z. and Atta-Ur-Rahman., J.Nat. Prod., 1990,53,1152.
- Dewick, P.M., In; Harborne, J.B., Eds., The Flavonoids: Advances in Research since 1980, Chapman and Hall, New York, 1988, 552-570.
- Agrawal, P.K., In; Carbon-13 NMR of Flavonoids, Elsevier Science, New York, 1989, 39, 192-211.
- Mabry, T. J., Markham, K.R. and Thomas, M.B., In;The Systematic identification of flavonoids, Springer-verlag, Berlin, 1970, 267.
- Tomczyk, M., Gudej, J. and MarekSochacki., Z. Naturforsch, 2002, 57c, 440.
- Angyal, S. J. and Odier, L., Carbohydr. Res., 1983, 123, 23.
- Blunt, J.W., Murray, H., Hunro, G. and Paterson, A.J., Aus. J.Chem., 1976, 29, 1115.
- Gay, C.A. and Gebicki, J.M., Anal. Biochem., 2002, 304, 42.
- McCord, J.M. and Fridovich, I., J. Biol. Chem, 1969, 244, 6049.