- *Corresponding Author:
- Abdelbagi Alfadil
Department of Clinical Microbiology and Immunology, King Abdulaziz University, Jeddah, Makkah Province 21589, Saudi Arabia
E-mail: aegmusa@kau.edu.sa
| Date of Received | 27 September 2023 |
| Date of Revision | 11 March 2024 |
| Date of Acceptance | 10 July 2024 |
| Indian J Pharm Sci 2024;86(4):1230-1238 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Inflammation, an adaptive response bring about by factors like infections, harmful chemicals, and immune reactions, induces the release of inflammatory mediators. These mediators play a role in significant protein denaturation and aggregation, which exacerbates the inflammatory condition. The existing anti-inflammatory medications, including non-steroidal anti-inflammatory drugs, come with undesirable side effects. Thus, there is a need to explore alternative therapeutics that offer anti-inflammatory benefits similar to non-steroidal anti-inflammatory drugs but with fewer adverse effects. Quinoxaline derivatives, categorized as small molecules, have exhibited promising anti-inflammatory activity and stand out as a potential avenue for further exploration. The objective of this study was to examine the inflammatory activity of 3-hydrazinoquinoxaline-2-thiol in a rat paw model and compare it to the effects of diclofenac. The in vivo activity of 3-hydrazinoquinoxaline-2-thiol was assessed using a rat paw model. The study employed histopathological examination and enzyme-linked immunosorbent assay as investigative methods. The anti-inflammatory efficacy of 3-hydrazinoquinoxaline-2-thiol was evident in the rat paw model, exhibiting a significant difference between the group treated with 3-hydrazinoquinoxaline-2-thiol and the positive control. Furthermore, no significant difference was observed between the group treated with 3-hydrazinoquinoxaline-2-thiol and the diclofenac group. Remarkably, the administration of 3-hydrazinoquinoxaline-2-thiol therapy resulted in a notable improvement in the inflammatory response, characterized by enhancements in inflammatory cell activity and the restoration of histological structures to a normal state. Suggesting that 3-hydrazinoquinoxaline-2-thiol has a good anti-inflammatory effect. The results of the study indicate that the topical administration of a hydrogel incorporating 0.2 % 3-hydrazinoquinoxaline-2-thiol (quinoxaline derivative) has proven to be successful in mitigating acute inflammation in the rat paw model. Subsequent research endeavors should delve into investigating different dosages of quinoxaline derivative with the aim of formulating a cost-effective and less toxic anti-inflammatory medication, potentially enhancing its utility in clinical settings.
Keywords
Inflammation, inflammatory mediators, non-steroidal anti-inflammatory drugs, 3-hydrazinoquinoxaline-2-thiol, rat paw, diclofenac, quinoxaline derivative
Inflammation represents an adaptive physiological response triggered by various factors, including infections, exposure to harmful chemicals, and immune reactions[1]. This response is initiated in the presence of tissue damage and manifests through observable symptoms such as swelling, redness, warmth, and pain, potentially leading to a decline in tissue functionality[2]. The inflammatory process involves the dilation of blood vessels and heightened activity of white blood cells, accompanied by the release of inflammatory mediators[3]. If left unaddressed, acute inflammation has the potential to evolve into chronic inflammation, a persistent and often prolonged state[4]. Chronic inflammation is closely linked to various health conditions, including diabetes, cardiovascular dysfunctions, cancer, and arthritis, but not limited to these[5]. The prolonged activation of the immune system and the sustained release of inflammatory mediators characterize chronic inflammation, contributing to the pathogenesis of these diverse and often serious medical conditions[5]. Recognizing the transition from acute to chronic inflammation is crucial for understanding the underlying mechanisms of these health issues and developing targeted interventions for their prevention and management[4].
The process of inflammation triggers the release of inflammatory mediators, encompassing Interleukin-1Beta (IL-1β), IL-6, C-Reactive Protein (CRP), and Tumor Necrosis Factor-Alpha (TNF-α). Notably, these mediators are found in both acute and chronic inflammatory conditions. Their presence plays a pivotal role in inducing substantial protein denaturation and aggregation, thereby amplifying the inflammatory response[6]. Additionally, inflammation sets in motion oxidative stress, which, in turn, serves as a catalyst for the escalation of protein denaturation. This interconnected relationship underscores the complexity of the inflammatory cascade, highlighting the multifaceted impact that inflammatory mediators and oxidative stress collectively exert on the progression and perpetuation of inflammation[7]. In the broader context, inflammation and its repercussions can set in motion a self-perpetuating cycle of deteriorating health conditions. The key strategy for effectively managing both acute and chronic inflammation is to identify and isolate phytochemicals with antioxidant properties and the ability to inhibit protein denaturation. These phytochemicals hold significant therapeutic promise in addressing the complexities of inflammatory processes[8]. An integral aspect of the inflammatory pathway involves Cyclooxygenase (COX), a key enzyme in prostaglandin biosynthesis, with two distinct isoforms, COX-1 and COX-2[9]. Conventional Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) function as non-selective inhibitors of these enzymes. Furthermore, selective COX-2 inhibitors are employed in the treatment of inflammatory diseases, aiming for a more targeted approach[10,11]. Another class of drugs, glucocorticoids, is frequently utilized for managing inflammation. Despite the associated side effects, glucocorticoids play an indispensable role in mitigating inflammation. Breaking the cycle of inflammation-related deterioration involves a multifaceted approach, including the exploration of phytochemicals with specific properties, the targeting of COX isoforms, and the cautious use of glucocorticoids. This comprehensive strategy is vital for addressing the intricate dynamics of inflammation and fostering effective therapeutic interventions[12]. In drug development, molecular design aims to create innovative compounds characterized by optimal efficacy and minimal side effects. The foundational quinoxaline scaffold plays a pivotal role in the synthesis of substances boasting diverse biological activities. Quinoxalines have been recognized for a spectrum of functions, encompassing antimicrobial[13], anti-inflammatory[14], bactericidal, bacteriostatic[15], anticonvulsant[16], Central Nervous System (CNS) depressant, antitumor[17], antioxidant[18], and neuroprotective properties[19].
The synthesis of quinoxalines can be achieved through various routes, and they are alternately referred to by different names such as 1,4-benzodiazine, benzopyrazine, and 1,4-diazonaphthalene[20,21]. Over the years, dedicated efforts within the domains of organic and medicinal chemistry have been consistently directed toward the design, synthesis, and evaluation of molecules specifically tailored to exhibit anti-inflammatory properties. This persistent exploration reflects a commitment to advancing the understanding of molecular structures that can contribute to the development of therapeutics with targeted anti-inflammatory effects[22].
The importance of quinoxaline molecules in medical research cannot be overstated. In light of the pressing need for alternative treatment modalities, our research is centered on a particular Quinoxaline Derivative (QD) (3-hydrazinoquinoxaline-2-thiol). This specific derivative, denoted as QD and characterized by the molecular formula C8H8N4S, is the focal point of our investigation. The primary aim of our study is to explore the potential anti-inflammatory effects of QD in a rat model and compare them to those of diclofenac. Additionally, we conducted a comprehensive assessment of the compound's in silico pharmacokinetic properties, scrutinizing aspects such as lipophilicity, skin permeation, and its overall toxicity profile. Through this evaluation, our intent is to gauge and appraise the therapeutic viability of QD.
Materials and Methods
Chemicals:
The compound with the molecular formula C8H8N4S, identified by the Chemical Identifier (CID) 781248 and coded as QD, as well as carrageenan, was procured commercially from Sigma Chemical in Munich, Germany. Diclofenac gel, specifically Voltaren Emulgel 1 % 50 g, was obtained from a local pharmacy. The ketamine injection, supplied by Hikma Pharmaceuticals PLC at a concentration of 10 mg/ml (10 ml), was sourced from the pharmacy department at King Abdul-Aziz University Hospital (KAUH). Additionally, xylazine hydrochloride was utilized in the studyIt is imperative to note that all solvents and reagents used in the experimentation were of pharmaceutical grade, ensuring the highest quality and standards for the research.
Preparation of plain and medicated hydrogel:
The formulation of the unmedicated Hydroxypropyl Methylcellulose (HPMC) gel at a concentration of 2 % w/w, totaling 100 g, commenced by dissolving 2 g of HPMC in an adequate amount of distilled water to achieve the desired weight. The meticulous procedure involved precisely measuring 2 g of HPMC, transferring it to a suitable beaker, and adding 98 g of distilled water. This mixture was covered and allowed to sit undisturbed for a duration of 24 h, facilitating complete hydration of HPMC and the formation of a uniform gel. In the case of the medicated gel, which incorporated the QD at 0.2 % w/w, the formulation process was executed with precision. Initially, 20 mg of QD was dissolved in 1g of Dimethyl Sulfoxide (DMSO). Subsequently, this solution was judiciously integrated into 9 g of the previously prepared plain HPMC gel with a continuous and gentle stirring mechanism to ensure a homogeneous distribution of the medication within the gel matrix. Both the unmedicated and QD-infused gels were then carefully transferred into suitable containers and stored under refrigeration at a temperature of 8° to preserve their stability and efficacy. It is noteworthy that these procedures adhere to established compounding standards prevalent in the United States Pharmacopeia (USP) references for pharmaceutical formulations[23,24].
Animals:
For this investigation, a total of 24 adult male Wister albino rats, weighing between 150 g and 200 g, were procured from the Faculty of Pharmacy at King Abdul-Aziz University. The rats were acclimated to standard laboratory conditions, provided with a regular laboratory diet, and had unrestricted access to water. Their housing environment adhered to a 12 h light-dark cycle, and the room temperature was consistently maintained between 22° and 25° within the animal house. Ethical considerations were paramount in the execution of all animal experiments. The biomedical ethics committee, having reviewed and approved the experimental protocol at the faculty of pharmacy, King Abdulaziz University, granted approval under the reference number (PH-1443-76). This ensured that the research involving the use of these animals was conducted in accordance with established ethical standards and guidelines.
Experimental design:
The experimental design involved the categorization of rats into four distinct groups, each comprising six animals (n=6), as follows: Group A served as the healthy control and received a subcutaneous injection of normal saline solution. Group B received carrageenan to induce inflammation and were treated with plain HPMC gel. Group C also received carrageenan and was subsequently treated with a gel containing 0.2 % w/w of the QD; and group D received carrageenan followed by treatment with a gel containing 1 % w/w of the anti-inflammatory drug diclofenac. This design aimed to evaluate the comparative efficacy of the treatments in mitigating inflammation induced by carrageenan.
To ensure the well-being and comfort of the animals during the procedures, all rats were anesthetized using a combination of xylazine hydrochloride (3 mg/kg) and ketamine hydrochloride (50 mg/kg) administered intraperitoneally. The control group received a subcutaneous injection of 0.2 ml saline on the plantar surface of the right hind paw. Groups B, C, and D received a similar injection of 0.2 ml carrageenan (1 % solution in isotonic saline) in the same location to induce inflammation, following the methodology outlined by Morris (2003). 1 h prior to the induction of inflammation, the respective gels (plain, QD, or diclofenac gels) were applied to the hind paws of the rats in Groups B, C, and D. The experiment concluded 4 h after the carrageenan injection. The rats were then anesthetized, euthanized, and their right hind paws were collected. Paw collection involved cutting the paws at the proximal end of the lateral malleolus. For subsequent histological examinations, portions of the tissue were preserved in a 10 % formalin solution for 24 h. Additionally, for biochemical analyses, other tissue samples were rinsed with ice-cold buffer, rapidly frozen in liquid nitrogen, and stored at -80° until further analysis[25,26].
Quantification of chosen inflammatory markers using an Enzyme-Linked Immunosorbent Assay (ELISA) methodology:
In the evaluation of inflammation, we employed ELISA immunoassay kits, specifically utilizing the ELISA inos kit. This kit comprised ELISA plates that were pre-coated with monoclonal antibodies designed to target the specific cytokine under investigation. The methodology employed a quantitative sandwich enzyme immunoassay technique. To precisely determine the levels of the selected inflammatory marker, standards, and samples were added to the wells of the ELISA plate. Following this, any unbound substances in the wells were removed using a wash buffer. Subsequently, an enzyme-linked polyclonal antibody specifically designed to target the cytokine of interest was introduced into the wells. After the addition of the enzyme-linked antibody reagent, washing steps were performed to eliminate any unbound antibodies. Finally, a substrate solution was introduced into the wells, and the development of color was terminated. The intensity of the resulting color was directly proportional to the quantity of the targeted cytokine that had bound, and this was quantified using a microplate reader[27].
Histopathological study:
Upon completion of the 7 d regimen and the administration of the final dose of the anti-inflammatory agent or saline solution, the animals were humanely euthanized. Euthanasia was carried out using an overdose of a ketamine-xylazine cocktail administered via intraperitoneal injection, followed by cervical dislocation. The tongues were extracted and fully immersed in Bouin solution for a minimum of 48 h to achieve fixation. Subsequently, the tongues were collected from the euthanized animals, fixed in a 20 % formalin solution, and embedded in paraffin. Thin 5 μm sections were meticulously prepared using a Leica microtome (Leica Microsystems Inc., Buffalo, NY, United States of America (USA)). These sections underwent staining with Hematoxylin and Eosin (H&E) as well as Periodic Acid-Schiff (PAS) stains to facilitate examination under a light microscope (Olympus Optical Co., Ltd., Japan) for comprehensive histopathological analysis[26].
Statistical analysis:
Statistical analysis for this study was conducted using GraphPad Prism. Each experiment was replicated at least twice, and average values were calculated. Unpaired t-tests were employed to evaluate significant differences and perform statistical comparisons across the experimental groups. A significance level of 0.05 was employed to determine statistical significance, with values equal to or less than 0.05 considered significant. The p-values were represented as follows: *p<0.05, **p<0.01, ***p<0.001, and ****p<0.0001.
Results and Discussion
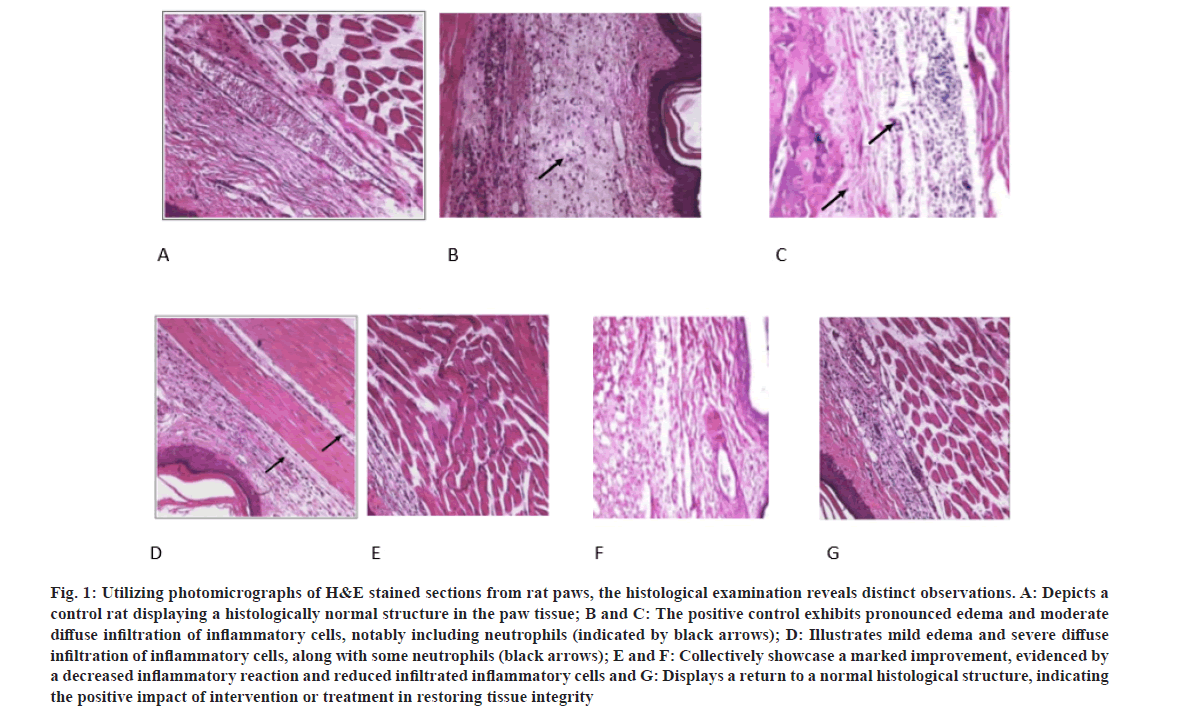
In our pursuit to comprehend the clinical manifestations associated with concurrent inflammation in the rat paw, we initiated a comprehensive examination utilizing histopathological analysis of tissue specimens. The histological landscape of the rat paw is distinctly characterized by identifiable tissue lesions, discernible in sagittal sections. These lesions exhibit a pathological spectrum, including prominent edema and a moderately diffuse infiltration of inflammatory cells, particularly neutrophils. Additionally, moderate lesions were observed, illustrating mild edema and severe diffuse infiltration of inflammatory cells, accompanied by a notable presence of neutrophils.
Significantly, upon the administration of 3-hydrazinoquinoxaline-2-thiol to the rat, histopathological slides unveiled notable enhancements in tissue conditions. These improvements were conspicuous, manifesting as a marked amelioration evidenced by a diminished inflammatory reaction and a reduction in infiltrated inflammatory cells, ultimately showcasing a restoration to a histological structure indicative of normalcy. This evidence underscores the positive impact of intervention or treatment in the restoration of tissue integrity, emphasizing the potential therapeutic efficacy of 3-hydrazinoquinoxaline-2-thiol in mitigating inflammatory responses in the rat paw model (fig. 1).
Fig. 1: Utilizing photomicrographs of H&E stained sections from rat paws, the histological examination reveals distinct observations. A: Depicts a control rat displaying a histologically normal structure in the paw tissue; B and C: The positive control exhibits pronounced edema and moderate diffuse infiltration of inflammatory cells, notably including neutrophils (indicated by black arrows); D: Illustrates mild edema and severe diffuse infiltration of inflammatory cells, along with some neutrophils (black arrows); E and F: Collectively showcase a marked improvement, evidenced by a decreased inflammatory reaction and reduced infiltrated inflammatory cells and G: Displays a return to a normal histological structure, indicating the positive impact of intervention or treatment in restoring tissue integrity.
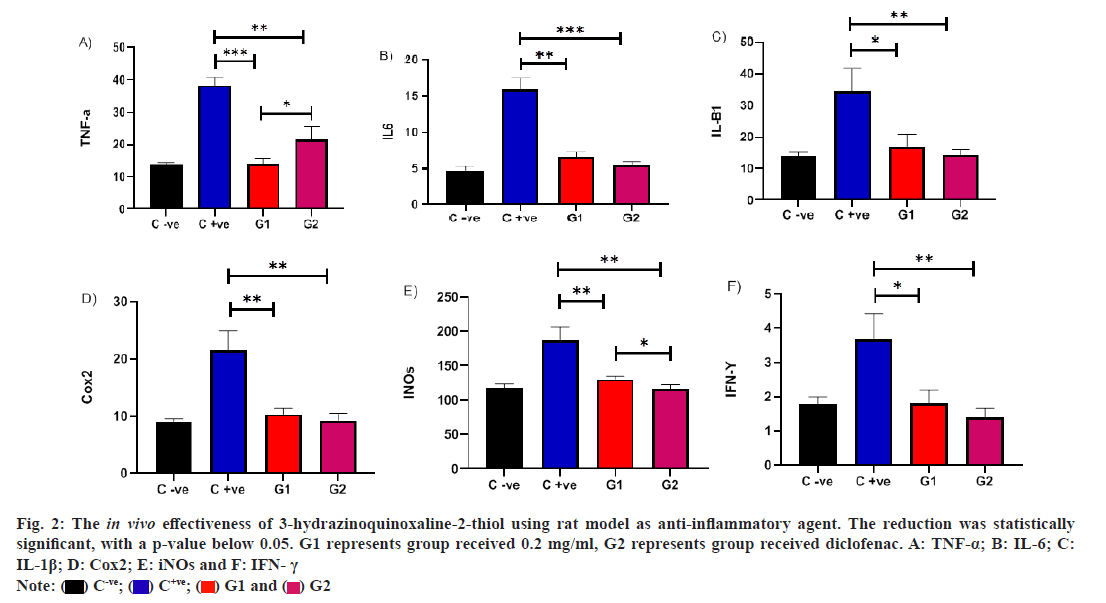
In a rat model, we evaluated the in vivo efficacy of QD in treating carrageenan-induced inflammation. Administering QD at concentrations (0.2 mg/ml) led to a notable decrease in TNF-α levels compared to the untreated infected rat group, with a p-value below 0.05. Additionally, a significant difference was observed between rats treated with diclofenac and those treated with QD. This indicates that QD exhibits anti-inflammatory effects akin to diclofenac against TNF-α, underscoring its potential to regulate immune responses during inflammation in vivo. Furthermore, a noteworthy decrease in IL-6 levels was evident in the QD-treated group compared to the untreated infected rat group, with a p-value below 0.05. Interestingly, the reduction in IL-6 levels was more pronounced in rats treated with diclofenac compared to those treated with QD. However, no significant difference was observed between these treated groups. Similarly, we observed a parallel outcome for IL-6 in the QD treated group. The decrease in IL-1β levels was apparent in the QD treated group compared to the untreated infected rat group, with a p-value below 0.05. Notably, the reduction in IL-1β levels was more pronounced in rats treated with diclofenac compared to those treated with QD. Nonetheless, no significant difference was observed between these treated groups.
Also, in a rat model, we conducted an assessment of the in vivo efficacy of QD in treating carrageenan-induced inflammation. The administration of QD at concentrations (0.2 mg/ml) resulted in a significant reduction in COX levels compared to the untreated infected rat group, with a p-value below 0.05. Notably, there was no significant difference observed between rats treated with diclofenac and those treated with QD. This suggests that QD possesses anti-inflammatory effects similar to diclofenac against COX, highlighting its potential to modulate immune responses during inflammation in vivo.
A noteworthy decrease in inducible Nitric Oxide synthase (iNOs) levels was observed in the QD-treated group compared to the untreated infected rat group, with a p-value below 0.05. Remarkably, the reduction in INOs levels was more pronounced in rats treated with diclofenac compared to those treated with QD. Importantly, a significant difference was observed between these treated groups. A significant reduction in Interferon?gamma (IFN?γ) levels was observed among the group treated with QD in comparison to the untreated infected rat group, with a p value below 0.05. Notably, the reduction in IFN?γ levels was more pronounced in rats treated with diclofenac compared to those treated with QD. However, there was no significant difference observed between these treated groups. This suggests that QD demonstrates anti-inflammatory effects comparable to diclofenac across various inflammatory markers, emphasizing its potential to modulate immune responses during inflammation in vivo (fig. 2).
Fig. 2: The in vivo effectiveness of 3-hydrazinoquinoxaline-2-thiol using rat model as anti-inflammatory agent. The reduction was statistically significant, with a p-value below 0.05. G1 represents group received 0.2 mg/ml, G2 represents group received diclofenac. A: TNF-α; B: IL-6; C: IL-1β; D: Cox2; E: iNOs and F: IFN- γ.
 .
.
Inflammation and pain persist as significant global issues, adversely affecting the overall well-being of numerous individuals worldwide. Moreover, the financial strain imposed by the high costs of pain inhibitors, including pain rehabilitation programs for chronic pain, exacerbates the challenge[28]. Presently, the existing medications for addressing inflammation and pain encompass NSAIDs and antinociceptive drugs. Although, these treatments frequently come with numerous adverse and toxic side effects. In cases where acute inflammation remains unresolved, a range of health complications may arise, potentially disrupting the normal functioning of the body's immune system. This can lead to the development of chronic inflammatory conditions such as rheumatoid arthritis and osteoarthritis[29]. Therefore, it is crucial to explore alternative therapeutics characterized by minimal side effects from a scientific standpoint. In a groundbreaking development, we have successfully showcased the anti-inflammatory effects of QD for the first time in a rat model. Histopathological analysis revealed a notable enhancement in the inflammatory response following QD therapy, characterized by increased inflammatory cell activity and the restoration of histological structures to normalcy. Additionally, we have presented evidence indicating that the inflammatory markers nearly reverted to baseline levels comparable to the negative control, with no statistically significant differences observed between the rats treated with QD and the negative control group. Furthermore, our findings revealed that the effectiveness of QD in reducing inflammatory markers is nearly equivalent to that of diclofenac, as no statistically significant distinctions were recorded between the two treatment groups. Notably, we observed that QD exhibited superior efficacy in reducing TNF-α compared to rats treated with diclofenac. Conversely, the reduction in iNOS was more pronounced in the group treated with diclofenac compared to the cohort treated with QD.
The exploration of novel anti-inflammatory therapies with minimal side effects presents an intriguing avenue for the development of new medicines. This approach is not only attractive but also imperative for addressing the limitations associated with existing treatments[30]. It has been reported that the methanol leaf extract derived from Ximenia caffra (X. caffra)has been demonstrated to exert inhibitory effects on the mRNA expression of pro-inflammatory genes, specifically IL-6, iNOS, and TNF-α, as assessed through Reverse Transcription-quantitative Polymerase Chain Reaction (RT-qPCR) in vitro. Notably, this extract exhibited a remarkable dose-dependent reduction of approximately 60 % in Nuclear factor kappa B (NF-κB) transcriptional activity compared to the control. Moreover, the extract elicited a substantial decrease in the expression of IL-6, reaching an almost 100 fold reduction when compared to untreated cells induced with lipopolysaccharide[31]. In a separate study, Aspalathus linearis, commonly known as rooibos tea, demonstrated significant efficacy in decreasing TNF-α and IL-6 levels in the liver of mice, as highlighted by Oguntibeju et al[32]. These findings underscore the potential anti-inflammatory properties of X. caffra and Aspalathus linearis, suggesting their promising roles as therapeutic agents in modulating inflammatory responses[31,32]. Our results are consistent with prior studies, the imperative to scrutinize and establish the safety profile of QD remains a pivotal aspect of its evaluation. This careful examination contributes to the broader landscape of medical knowledge, enhancing our understanding of the potential benefits and risks associated with this medicine and ultimately fostering a safer and more effective therapeutic approach.
Emerging scientific investigations have brought to light a recently identified group of compounds, namely 4-alkoxy-6,9-dichloro triazolo[4,3-a]quinoxalines, showcasing notable inhibitory capabilities against pro-inflammatory cytokines, particularly TNF-α and IL-6. The discernment of this inhibitory effect hints at a conceivable correlation between the efficacy of quinoxalines as anti-inflammatory agents and their specific targeting prowess directed at these cytokines. This revelation underscores the potential significance of QD in modulating inflammatory responses through the precise inhibition of key mediators like TNF-α and IL-6, thus presenting a promising avenue for further exploration in the realm of anti-inflammatory therapeutics[14]. The preceding investigation aligns with our own research findings, wherein we demonstrated the capacity of the QD to diminish inflammatory markers, encompassing but not limited to COX, IFN-γ, IL-6, IL-1β, iNOS and TNF-α. This evidence strongly implies an anti-inflammatory effect exerted by QD. However, a comprehensive exploration of the underlying mechanistic pathways and safety profiles is imperative to validate and elucidate the potential therapeutic implications of QD in the context of inflammation.
Topical drug administration targets localized areas through routes such as ophthalmic, rectal, vaginal, and skin applications. The skin, being easily accessible, is the primary route for delivering medications, especially for treating fungal infections like athlete's foot and ringworm. It has been shown that miconazole, an imidazole antifungal, effectively treats both local and systemic fungal infections, but its oral use is limited due to significant side effects. To enhance patient compliance, reduce dosage, and minimize side effects, a topical gel formulation of miconazole was developed. This formulation was characterized for drug content, pH, viscosity, diffusion, antifungal activity, and skin irritation, with the F1 formulation identified as the best[33]. Topical drug administration provides a localized method for delivering medication through various routes, including ophthalmic, rectal, vaginal, and skin applications. Among these, the skin is the most accessible and commonly used route for topical therapies, especially for treating dermatological conditions such as fungal infections, including athlete’s foot, ringworm, and candidiasis. Miconazole, an imidazole antifungal agent, is effective in treating both local and systemic fungal infections. However, the oral administration of miconazole is often associated with undesirable side effects, such as liver and kidney damage. To address these issues and improve patient outcomes, a topical gel formulation of miconazole has been developed. This approach aims to provide a more effective and targeted delivery of the antifungal medication directly to the affected area. The gel formulation offers several advantages; it enhances patient compliance by making the application process more convenient and less intrusive compared to oral medications. Additionally, it allows for a reduced dosage of the drug while minimizing systemic side effects, which are often a concern with oral administration[33]. This could enhance using gel formulation it may represent a significant advancement in topical antifungal therapy. It enhances the drug’s delivery to the site of infection, reduces the required dose, and minimizes the risk of systemic adverse effects, making it a promising approach for more effective and safer treatment of fungal infections. Another study has shown possible novel antifungal agents. Study focused on developing a topical hydrogel formulation of oxiconazole nitrate, an antifungal agent. It demonstrated good antifungal activity with a 20 mm zone of inhibition for Aspergillus Niger[34].
Conclusion
For the first time, the study's outcomes indicate that the topical application of a hydrogel containing 0.2 % QD has demonstrated efficacy in mitigating acute inflammation in a rat paw model. This anti-inflammatory effect is likely attributable to the inhibition of the inflammatory marke such as iNOS and COX. As a result, QD emerges as a promising candidate for a potent anti-inflammatory agent. Nevertheless, further in depth investigations into the underlying mechanisms are essential to substantiate these initial observations. Future research endeavors should delve into exploring different dosages of QD with the aim of developing an economically viable and less toxic anti-inflammatory medication, potentially yielding greater clinical benefits.
Funding:
This project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, under grant no. (G235-1440-1441). The authors therefore acknowledge with thanks DSR for their technical and financial support.
Conflict of interest:
The authors declared no conflict of interests.
References
- Medzhitov R. Origin and physiological roles of inflammation. Nature 2008;454(7203):428-35.
[Crossref] [Google Scholar] [PubMed]
- Smith LL. Acute inflammation: The underlying mechanism in delayed onset muscle soreness? Med Sci Sports Exerc 1991;23(5):542-51.
[Google Scholar] [PubMed]
- Spector WG, Willoughby DA. The inflammatory response. Bacteriol Rev 1963;27(2):117-54.
[Crossref] [Google Scholar] [PubMed]
- Voscopoulos C, Lema M. When does acute pain become chronic? Br J Anaesth 2010;105:i69-85.
[Crossref] [Google Scholar] [PubMed]
- Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, et al. Chronic inflammation in the etiology of disease across the life span. Nat Med 2019;25(12):1822-32.
[Crossref] [Google Scholar] [PubMed]
- Abdulkhaleq LA, Assi MA, Abdullah R, Zamri-Saad M, Taufiq-Yap YH, Hezmee MN. The crucial roles of inflammatory mediators in inflammation: A review. Vet World 2018;11(5):627.
[Crossref] [Google Scholar] [PubMed]
- Arndt D, Unrine J. Redox interactions between nanomaterials and biological systems. Oxid Stress Biomater 2016:187-206.
- Kong M, Xie K, Lv M, Li J, Yao J, Yan K, et al. Anti-inflammatory phytochemicals for the treatment of diabetes and its complications: Lessons learned and future promise. Biomed Pharmacother 2021;133:110975.
[Crossref] [Google Scholar] [PubMed]
- Tanabe T, Tohnai N. Cyclooxygenase isozymes and their gene structures and expression. Prostaglandins Other Lipid Mediat 2002;68:95-114.
[Crossref] [Google Scholar] [PubMed]
- Bertolini A, Ottani A, Sandrini M. Selective COX-2 inhibitors and dual acting anti-inflammatory drugs: critical remarks. Curr Med Chem 2002;9(10):1033-43.
[Crossref] [Google Scholar] [PubMed]
- Jahnavi K, Reddy PP, Vasudha B, Narender B. Non-steroidal anti-inflammatory drugs: An overview. J Drug Deliv Ther 2019;9(1-s):442-8.
- Barnes PJ. Glucocorticosteroids: Current and future directions. Br J Pharmacol 2011;163(1):29-43.
[Crossref] [Google Scholar] [PubMed]
- Kaplum V, Cogo J, Sangi DP, Ueda-Nakamura T, Corrêa AG, Nakamura CV. In vitro and in vivo activities of 2, 3-diarylsubstituted quinoxaline derivatives against Leishmania amazonensis. Antimicrob Agents Chemother 2016;60(6):3433-44.
[Crossref] [Google Scholar] [PubMed]
- Guirado A, Sánchez JI, Ruiz-Alcaraz AJ, Bautista D, Gálvez J. Synthesis and biological evaluation of 4-alkoxy-6, 9-dichloro [1, 2, 4] triazolo [4, 3-a] quinoxalines as inhibitors of TNF-α and IL-6. Eur J Med Chem 2012;54:87-94.
[Crossref] [Google Scholar] [PubMed]
- Parhi AK, Zhang Y, Saionz KW, Pradhan P, Kaul M, Trivedi K, et al. Antibacterial activity of quinoxalines, quinazolines, and 1, 5-naphthyridines. Bioorg Med Chem Lett 2013;23(17):4968-74.
[Crossref] [Google Scholar] [PubMed]
- Elhelby AA, Ayyad RR, Zayed MF. Synthesis and biological evaluation of some novel quinoxaline derivatives as anticonvulsant agents. Arzneimittelforschung 2011;61(07):379-81.
[Crossref] [Google Scholar] [PubMed]
- Waring MJ, Ben-Hadda T, Kotchevar AT, Ramdani A, Touzani R, Elkadiri S, et al. 2, 3-Bifunctionalized quinoxalines: Synthesis, DNA interactions and evaluation of anticancer, anti-tuberculosis and antifungal activity. Molecules 2002;7(8):641-56.
- Burguete A, Pontiki E, Hadjipavlou?Litina D, Ancizu S, Villar R, Solano B, et al. Synthesis and biological evaluation of new quinoxaline derivatives as antioxidant and anti?inflammatory agents. Chem Biol Drug Des 2011;77(4):255-67.
[Crossref] [Google Scholar] [PubMed]
- Selvaraj B, Kim DW, Park JS, Kwon HC, Lee H, Yoo KY, et al. Neuroprotective effects of 2-heptyl-3-hydroxy-4-quinolone in HT22 mouse hippocampal neuronal cells. Bioorg Med Chem Lett 2021;49:128312.
[Crossref] [Google Scholar] [PubMed]
- Karami B, Khodabakhshi S. A facile synthesis of phenazine and quinoxaline (new 1, 4-benzo diazine) derivatives using magnesium sulfate heptahydrate as a catalyst. J Serb Chem Soc 2011;76(9):1191-8.
- Hojati SF, Nematdoust Z, Zeinali T. The preparation of quinoxaline and 2, 3-dihydropyrazine derivatives using selectfluor as an efficient and reusable catalyst. Iran Chem Commun 2015;3:6-15.
- Meshram MA, Bhise UO, Makhal PN, Kaki VR. Synthetically-tailored and nature-derived dual COX-2/5-LOX inhibitors: Structural aspects and SAR. Eur J Med Chem 2021;225:113804.
[Crossref] [Google Scholar] [PubMed]
- Khedekar YB, Mojad AA, Malsane PN. Formulation, development, and evaluation of silymarin loaded topical gel for fungal infection. J Adv Pharm 2019;8(01):6-9.
- Maanvizhi S, Iyyappan V, Bhavishi PG. Evaluation of an antifungal luliconazole gel formulation using semi-automatic diffusion cell apparatus and application of mathematical models in drug release kinetics. Eur J Mol Clin Med 2021;8(4):231-41.
- Halawi MH, Yassine W, Nasser R, Yusef H, Borjac J, Al Sagheer T, et al. Two weeks of chronic unpredictable stress are sufficient to produce oral candidiasis in balb/C mice. Asian J Microbiol Biotech Env Sci 2020;22(2):254-64.
- Takakura N, Sato Y, Ishibashi H, Oshima H, Uchida K, Yamaguchi H, et al. A novel murine model of oral candidiasis with local symptoms characteristic of oral thrush. Microbiol Immunol 2003;47(5):321-6.
[Crossref] [Google Scholar] [PubMed]
- Fathy M, Nikaido T. In vivo modulation of iNOS pathway in hepatocellular carcinoma by Nigella sativa. Environ Health Prev Med 2013;18:377-85.
[Crossref] [Google Scholar] [PubMed]
- Turk DC. Clinical effectiveness and cost-effectiveness of treatments for patients with chronic pain. Clin J Pain 2002;18(6):355-65.
[Crossref] [Google Scholar] [PubMed]
- Porcelli EG. Chronic inflammation. J Am Dent Assoc 2018;149(9):750-1.
[Crossref] [Google Scholar] [PubMed]
- Khumalo GP, Van Wyk BE, Feng Y, Cock IE. A review of the traditional use of southern African medicinal plants for the treatment of inflammation and inflammatory pain. J Ethnopharmacol 2022;283:114436.
[Crossref] [Google Scholar] [PubMed]
- Zhen J, Guo Y, Villani T, Carr S, Brendler T, Mumbengegwi DR, et al. Phytochemical analysis and anti?Inflammatory activity of the extracts of the African medicinal plant Ximenia caffra. J Anal Methods Chem 2015;2015(1):948262.
[Crossref] [Google Scholar] [PubMed]
- Oguntibeju OO. Medicinal plants with anti-inflammatory activities from selected countries and regions of Africa. J Inflamm Res 2018:307-17.
[Crossref] [Google Scholar] [PubMed]
- Chandrakala V, Mamatha HS, Usha A, Priya B. Formulation and evaluation of solid lipid nanoparticles-based gel containing miconazole nitrate (an antifungal agent). Adv Concepts Pharm Res 2024;5(2):108-19.
- Dombe S, Disale A, Pandhare R, Sutar S. Development and evaluation of antifungal gel by using natural polymer. Int J App Pharm 2018;7(12):960-77.