- *Corresponding Author:
- Z. Al-Qodah
Chemical Engineering Department, Al-Balqa Applied University, Amman, Jordan
E-mail: z_alqodah@hotmail.com
| Date of Submission | 23 July 2016 |
| Date of Revision | 19 January 2017 |
| Date of Acceptance | 19 May 2017 |
| Indian J Pharm Sci 2017;79(4):559-567 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
In the present study, fungi from waste water of different sources in Madinah, KSA, was isolated in order to determine the effective dose and shape of silver nanoparticles necessary for treating them. Waste water was collected from two sources, homes and hospitals (Ohud Hospital). Additionally, bottled drinking water (Taibah) and autoclaved distilled water was used as control. Uncoated silver nanoparticles were used. The particles were with two shapes (rod, cube) and four concentrations (0, 1, 10 and 100 μg/ml) were selected and distilled water was used as a solvent. Fungi were isolated and purified on potato dextrose agar media. A total of eight genera and nine fungal species were identified: Aspergillus flavus, A. niger, A. terreus, Fusarium oxysporium, F. solani, Geotrichum candidum, Mucor hiemalis, Penicillium chrysogenum, Rhizopus oryzae, Trichoderma harizianum and Trichophyton sp., Aspergillus sp. was the highest in its number collectively, thus was considered for further experiment. Silver nanoparticles were tested on two Aspergillus sp. i.e. A. niger and A. terreus. The gradual growth reduction was clear in both Aspergillus species as the concentration of the silver nanoparticles increased. A. terreus had higher reduction compared to A. niger. No significant differences were found among the 1, 10, and 100 µg/ml concentrations. The rod shaped nanoparticles showed less growth for the fungi studied compared to the cube shaped. It is possible to use silver nanoparticles as antifungal substances; however, more considerations should be taken.
Keywords
Silver nanoparticles, fungi, Madinah, Aspergillus sp.
Water is considered one of the main components in the development processes in the Kingdom of Saudi Arabia (KSA). Problems associated with water demands include the increase in the number of private and farming wells, uncontrolled pumping, water quality deterioration, uncontrolled agricultural practices, overirrigation all of which increase soil salinity and leakage from water supply systems [1].
Water resources in the Kingdom include surface water (10-48%), ground water (49-80%), desalinated water (3-6%), and reclaimed water (0-5%) [2]. A combination of growing population and limited potable water resources [3] are reducing the availability and quality of drinking water. In addition, problems resulting from the disposal of waste water continue to appear. Therefore, waste water management practices are vital. Increasing the safe use of recycled water can greatly assist in meeting water requirements, enhance the environment and benefit public health by preserving resources upon which public health protection is based [3].
Water contains soluble and insoluble ingredients such as different salts, gases, plants, and microorganisms. These components are considered impurities in water and their presence may be useful to a certain limit, however, when increased, it may become harmful and preventive measures become needed [4].
Waste water is the water that contains unwanted substances, which affect its quality and thus making it unsuitable for use [5]. Waste water is considered an important source for agricultural use in KSA because of the expected high demand on water in the coming 25 y [6]. Additionally, the population of the Kingdom is expected to reach 45 million after 10 y, and the expected waste water will account for 67% of the total used water in agriculture. However, the use of waste water has disadvantages. It may cause health problems for human and animals. Contaminates in ground water and waste water includes heavy metals and chemicals that affect not only humans, but also animals and plants [7].
Many microorganisms have been reported in waste water in published studies. The most isolated bacterial genera from waste water were Escherichia coli, Proteus vulgaris, Pseudomonas aeruginosa, and Acinetobacter radioresistens [8]. Dematiaceous fungi constituted 63% of the isolated fungi, and included Cladosporium (27%), Phoma (9%), Alternaria (7%), and Exophiala (7%) [9]. In another study, the most prevalent genera of fungi were Penicillium, Aspergillus, Acremonium, and Candida [10]. Moreover, viruses such as poliovirus, coxsackie virus, rotavirus, echovirus, and hepatitis virus [11], and nematodes Ascaris sp., hookworms [12], parasites and protozoa [13] may also exist in waste waters and their existence is considered risky to human beings and requires high attention.
Various methods have been used to resolve water quality problems in natural environments [14,15]. Nowadays, intensive research is conducted to use nanotechnology in water purification [5]. Nanotechnology is used in detecting and removing different pollutants from waste water as a treatment method [16]. When using nanoparticles as adsorbents, nano filtration membranes remove or separate pollutant from water, whereas when using nanoparticles as catalysts for chemical or photochemical oxidation, it affect the destruction of contaminants present [17].
Nanoparticles are one of those methods with great interest due to properties that makes them a good choice for many disciplines [18] such as biology, medicine [19], and much more. Gold, silver, copper, magnesium and other materials have been used as nanoparticles against microbes, however, silver was the most efficient [20]. Silver nanoparticles (AgNPs) are currently attracting and increasing attention in many applications including wastewater treatment. These powders have shown unique such as large surface area, quantum confinement and high stability. The most applied method for AgNPs preparation is by the reduction of Ag+ in aqueous solution. This reduction usually yields colloidal AgNPs with diameters of several nanometres. This colloidal behaviour causes their dispersion in water or organic solvents [21]. Different microbes vary in their response to AgNPs. For example, AgNPs have adverse effect against Gram-negative bacteria compared to Gram-positive due to the peptidoglycan layer thickness and charge [22,23]. The objectives of the current study were to isolate, identify, and quantify the fungal microorganisms in different waste water sources in Madinah and to determine the effective concentration dose and shape of nanoparticles necessary for treating the most prevalent fungus isolated from the different water sources.
Materials and Methods
Waste water was collected from two sources in Madinah: homes (raw water, primary treated, secondary treated, tertiary treated) from Water Authority in Al-Kheleel; and hospitals (Ohud Hospital). Additionally, bottled drinking water (Taibah brand from local market) was used based on a preliminary experiment where it showed few microbes among many drinking water sources. Moreover, autoclaved distilled water was used as control. Collection frequency was conducted every month for six months. Five replicates were conducted per water source. Temperature and relative humidity during the collection period was obtained. Temperature ranged from 16-38° and relative humidity was between 0.18-16% during the time of study.
Fungi isolation, purification, and identification
A 50 μl from each replicate were plated on potato dextrose agar (PDA) media (HiMedia Laboratories Pvt. Ltd), and then incubated at 25° for one week. After that, fungal colonies were purified on PDA and long term stored at -20° for further use. The isolates were identified morphologically using Barnett and Hunter [24].
AgNPs treatment
Same AgNPs used in Alananbeh et al. [25] were used here. Uncoated AgNPs (15±3 nm mean diameter) were purchased from Nano Tech, Egypt and were at least 99.99% pure. Chemical reduction method was used to prepare the particles [26]. A solution of AgNO3 was used as the Ag+ ion precursor and sodium borohydride was used as a mild reducing and stabilizing agent. The AgNPs solution had grayish yellow color, indicating the reduction of Ag+ ions to AgNPs. The particles were with two shapes (rod, cube) and had an optical absorption peak at 410 nm, and four concentrations (0, 1, 10, 100 μg/ml) diluted with distilled water were used. Fungal species were selected based on their prevalence in the waste water samples collected.
Treatment assay
Each fungus was grown for two days on PDA, then a plug (5 mm) of mycelium was cut from the edges of the colony (hyphal tip) and placed on a new PDA Petri dishes. After that 50 μl of the two shapes and the four concentrations of AgNPs were added to the mycelium plug. Plates were incubated at 28° for 10 d. Mycelium growth measurement (diameter) was carried out every 2 d. Three replicates per concentration were conducted and the experiment was repeated twice. The fungal species chosen were studied at the same time. Ag-untreated samples were employed as controls for comparison.
Statistical analysis
Minitab 17 software was used for data analysis. Analysis of variance (ANOVA) (one way) and descriptive statistics including mean and standard deviation were used to study each factor considered in the collection separately. Means for each factor was grouped based on Tukey's method.
Results and Discussion
This discussion will consider the main results obtained in this investigation including total cell count, fungi count based on collection date, fungi count based on water source and AgNPs treatment. Fungal isolation was conducted monthly throughout 6 mon. The isolates were counted based on their numbers from the different water sources (Ohud Hospital, raw waste water, primary, secondary, tertiary, Taibah bottled water, and distilled water). A total of 4120 colony/1 ml were counted for the different species of fungi.
Based on the date of water samples collection, ANOVA showed significant differences among dates for fungi (Table 1). CFU of fungi (n=28) means were found to be the highest in January, however, no significant difference was found between January, November and December (Table 2). The CFU were lower and significant in February through April.
| Source | Df1 | Adj MS2 | F-value | P-value |
|---|---|---|---|---|
| Date | 5 | 1179.6 | 4.03 | 0.003 |
| Error | 66 | 292.7 | ||
| Water source | 5 | 1577.6 | 6.01 | 0.000 |
| Error | 66 | 262.5 |
1Degrees of freedom, 2adjusted mean square
Table 1: Total fungal CFU based on date and water source
| Variable | Total fungal CFU | ||
|---|---|---|---|
| Mean1 | SD2 | ||
| Date | 30/11/2013 | 15.92ab | 13.19 |
| 29/12/2012 | 12.92ab | 33.79 | |
| 28/01/2013 | 28.50a | 17.60 | |
| 26/02/2013 | 4.92b | 8.31 | |
| 28/03/2013 | 3.50b | 5.54 | |
| 26/04/2013 | 2.92b | 5.50 | |
| Water source | Ohud | 22.42a | 32.29 |
| Raw | 26.50a | 15.62 | |
| Primary | 15.08ab | 15.99 | |
| Secondary | 1.83b | 2.17 | |
| Taibah | 0.08b | 0.29 | |
| Tertiary | 2.75b | 5.31 | |
1Means followed with similar letters are not significantly different, 2standard deviation
Table 2: Means and grouping analysis for the fungal CFU based on date and water source
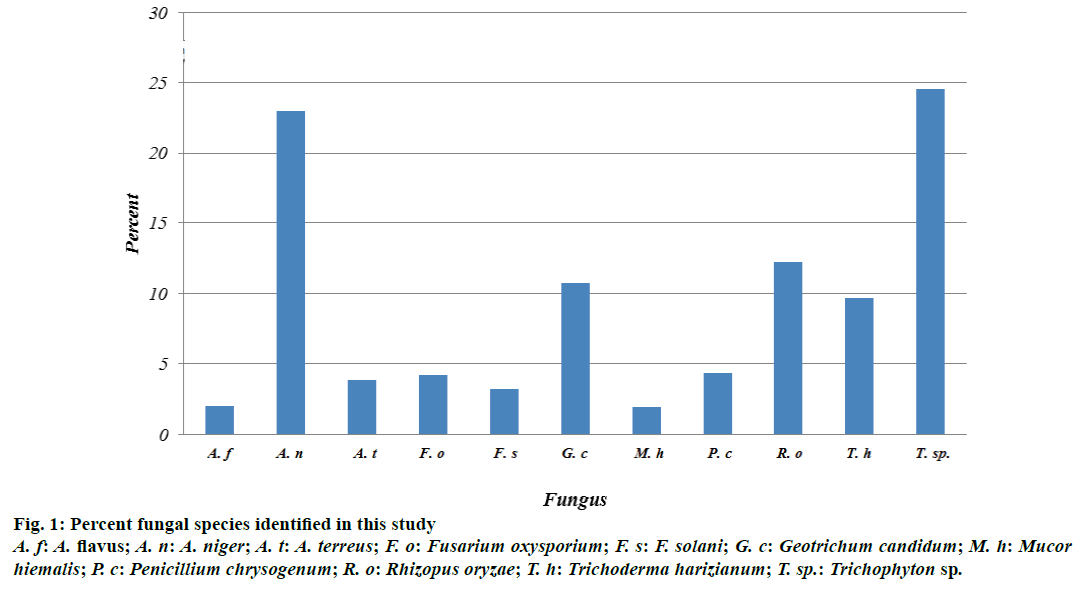
Fungal CFU were counted in the different sources of water samples collected. The highest number of fungal colonies was found in Ohud Hospital and raw waste water with a mean of 22 and 26, respectively. No significant differences were found between Ohud, raw and primary water sources (Table 2). There were eight genera and nine species identified (Figure 1). The percent of colonies for each species for the two replicates was as follows: A. flavus (2.06%), A. niger (22.94%), A. terreus (3.88%), Fusarium oxysporium (4.25%), F. solani (3.28), Geotrichum candidum (10.80), Mucor hiemalis (1.94%), P. chrysogenum (4.37%), Rhizopus oryzae (12.26%), Trichoderma harizianum (9.71%), and Trichophyton sp. (24.51%) (Figure 1). Aspergillus sp. was the highest in its number collectively.
Figure 1: Percent fungal species identified in this study
A. f: A. flavus; A. n: A. niger; A. t: A. terreus; F. o: Fusarium oxysporium; F. s: F. solani; G. c: Geotrichum candidum; M. h: Mucor hiemalis; P. c: Penicillium chrysogenum; R. o: Rhizopus oryzae; T. h: Trichoderma harizianum; T. sp.: Trichophyton sp.
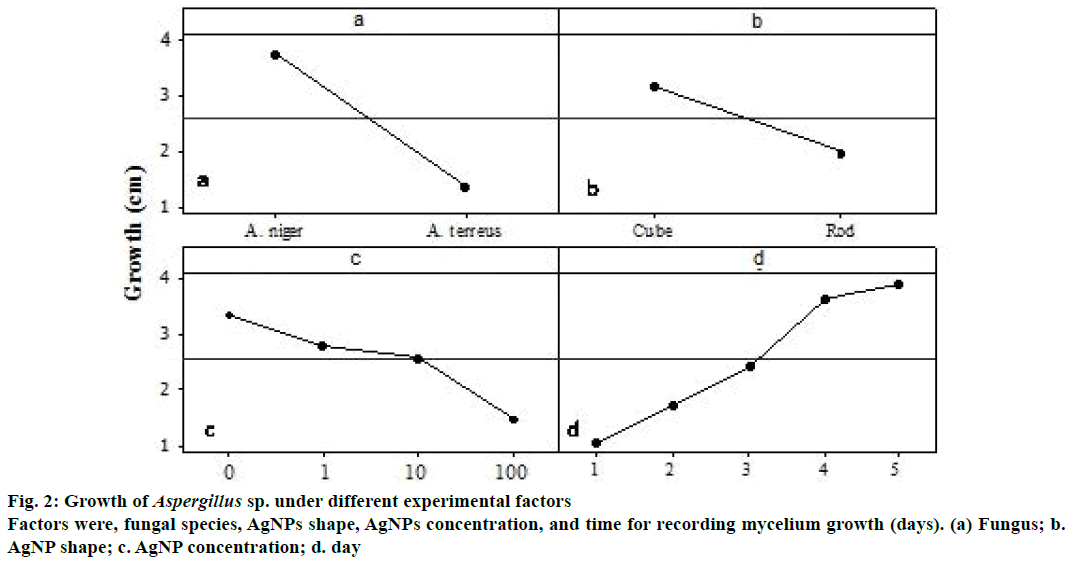
The fungus, A. niger was found to be the highest isolated either based on its number or based on its occurrence (Figure 2), therefore, it was chosen. Another species, A. terreus was also tested in response to AgNPs treatment.
Based on the ANOVA, growth reduction data for fungi studied, the model showed significance at α=0.05. There was no significance between the two experiments and replicates. However, fungus, shape, concentration, day and the interactions between the four factors were all highly significant at α=0.05 (Table 3).
| Source | DF | Mean square | F | P |
|---|---|---|---|---|
| Regression | 70 | 32.21 | 149.63 | 0.0000 |
| Experimenta | 1 | 0.12 | 0.50 | 0.4792 |
| Fungusb | 1 | 672.84 | 3125.31 | 0.0000 |
| Ag NP shape | 1 | 174.73 | 811.59 | 0.0000 |
| Ag Np concentration | 3 | 72.99 | 339.04 | 0.0000 |
| Day | 4 | 140.86 | 654.30 | 0.0000 |
| Replicate | 2 | 0.07 | 0.32 | 0.7251 |
| Fungus´AgNP shape | 1 | 2.09 | 9.72 | 0.0019 |
| Fungus´AgNp concentration | 3 | 22.86 | 106.16 | 0.0000 |
| Fungus´day | 4 | 65.56 | 304.51 | 0.0000 |
| AgNP shape´AgNp concentration | 3 | 45.63 | 211.93 | 0.0000 |
| AgNP shape´day | 4 | 2.06 | 9.57 | 0.0000 |
| AgNp concentration´day | 12 | 5.77 | 26.79 | 0.0000 |
| Fungus´AgNP shape´AgNp concentration | 3 | 13.24 | 61.50 | 0.0000 |
| Fungus´AgNP shape´day | 4 | 0.37 | 1.70 | 0.1500 |
| AgNP shape´AgNp concentration ´day | 12 | 1.72 | 8.01 | 0.0000 |
| Fungus´AgNP shape´Ag-Np concentration´day | 12 | 1.30 | 6.08 | 0.0000 |
| Error | 409 | 0.22 | ||
| Total | 479 |
aTwo experiments were conducted at the same time, btwo fungi were studied: A. niger, A. terreus, ctwo shapes were tested: rod and cube, dfive concentrations were studied: 0, 1, 10, and 100 μg/μl, ethree replicates were conducted for each concentration, fmeasurements were recorded five times after the fungi were treated (1,3, 5, 7, 9) days
Table 3: ANOVA for fungi mycelium growth inhibition data measured as diameter (cm)
There were differences between the Aspergillus sp. using AgNPs for growth reduction. A. terreus had higher reduction with a mean of 1.38 cm compared to A. niger with a mean of 3.74 cm (Figure 2a). There was difference between the rod and the cube nanoparticle shapes for growth reduction of fungus used. However, rod nanoprticles induced higher reduction with a mean of 1.96 cm compared to cube nanoparticles with a mean of 3.16 cm (Figure 2b).
Based on the nanoparticle concentration, there was gradual growth reduction as the concentration increases, however, no significant differences were found among the 1, 10, and 100 μg/μl concentrations, which had 2.82, 2.58, and 1.49 cm growth reductions, respectively (Figure 2c). There was significant difference between the five days with a mean of 1.06, 1, 75, 2.44, 3.63, and 3.91 cm for day 1, 2, 3, 4, and 5, respectively (Figure 2d).
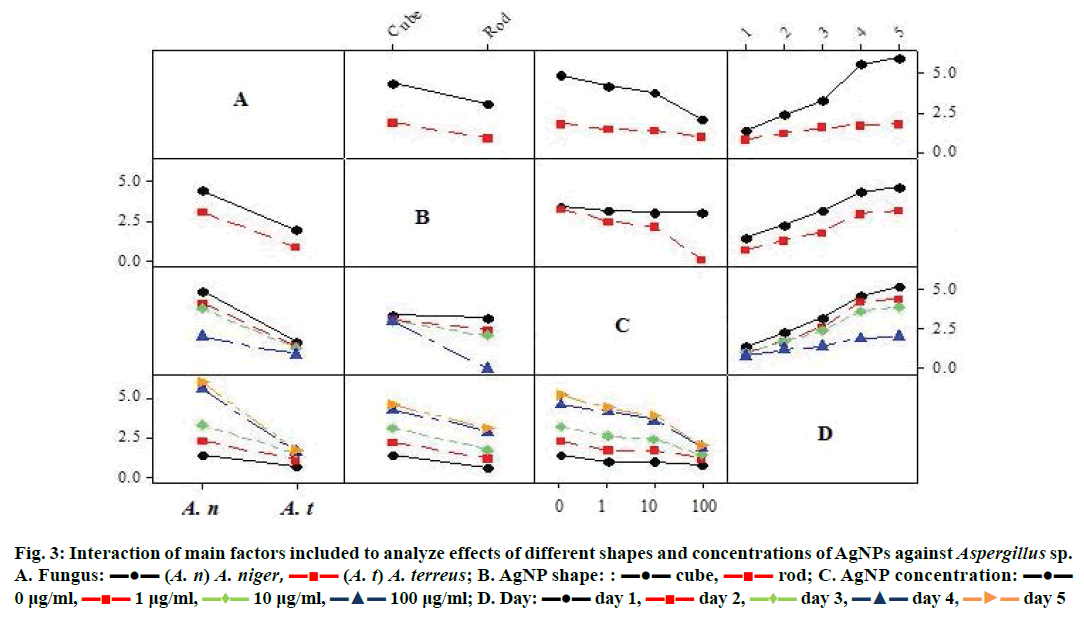
For the two shapes of AgNPs, there was significant difference of growth reduction for the two studied fungi. However, the interaction between the rod shape and the Aspergillus sp. showed 0.84 and 3.07 cm growth in the case of A. terreus and A. niger, respectively, compared to the cube×fungus effect, which had a mean growth of 1.91 and 4.41 cm for the A. terreus and A. niger, respectively (Figure 3).
Figure 3: Interaction of main factors included to analyze effects of different shapes and concentrations of AgNPs against Aspergillus sp.
A. Fungus: ??? (A. n) A. niger,  (A. t) A. terreus; B. AgNP shape: : ??? cube,
(A. t) A. terreus; B. AgNP shape: : ??? cube,  rod; C. AgNP concentration: ??? 0 μg/ml,
rod; C. AgNP concentration: ??? 0 μg/ml,  1 μg/ml,
1 μg/ml,  10 μg/ml,
10 μg/ml,  100 μg/ml; D. Day: ??? day 1,
100 μg/ml; D. Day: ??? day 1,  day 2,
day 2,  day 3,
day 3,  day 4,
day 4,  day 5
day 5
The gradual growth reduction was clear in both Aspergillus species as the concentration of the AgNPs increased. However, the interaction between the A. terreus and the 100 and 10 μg/ml concentration showed 0.93 and 1.32 cm growth, respectively, compared to the A. niger×concentration effect, which had a growth mean of 2.06 and 3.84 cm for the 100 and 10 μg/ml, respectively (Figure 3). The mycelium growth increased with days in both Aspergillus sp., however, the interaction was more in A. niger compared to A. terreus (Figure 3). The gradual growth reduction was clear in both shapes as the concentration of the nanoparticles increased. The interaction between the rod shape and the 100 and 10 μg/μl concentrations showed 0.00 cm and 2.11 cm growth reduction, respectively, compared to the cube×concentration effect, which had a growth mean of 2.99 and 3.05 cm for the 100 and 10 μg/ml, respectively (Figure 3). The mycelium growth increased with time in both shapes. However, the rod shape nanoparticles had less growth compared to the cubic shape (Figure 3). The whole possible interactions among the main effects (fungus, shape, concentration, days) were all highly significant (Figure 3, Table 3) except the fungus× shape×day interaction.
According to the best of our knowledge, the current study is considered the first for fungal genera and species isolation from waste water sources in the KSA over a period of time. Waste water can be re-used in agriculture and aquaculture. However, the health risks can be major from such practice. There are regulations of using waste water published by WHO in 2006 and also there are local measurements for countries in addition to KSA to monitor the use of waste water.
Different genera of fungi have been isolated from different waste water sources in Madinah. The number and the importance of the isolated microorganisms varied based on the type of waste water from which they were isolated. In April 2013, fungi numbers were more during the first three months then started to decrease. This could be attributed to the temperature at that period. The temperature during the collection period was within the range of 16-38°. In the cold months (November-February), fungal genera Asprigillus and Trichophyton sp. dominated the isolates, while in warmer months (March-April), A. niger was the most dominant microorganism isolated.
There are some studies in the literature reporting some correlation between the time and the type of microorganism. In one study, fungal flora of hospital tap water was evaluated in Iran [27] over a one year period. In that study, different fungal genera were identified: Aspergillus was the most recovered genera followed by Cladosporium and Penicillium. The colony counts varied based on the time of year where Aspergillus was more predominated in autumn, Cladosporium in winter and spring and Penicillium in summer. In another study a total of 340 taxa were isolated from drinking water [28], and filamentous fungi were found more in winter months, while bacteria and yeast were detected in warmer months. In that study, Penicillium and Acremonium were the most frequently isolated fungi. Drinking water quality is determined by its pathogenic bacteria content. However, the water-borne spores of different fungal genera became potential and more recognized [29].
As mentioned in the above results, the different waste water sources in Medina were used to isolate eight fungal genera and nine species. On the other hand, different fungal genera such as Phialophora sp., Cladosporium sp., R. stolonifer, Chaetomium sp., Alternaria sp., Aspergillus sp., were isolated from tap water in Jeddah city, KSA [28]. This study assured that bottled water has no microbes, whereas two other previous studies reported that bottled samples contaminated with bacteria [30,31].
Some of the isolated microorganisms like Aspergillus, Geotrichum, Clostridum, E. coli, Gardrenella vaginalis were found in human bodies and considered as natural flora to keep human body functioning normally. However, if their numbers become higher, they might cause sickness [32]. Aspergillus sp. may cause Aspergillosis, however certain species like A. flavus is 100-fold more virulent than other species and it is a toxin producer [27]. On the other hand, A. niger is considered non-pathogenic to human and is widely distributed in nature, however, it can colonize the human body as an opportunistic fungus especially in patients who have immunosuppressive treatment or with severe illness [33]. Similarly, Fusarium sp. [34], Trichodermas sp. [35], Penicillium sp. [36], Geotrichum sp. [37] are fungi that infect immunocompromised patients. Moreover, Mucor sp. is responsible about mucormycosis and Rizopus sp. about mucormycetes infections in humans with immunocompromised and immunocompatent individuals [38].
It should be noted that the number of isolated microbes varied according to the type of water. In this study, Ohud hospital and raw waste waters had the maximum number of fungi compared to the other water sources. Treated water (primary, secondary, and tertiary) also had fungi but less than raw and Ohud hospital waste waters. This was not surprising; hospital waste waters and the raw waste water are rich with different substances such as inorganic like different metals, nutrients nitrogen and phosphorus, organic matter, and oil and grease. These substances promote microbial growth. Moreover, organisms require small amounts of nutrients to support their growth, thus, even treated waste water may contain enough nutrients for microorganisms (Eutrophication) [39]. Sources of pathogens that were found in hospital waste waters and other water originate from people or animals carriers or infected with a disease and considered a huge risk to public health. Even municipal water and bottled water may contain risk for pathogens [30]. For that reason treating water is important to public health.
AgNPs effect was evaluated with two shapes and four concentrations against different Aspergillus species using the Petri dishes method. This method was simple and successful in studying the effect of AgNPs. It was successfully used as in disk diffusion method [40], agar over layer [41], and sol-gel method [42].
Results showed that rod shaped AgNPs at 100 μg/μl produced very high percent inhibition of the test fungi. However, other concentrations also inhibited fungal growth, but not as effectively as the 100 μg/μl concentration. These results suggested that rod AgNPs could be used as antifungal agents. Previous studies reported successful application of different AgNPs shapes and concentrations against fungi such as Fusarium, Aspergillus and Alternaria alternate, and results showed high inhibition [43]. Moreover, dermatophytes such as Trichophyton mentagrophytes [44] and T. rubrum [45], were also inhibited by AgNPs, and thus could be considered for clinical applications. AgNPs exhibit high antifungal activity against pathogenic Candida sp. at the concentrations of 1 mg/l of AgNPs [46]. Antifungal activity of the AgNPs was comparable with those of ionic silver. However, ionic silver remains cytotoxic at those concentrations that inhibit the growth of the tested yeasts. The AgNPs were found to inhibit growth of the yeasts at very low concentrations that are comparable to those of common antifungals, and they proved that the AgNPs exhibit no cytotoxic effects on human fibroblasts at these concentrations [47].
There are several explanations for the AgNPs mode of action. Metal depletion is one of AgNPs mode of actions that forms irregularly shaped pits in the outer membrane and change membrane permeability, which is caused by progressive release of lipopolysaccharide molecules and membrane proteins [48]. Although their inference involved some sort of binding mechanism that involves interaction between Ag nanoparticles and component(s) of the outer membrane is still unclear. Ag-generated free radicals was reported by using the electron spin resonance (ESR) of Ag nanoparticles [49]. Antimicrobial mechanism of Ag nanoparticles is related to the formation of free radicals and subsequent free radical-induced membrane damage. The antioxidant N-acetylcysteine was used to test whether the antioxidant could influence Ag nanoparticles-induced antimicrobial activity. Other modes of action include DNA condensation, and dehydration of microbial cell was suggested by other authors [50].
Toxicity is a concentration- and size-dependent. Moreover, effectiveness of AgNPs against microbes depends on its shape and size [46,47,51]. The antimicrobial activity varies as AgNPs sizes decrease; it was found that 7 nm was the most effective against bacteria [52] compared to the other sizes because of its small sizes, which can reach the bacterial nuclear content and can be in contact with more surface area [53]. In our study 50 nm was the size for the rod-shaped AgNPs, yet they inhibited the different microbes. This could be explained by the fact that larger AgNPs can persist longer and could serve as continuous Ag ions source [54,55].
This study showed that it is possible to use AgNPs as antifungal substances. AgNPs are considered less harmful, toxic, and cost-effective than other methods. However, in order to get more satisfied results for waste water treatments, future investigations are needed to study other particles against different microbes such as gold nanoparticles, to use smaller sizes of AgNPs to avoid the water characteristics alterations, to study more AgNPs shapes (sphere, triangular, beam) and concentrations, study methods for removing AgNPs from water either by filtration or by using non-pathogenic microorganisms to adsorb them, and combine AgNPs with other waste water treatment methods such as UV light, copper ions, or oxidizers, to test the possible synergistic effect.
Acknowledgments
Authors thank the Taibah University, Department of Biology for providing materials and space to conduct this study.
Conflict of interest
The authors declare no conflict of interest.
Financial support and sponsorship
Nil.
References
- http://www.idrc.ca/en/ev-93954-201-1-DO-TOPIC.html.
- Zaharani KH, Al-Shayaa M, Baig MB. Water conservation in the Kingdom of Saudi Arabia for better environment: implications for extension and education. Bulg J Agricul Sci 2011;17 : 389-95.
- Angelakis AN, Bontoux L. Waste water reuse and reclamation in European Countries. Water Pol 2001;3:47-59.
- Kulshreshtha SN. A global outlook for water resources to the year 2025. Water Res Manag 1998;12 : 167-84.
- Tiwari DK, Behari J, Sen P. Application of nanoparticles in waste water treatment. World Allied Sci J 2008;3:417-33.
- Kajenthira A, Sidiqqi A, Anadon LD. A new case for waste water reuse in Saudi Arabia: bringing energy into the water equation. J Environ Manage 2012;102:184-92.
- Mosleh YI, Almagrabi OA. Heavy metal accumulation in some vegetables irrigated with treated waste water. Int J Green Herb Chem 2013;2 : 81-90.
- Moura A, Henriques I, Ribeiro R, Correia A. Prevalence and characterization of integrons from bacteria isolated from a slaughterhouse waste water treatment plant. J Antimicrob Chemother 2007;60 : 1243-50.
- West PR. Isolation rates and characterization of fungi in drinking water distribution systems. In: Proceedings of the Water Quality Technology Conference. Denver, Colorado: American Water Works Association 1986. p. 29-36.
- Arvanitidou M, Kanellou K, Constantinides TC Katsouyannopoulos V. The occurrence of fungi in hospital and community potable waters. Lett Appl Microbiol 1999;29:81-4.
- Lane SJ, Fujioka M. The impact of changes in irrigation practices on the distribution of foraging egrets and herons (Ardeidae) in the rice fields of central Japan. Biol Conserv 1998;83:221-30.
- Mara DD, Silva SA. Removal of intestinal nematode eggs in tropical waste stabilization ponds. J Trop Med Hyg 1986;89:71-4.
- Hachich EM, Galvani AT, Padula JA, Stoe NC, Garcia SC, Bonanno VMS, et al. Pathogenic parasites and enteroviruses in waste water: support for a regulation on water reuse. Water Sci Technol 2013;7:1512-8.
- www.observatorynano.eu/project/document/2790.
- Zhuang J, Gentry RW. Environmental Application and Risks of Nanotechnology. In: Ripp S, Henry TB, editors. Biotechnology and Nanotechnology Risk Assessment: Minding and Managing the Potential Threats around Us. Washington, D.C.: ACS Publications; 2011. p. 41-67.
- Tyagi KP, Singh R, Vats S, Kumar D, Tyagi S. Nanomaterials use in waste water treatment. Int Conf Nanotechnol Chem Eng 2012;2:65-9.
- Nowack B. Pollution prevention treatment using nanotechnology. Nanotechnology 2008;2:15-20.
- Ollis DF, El-Akabi H. Photocatalytic purification and treatment of water and air. Amsterdam: Elsevier Science Ltd.; 1993. p. 957-61.
- Gong P, Li H, He X, Wang K, Hu J, Tan W. Preparation and antibacterial activity of Fe3O4 Ag nanoparticles. Nanotechnology 2007;18:604-11.
- Ahmad Z, Pandey R, Sharma S, Khuller GK. Alginate nanoparticles as antituberculosis drug carriers: formulation development, pharmacokinetics and therapeutic potential. Indian J Chest Dis Allied Sci 2005;48:219-26.
- Abou El-Nour Kh, Eftaiha A, Al-Warthan A, Ammar R. Synthesis and applications of silver nanoparticles. Arab J Chem 2010;3:135-40.
- Kawahara K, Tsuruda K, Morishita M, Uchida M. Antibacterial effect of silver-zeolite on oral bacteria under anaerobic conditions. Dent Mater 2000;16:452-5.
- Dibrov P, Dzioba J, Gosink KK, Hase CC. Chemiosmotic mechanism of antimicrobial activity of Ag (+) in Vibrio cholerae. Antimicrob Agents Chemother 2002;46:2668-70.
- Barnett HL, Hunter BB. Illustrated genera of imperfect fungi. Amer Phytopathol Soc 1998;2:234.
- Alananbeh KM, Al-Qudah Z, El-Adly A, Al Refaee WJ. Impact of Silver Nanoparticles on Bacteria Isolated from Raw and Treated Wastewater in Madinah, KSA. Arab J Sci Eng 2017;42:85-93.
- Solomon SD, Bahadory M, Jeyarajasingam AV, Rutkowsky SA, Boritz C. Synthesis and study of silver nanoparticles. J Chem Educ 2007;84:322-5.
- Hedayati MT, Pasqualotto AC, Warn PA, Bowyer P, Denning DW. Aspergillus flavus: human pathogen, allergen and mycotoxin producer. Microbiology 2007;153:1677-92.
- Goncalves AB, Russell R, Paterson RRM, Lima N. Survey and significance of filamentous fungi from tap water. Int J Hyg Environ Health 2006;209:257-64.
- Sammon NB, Harrower KM, Fabbro LD, Reed RH. Incidence and distribution of microfungi in a treated municipal water supply system in sub-tropical Australia. Int J Environ Res Publ Health 2010;7:1597-611.
- Abed KF, Alwakeel SS. Mineral and microbial contents of bottled and tap water in Riyadh, Saudi Arabia. East J Sci Res 2007;2:151-6.
- Al-Turk IM, Diab AM. Bacteriological drinking water potability at Al-Madinah Al-Mounwwarah in relation to plasmid-linked multidrug-resistance. J Int Environ Appl Sci 2009;4:214-30.
- https://scholarworks.iupui.edu/bitstream/handle/1805/747/understandingmicrobes,insicknessandinhealth.pdf?sequence=1.
- Schuster E, Dunn-Coleman N, Frisvad JC, van Dijck PWM. On the safety of Aspergillus niger. Appl Microbiol Biotechnol 2002;59:426-35.
- Boutati EI, Anaissie EJ. Fusarium, a significant emerging pathogen in patients with hematologic malignancy: ten years' experience at a cancer centre and implications for management. Blood 1997;90:999-1008.
- Kuhls K, Lieckfeldt E, Borner T, Gueho E. Molecular reidentification of human pathogenic Trichoderma isolates as Trichoderma longibrachiatum and Trichoderma citrinoviride. Med Mycol J 1999; 37:25-33.
- Andrianopoulos A. Control of morphogenesis in the human fungal pathogen Penicillium marneffei. Int J Med Microbiol 2002;292:331-47.
- Verghese S, Ravichandran P. Geotrichum candidum infection in a renal transplant recipient. Indian J Nephrol 2003;13:72-4.
- Kontoyiannis P, Lewis E. Invasive zygomycosis: update on pathogenesis, clinical manifestations, and management. Infect Dis Clin North Am 2006;20:581-607.
- Burks, Bennete D, Minnis MM. Onsite waste water treatment systems. Madison, WI: Hogarth House Ltd.; 1994. p. 248-54.
- Sadeghi B, Sadjadi MAS, Vahdati RAR. Nanoplates controlled synthesis and catalytic activities of silver nanocrystals. Superlattices Microstruct 2009;46:858-63.
- Geoprincy G, Saravanan P, Gandhi NN, Renganathan S. A Novel approach for studying the combined antimicrobial effects of silver nanoparticles and antibiotics through agar over layer method and disk diffusion method. Dig J Nanomater Biostruc 2011;6:1557-65.
- Lkhagvajava N, Ya?ab I, Çelikc E, Koizhaiganovaa M, Saria O. Antimicrobial activity of colloidal silver nanoparticles prepared by sol-gel method. Dig J Nanomater Biostruct 2011;6:149-54.
- Gao C, Xu Y, Xu C. In vitro activity of nano-silver against ocular pathogenic fungi. Life Sci J 2012;9:750-3.
- Keuk-Jun K, Sung WS, Moon S, Choi J, Kim JG, Lee DG. Antifungal effect of silver nanoparticles on dermatophytes. J Microbiol Biotechnol 2008;18:1482-84.
- Rezaie S, Shahverdi AR. Antifungal effects of silver nanoparticle alone and with combination of antifungal drug on dermatophyte pathogen Trichophyton rubrum. International Conference on Bioscience, Biochemistry and Bioinformatics 2011;5:364-67.
- Panacek A, Kvitek L, Prucek R, Kolar M, Vecerova R, Pizurova N. Silver colloid nanoparticles: synthesis, characterization, and their antibacterial activity. J Phys Chem B 2006;110:16248-53.
- Panacek A, Kolar M, Vecerova R, Prucek R, Soukupova J, Rystof V, et al. Antifungal activity of silver nanoparticles against Candida sp. J Biomater 2009;30:6333-40.
- Amro NA, Kotra LP, Wadu-Mesthrige K, Bulychev A, Mobashery S, Liu G. High-resolution atomic force microscopy studies of the Escherichia coli outer membrane: structural basis for permeability. Langmuir 2000;16:2789-96.
- Danilczuk M, Lund A, Saldo J, Yamada H, Michalik J. Conduction electron spin resonance of small silver particles. Spectrochim Acta A Mol Biomol Spectrosc 2006;63:189-91.
- Feng QL, Wu J, Chen GQ, Cui FZ, Kim TN, Kim JO. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J Biomed Mater Res 2000;52:662-8.
- Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ramirez JT. The bactericidal effect of silver nanoparticles. Nanotechnology 2005;16:2346-53.
- Martinez-Castanon GA, Nino-Martinez N, Martinez-Gutierrez F, Martinez-Mendoza JR, Ruiz F. Synthesis and antibacterial activity of silver nanoparticles with different sizes. J Nanopart Res 2008;10:1343-8.
- Lok CN, Ho CM, Chen R, He QY, Yu WY. Proteomic analysis of the mode of antibacterial action of silver nanoparticles. J Proteome Res 2006;5:916-24.
- Dobias J, Bernier-Latmani R. Silver release from silver nanoparticles in natural waters. Environ Sci Technol 2013;47:4140-6.
- Al-Qodah Z, AT Shawaqfeh AT, Lafi WL. Two-resistance mass transfer model for the adsorption of the pesticide deltamethrin using acid treated oil shale ash. Adsorption 2007;13:73-82.