- *Corresponding Author:
- Saini Navjot Kaur
Department of Pharmaceutics, Amar Shaheed Baba Ajit Singh Jujhar Singh Memorial College of Pharmacy, Bela, Punjab 140111, India
E-mail: saini.navjotkaur@gmail.com
| Date of Received | 20 May 2023 |
| Date of Revision | 20 February 2023 |
| Date of Acceptance | 20 May 2023 |
| Indian J Pharm Sci 2023;85(3):686-697 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The well-known plant components for their anti-diabetic properties are Azadirachta indica leaves, Pterocarpus marsupium heartwood, Picrorhiza kurroa rhizomes and Withania coagulans berries and fruit coat. The current study's objective was to create a polyherbal dosage form and assess its effectiveness and stability. The various methanolic extracts of all four medicines were combined in the 1:1:1:1 ratios to create the polyherbal extract. Both in vitro antioxidant and in vitro antidiabetic activity of the extract were evaluated. For their preformulation investigations, a total of six polyherbal compositions were examined. Through Fourier transform infrared spectroscopic analysis, the phytochemicals present were identified. The medication was then put into capsules with a 0 size and evaluated for every aspect of a capsule. The stability of the most recent medicine was examined under diverse circumstances. The 2,2-diphenyl-1- picrylhydrazyl half maximal inhibitory concentration value for the polyherbal extract was 60.56 μg/ml indicating in vitro antioxidant activity. The anti-diabetic action was equivalent to the industry standard and dose-dependent, with an half maximal inhibitory concentration value of 59.82 μg/ml for inhibiting alpha-amylase and 67.28 μg/ml for inhibiting alpha-glucosidase. The polyherbal formulation 1 displayed the best flow qualities. According to Fourier transform infrared spectroscopic data, granules may include terpenes, tannins, phenols and flavonoids. In 30 min, the capsules with polyherbal formulation 1 had a 95.77 % cumulative drug release value. The capsules were discovered to be stable under many types of lighting, however they disintegrate above 70 % humidity and over 55°. As a result, it was discovered that the polyherbal capsules were stable and effective in treating hyperglycemia.
Keywords
Polyherbal capsules, in vitro antidiabetic, antioxidant, stability study, Fourier transform infrared spectroscopy
Diabetes is a serious public health concern across the world. Hyperglycemia is a symptom of diabetes mellitus, which is triggered by an insulin shortage or it’s less binding on receptors or both. It is one of the most serious health issues that have now turned into a world-wide spread. Diabetes' global incidence has risen quickly during the last four decades. However, the World Health Organization has programmed to inhibit the rise in diabetes incidence and premature mortality from by 1/3rd by year 2030 but the results are not promising. It has been estimated recently that the cases of diabetes from 20-79 age will upsurge from 41 million in 2010 to 51 million in 2030 (1 in 10 adults)[1].
In allopathic system of medicines, insulin and oral anti-diabetic medications used now a day to treat diabetic complications are efficient in lowering blood glucose levels along with a variety of adverse effects. Several herbal medicines and minerals are mentioned in the literature for the effective control of diabetes mellitus. The herbal medicines are considered safe as they have fewer side-effects than allopathic system of medicines[2,3].
Evaluating the hypoglycemic ability of medicinal plants has therefore become essential. In the current study, in vitro antidiabetic activity is being investigated, a polyherbal extract made from an equal mixture of Azadirachta indica leaves, Picrorhiza kurroa rhizomes, Pterocarpus marsupium heartwood and Withania coagulans berries and fruit coat is prepared, polyherbal capsules are made, evaluated, and their stability is being studied.
Materials and Methods
Azadirachta indica:
Azadirachta indica (Family: Meliaceae) is used traditionally in different systems of drugs for its therapeutic purposes. Because of its many uses, it has been dubbed Limbo, Nim, Nimba, Medusa, Vempu and "village pharmacy"[4]. Each plant part like leaves, fruits, bark, twigs etc. are used in Ayurveda, Unani, Siddha and Homeopathic systems since long ago and now it is being practiced in modern system too for the welfares of mankind[5]. The drug contains various phytoconstituents like nimbin, azadirachtin, nimbidin, epicatechin, catechin, margolone and gallic acid. Because of the antibacterial qualities, the plant's twigs are used in dental care for toothaches, cleansing and foul breath[6].
Neem is used to healing a range of ailments, including headaches, ulcers, oral cancer, diabetes, malaria, leprosy, dengue fever, chicken pox, respiratory difficulties and skin issues. The drug works as an astringent, antifertility, antinociceptive, immunomodulatory, antiinflammatory, antiviral, nematicidal, insecticidal, cardioprotective, neuroprotective, antimicrobial, hepatoprotective, antidiabetic and antioxidant. Insecticides, herbicides, and/or anti-feedants are all utilized with neem[7-9]. Neem does not alter libido or other secondary sexual characteristics as an antifertility medication, but it does exhibit transient and reversible contraceptive activity[10]. Nowadays, the medication is used to treat eczema, ulcers, burns, and sores[4].
Pterocarpus marsupium:
Pterocarpus marsupium Roxb. (English: Red Kino Tree, Hindi: Bijasal, Kannada: Raktahonne) is an inborn plant of India, Nepal and Sri Lanka. The medication has been used as an anti-inflammatory, anthelminthic, aphrodisiac and antipyretic and in the management of mental disorders and ulcers. Stomach-ache, cholera, dysentery, urinary problems, tongue disorders and toothache are all treated with the bark. The anti-diabetic properties of Pterocarpus marsupium's heartwood and bark are well-known[11]. The kino tree contains a number of phytochemicals, including 2,6-dihydroxyphenyl glucopyranoside, ethanedione, pteroside, pterocarposide C-D-glucopyranosyl-2, 6-dihydroxyl benzene pterosupol, marsuposide, epicatechin, formononetin, vanillic acid, quercetin and naringenin derivative along with sesquiterpenes[12,13].
The three major phenolic components of this plant's heartwood, marsupsin, pterosupin and pterostilbene, have been shown to dramatically reduce blood glucose levels[12]. The medication also aids in the healing of diabetic wounds[14]. Pterostilbene cream (0.4 %) was shown to be extremely effective in improving skin tone, skin moisture suppleness while reducing age indicators like wrinkles and fine lines and inhibits melanogenesis without any side effects[14].
Pterocarpus marsupium is capable of restoring all the ovarian parameters to normal while reducing ovarian cysts[15]. Because of its antioxidant properties, the medication appears to be a potential choice for skin whitening[16]. The problems like axonal degeneration, myelin fibrosis and Schwann cell hyperplasia get significantly restored by Pterocarpus[17]. The medication has shown its potential in cancer treatment as well as the treatment of hyperlipidemia and diabetes[18].
Picrorhiza kurroa:
Picrorhiza kurroa Royle ex Benth. (Scrophulariaceae), is a well-known medicinal plant for rhizomes and roots. Katki (Hindi), Karru, and Kaur (Punjabi), Kadvi (English)[19]. Deepan (repair of Agni), pachan (digestion), lekhan (scraping), and Bhedan (purgative) are all characteristics of Katuka (Picrorhiza kurroa). Vayu and aakash mahabhutas are the most prominent. It lowers the overabundance of kleda and meda in the body due to all of these qualities (lipid, fat reduces). It also possesses choleretic and cholegogue-Virechak (purgative) properties, which cause bile excretion in the faeces and a decrease in fat and lipid absorption in the gut, resulting in a drop-in serum lipid content[20].
Picrorhiza kurroa is an essential plant in Ayurvedic medicine in India. It has been discovered to have antiallergic, antiasthmatic, anticancer, cardiovascular, hypolipidemic, cholerectic, hypoglycemic, antiviral, purgative, antiphosphodiesterase, neurogenic, molluscicidal, and leishmanicidal properties, as well as antiallergic, antiasthmatic, anticancerous, cardiovascular, choleretic, hypoglycemic, hypolipidemic, It has also been used to treat skin problems, gastric ulcers, neuralgia, vitiligo, and rheumatoid arthritis[19].
Roots and rhizomes are the most commonly utilized components. Kutkin is a Kutaki active principle that includes kutkoside as well as picrosides I, II, III and V. Further phytoconstituents include apocynin, nine cucurbitacin glycosides and drosin[21]. P. kurroa has mussaenosidic acid, boschnaloside and bartsioside. The active iridoid glycosides are responsible for the antioxidant, hepatoprotective, anti-allergic, antiinflammatory, choleretic, anti-ulcerative colitis, immunomodulator, anti-asthmatic, antibacterial and anti-cancerous activities. It's also used to treat persistent fevers, dyspepsia, diarrhoea, and scorpion stings. Picrosides have been found to exhibit anticancer properties such as metal ion chelating, free radical scavenging, detoxifying, apoptotic induction and cell cycle modulation[22,23]. Cucarbitacins are naturally cytotoxic agents so the drug is used in treatment of Hep3B (human hepatocellular cancer), also used in the treatment of vitiligo in combination with phytotherapy[24,25]. The drug helps in cell regeneration, as well as increased insulin production and hypoglycemic properties, meanwhile protects hepatic and renal functioning against oxidative damage[26].
Withania coagulans:
Withania coagulans also known as 'tukhm-e-hayat' (fruit of life) is well-known in herbal therapeutic systems. The shrub is widely distributed in North West areas of India, like in Punjab, Rajasthan and Shimla. The fruit is a berry with a pleasant flavor and sedative, emetic and diuretic effects, according to reports hypoglycemic, hypolipidemic, immunosuppressive, hepatoprotective, antiflatulent, anti-inflammatory, wound healer, anticancer, cardiovascular, digestive, sedative, antifungal, antibacterial and cytotoxic characteristics have all been discovered in the plant. Withanolides, recognised anticancer agents, and flavonoids the primary active phytoconstituents are present in Withania genus. The primary withanolides found in the genus Withania are Withanolide A, Withaferin A, Withaferin and Withanone. The apoptotic activity evaluated against normal epithelial cell lines as well as human breast cancer cell lines[27,28]. Different prostate cancer cell lines showed enhanced apoptotic induction and reduced cell proliferation, cell viability, invasion as well as migration[29].
The plant is used to treat asthma, insomnia, liver problems, as well as act as emetic, diuretic, antimicrobial, antitumor, antihyperglycemic, immunosuppressive, and depressant on central nervous system. The numerous therapeutic uses of withanolides found in Withania coagulans have piqued the scientific community's curiosity. The medication contains a novel withanolide glycoside, withacogulanoside-B, coagulin E, withaperuvin C, withanolid J, 27-hydroxywithanolide I, and ajugin E Myrcetin, quercetin, gallic acid, and p-hydroxybenzoic acid are all included in the medication[30,31]. The fruit of Withania coagulans has long been used as a milk coagulant. It might be used to make cheeses with reduced salt content[32]. Coagulansin A was discovered to be non-toxic and to have significant urease inhibitory action[33]. Withania coagulans has a defensive effect against kidney injury induced through cisplatin, owing to its anti-inflammatory and free radical scavenging properties[34].
It is found in the literature that there are a number of formulations present in market which contain Azadirachta indica (neem) and Pterocarpus marsupium (vijaysar) in combination for antidiabetic effect. For eg., A diabetegon, madhu har, diabenil, diabetter plus, Picrorhiza kurroa (Kutki) in trigo diab granules, Glucotone granules, dibadac, ojamin, Withania coagulans (Paneer dodi) in paneer doda polyherbal powder
It has been seen that above three drugs are present in combination with each other in a number of formulations but Withania coagulans with pronounced antidiabetic activity is not found in combination with above mentioned drugs anywhere in literature. Therefore, we are going to prepare a polyherbal formulation in the present study that could work on different targets and at the same time show improved patient adherence and therapeutic behavior.
Collection as well as authentication of the plant material:
Herbal drugs were gathered from the local areas of Ropar and authenticated from Natural Product Field Laboratory and Nursery, NIPER SAS Nagar, Pb. The drugs have all been verified Azadirachta indica (Neem) leaves, Picrorhiza kurroa (Kutki) rhizomes, Pterocarpus marsupium (Vijaysar) wood and Withania coagulans (paneer dodi) fruit.
Preparation of extracts:
All the four authenticated drugs were individually dried, powdered and extracted with methanol for 18 h. The extracts concentrated below 60o were stored at cool area. The dried extracts were mixed in ratio 1:1:1:1 in order to get a polyherbal extract.
Preliminary phytochemical screening:
Standard methods were used to evaluate the incidence of alkaloids, glycosides, tannins, phenolics, flavonoids, saponins, terpenes and sterols in the extracts[35,36].
Total phenolic content: The reagent used was Folin-Ciocalteau technique for total phenolic content determination in the polyherbal extract. The Folin-Ciocalteau Reagent (FCR) oxidises phenolic in plant extract, and Ultraviolet (UV)- visible monitors changes in the dark blue colour (K=750 nm). The method using Gallic acid (0-150 mg/l) as standard (y= 2.341x+0.0987,R2=0.9923). The results were expressed as mg of gallic acid equivalent (GAE)/1g of Dry Plant Extract (DPE). Methanol was used in order to prepare blank solution. In this method 1 ml of each extract in (1 mg/ml) was added to 10 ml deionized water. The mixture was incubated for 5 min, after adding 1 ml FCR. To the mixture 2 ml of 20 % Na2CO3 was added and incubated for 60 min in dark and absorbance was measured[37].
Total Flavonoids contents: The total flavonoid content was estimated using UV spectrophotometer by measuring absorbance at 510 nm using rutin trihydrate as (0-300 mg/l) standard (y=0.6813x+0.0257, R2=0.9826) while expressing the results as rutin trihydrate equivalents (RE)/1 g of DPE and methanol as blank. In this method 1ml of each extract in (1 mg/ml) was added to 4 ml deionized water. To above mixture 0.3 ml (5 % w/v) NaNO3 was added and the mixture was incubated for 5 min. Added 0.3 ml of 10 % w/v AlCl3, after which 2 ml (1 M) NaOH was added to mixture, making up the volume up to 10 ml and the absorbance was measured[37].
Antioxidant activity:
2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay: In this 1.0 ml of (0.3 mM) methanolic DPPH, 1 ml of the polyherbal methanolic extract and methanol were combined to total 3.0 ml of reactants. The resulting combination was stored in dark for 10 min prior to determining the absorbance at 517 nm. Using ascorbic acid as controls the following formula can be used to compute the inhibition (%).
Inhibition (%)=(AC–AT/AC)×100
Where AC=control absorbance and AT=test absorbance[38].
Reducing power anti-oxidant activity: Reducing power has been linked to antioxidant activity as the constituents having reducing power or the edonors can reduce oxidised components in lipid peroxidation processes, making them as primary and secondary antioxidants. The method given by Chanda et al.[35] was used to determine the reducing power anti-oxidant activity at 700 nm with positive controls ascorbic acid[38]. The results obtained in above two methods are mentioned in Table 1.
| Concentration (µg/ml) | Reducing power | DPPH method | ||
|---|---|---|---|---|
| Std. | PHE 500 | STD. | PHE 500 | |
| 10 | 0.143± 0.005 | 0.092± 0.007 | 46.31± 6.7 | 33.96 ± 5.3 |
| 20 | 0.172± 0.006 | 0.136± 0.005 | 56.71± 5.1 | 40.36 ±4.9 |
| 50 | 0.189± 0.006 | 0.146± 0.007 | 63.14± 2.8 | 51.82 ± 7.5 |
| 100 | 0.258± 0.003 | 0.173± 0.003 | 77.69± 4.9 | 60.94 ± 4.6 |
| 200 | 0.463± 0.007 | 0.273± 0.005 | 90.67±7.3 | 76.18 ±1.9 |
| 500 | 0.994± 0.004 | 0.491±0.004 | 93.29±4.9 | 90.96 ± 1.8 |
| IC50 (µg/ml) | 46.73 | 60.56 | ||
Note: DPPH: α-, α-Diphenyl-β-picryl-hydrazyl and PHE: Polyherbal Extract
Table 1: In Vitro Antioxidant Activity of Polyherbal Extract
In vitro antidiabetic activity:
α-Amylase inhibition activity: To estimate the in vitro antidiabetic activity, the procedure given by Demelash et al.[36] was utilized. The absorbance was estimated at 540 nm using UV-Visible spectrophotometer. A sample blank reaction was produced in the same way using the plant extract without enzyme. Acarbose as positive control was used. Percentage inhibition was calculated using following equation
Inhibition (%) =(AC-AB)-(AS-ASB)/(ACACB) ×100
Where A is absorbance; C is control; CB is Control Blank; S is absorbance of sample and SB is sample blank. The results were expressed in IC50 value[39].
α-glucosidase assay:
In order to estimate the in vitro antidiabetic activity by α-glucosidase assay the method given by Rege et al.[37] was used. It was estimated as a percentage inhibition.
Inhibition (%) =AN-AT/AN×100
Where AN is absorbance of negative control; AT is Absorbance of test sample. The result is stated as IC50 value and mentioned in Table 2[40].
| Concentration (µg/ml) | α- amylase | α- glucosidase | ||
|---|---|---|---|---|
| Acarbose | PHE | Acarbose | PHE | |
| 10 | 27.36±0.25 | 23.96±0.21 | 19.13±1.6 | 16.39±2.3 |
| 20 | 37.64±0.15 | 36.61±0.37 | 28.33±2.1 | 20.34±3.5 |
| 50 | 48.37±0.29 | 47.31±0.49 | 49.12±2.9 | 40.32±5.4 |
| 75 | 67.28±0.18 | 60.31±0.30 | 70.30±5.4 | 58.42±5.9 |
| 100 | 76.21±0.73 | 73.41±0.47 | 76.39±7.3 | 74.36±4.2 |
| IC50 | 50.21 | 59.82 | 56.34 | 67.28 |
Note: PHE: Polyherbal Extract
Table 2: In Vitro Antidiabetic Activity of Polyherbal Extract
Formulation of Polyherbal granules:
The granules from polyherbal methanolic extract were prepared through wet granulation method. In this method the four herbal extracts were taken in same ratio and mixed with the excipients in the ratios mentioned below in Table 3.
| Ingredients | PHF 1 | PHF 2 | PHF 3 | PHF 4 | PHF 5 | PHF 6 |
|---|---|---|---|---|---|---|
| Azadirachta indica | 60 | 60 | 60 | 60 | 60 | 60 |
| Withania coagulans | 60 | 60 | 60 | 60 | 60 | 60 |
| Picrorhiza kurroa | 60 | 60 | 60 | 60 | 60 | 60 |
| Pterocarpus marsupium | 60 | 60 | 60 | 60 | 60 | 60 |
| Lactose / Mannitol | 235 | 225 | 215 | 205 | 195 | 185 |
| Pregelatinised Starch | 0 | 10 | 20 | 30 | 40 | 50 |
| Talc | 20 | 20 | 20 | 20 | 20 | 20 |
| Sodium Benzoate | 5 | 5 | 5 | 5 | 5 | 5 |
| Total (mg) | 500 | 500 | 500 | 500 | 500 | 500 |
Note: PHF: Polyherbal Formulation
Table 3: Formulation of Polyherbal Granules by Wet Granulation Method
Pre-formulation study:
It includes assessment of different parameters like bulk and tap density, Hausner’s ratio, Carr’s index, angle of repose in order to determine the flow properties polyherbal granules[41].
Fourier-Transform Infrared (FTIR) spectroscopy:
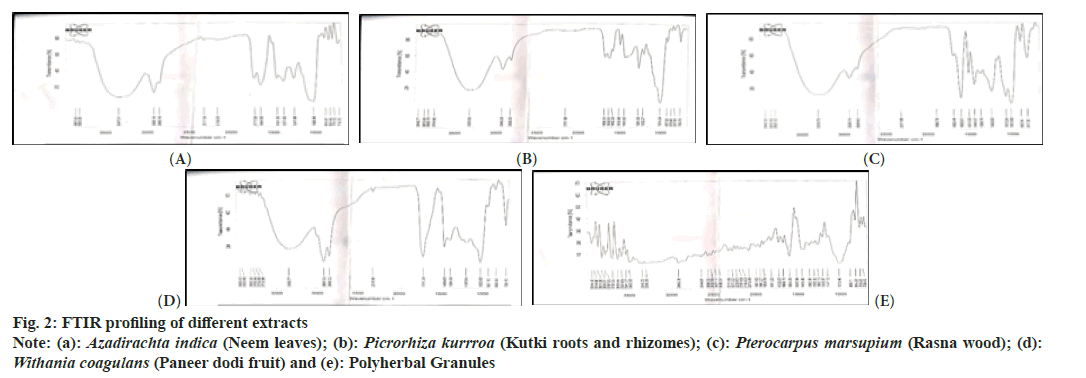
The Infrared (IR) spectrum of all the four drug extracts and polyherbal granules were drawn using Bruker Α-E IR spectrum of drugs available at Instrument lab at ASBASJS college of Pharmacy, Bela, Ropar and Pb. The KBr was used to make the sample disks. The samples were examined at 700-4000 cm-1.
Capsule evaluation parameters:
The parameters like organoleptic characters, average weight, weight variation, disintegration time, moisture content and dissolution rate were determined in order to evaluate the capsules of polyherbal granules.
Average weight of capsules: Average weight of 20 capsules was calculated using a digital weighing balance.
Weight variation: Randomly 20 capsules were selected, weighed individually and their average weight was compared with weight of individual capsule[42].
Drug Content: Six capsules' contents were poured into a mortar, the dose-equivalent quantity (20 mg) was measured, and the drug content of the capsules was ascertained using the method described in IP[43].
Disintegration time: In this method six random capsules were placed in all the six tubes of the Basket rack and dipped into the beaker containing 900 ml medium. The Simulated Gastric fluid (SGF) was upheld at 37±2°. The basket was moved 5-6 cm in height, at the rate of 28-32 cycles per minute through a motor driven shaft. The time was noted in which the capsules pass through a 10 mesh screen and have disintegrated[44].
Moisture content: It was determined by taking 5 g of sample and drying it in the hot air oven at 100-110° until the persistent weight is obtained. Any change in the weight of dried sample was calculated[45].
In vitro dissolution studies: It is done to govern absorption rate, bio equivalency and bioavailability of the capsules. The method used by Kaur et al.[42], was used. The samples so taken were analyzed using UV Spectrophotometer (Shimadzu 2700) at 260 nm[46].
Stability study:
To determine the durability of the pharmaceutical formulations, they must be exposed to augmented temperature, humidity and light intensities. Researchers examined the effects of extrinsic factors on the capsule's physical, chemical, and medicinal properties[47].
Results and Discussion
The phytoconstituents contained in the separate medicines were discovered in the polyherbal extract, where they are predicted to operate in synergistic way for the treatment of diabetes. In present study all the four drugs Azadirachta indica (Neem) leaves, Picrorhiza kurroa (Kutki) rhizomes, Pterocarpus marsupium (Vijaysar) wood and Withania coagulans (paneer dodi) fruit. The phytochemical analysis showed that all the four drugs have phenolic, flavonoid and steroidal content.
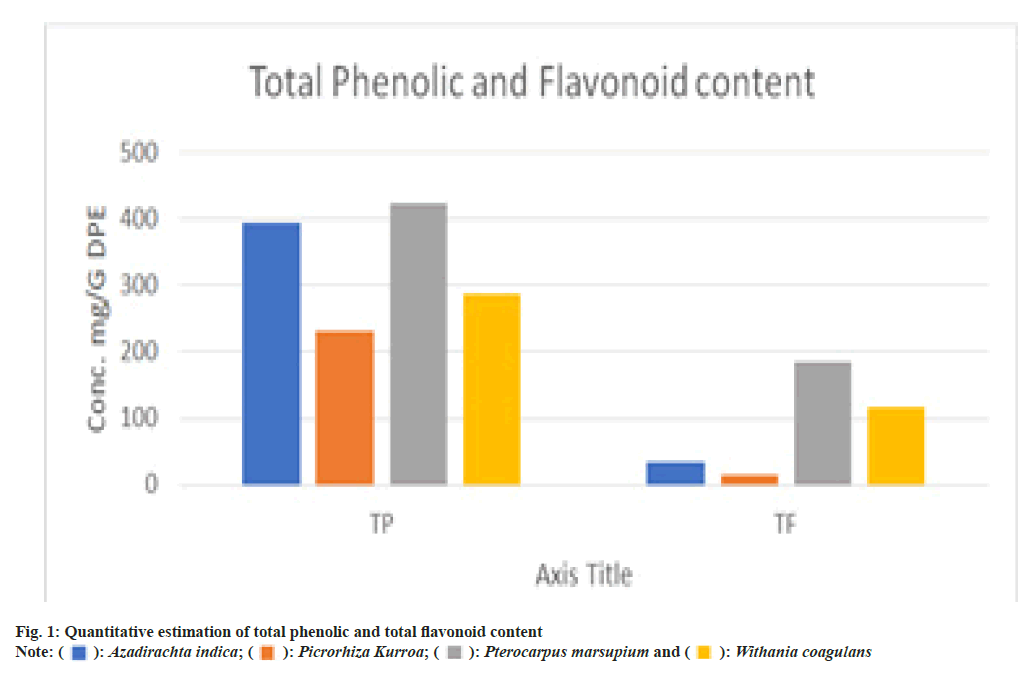
As a consequence of these tests, the drug's total phenolic and flavonoid content was determined. The drug's antioxidant activity was also determined to be substantial. Polyherbal extract may have antioxidant action owing to phenolic and flavonoid levels in the extract. An excellent structural chemistry exists in plant polyphenols for their free radical scavenging action. They act as electron donors and have capacity to stabilize and delocalize unpaired electrons and their capacity to bind metal ions are all responsible for their antioxidative characteristics. The phenolic content of neem leaves (394.53±.510) (mg GAE/g DPE) was found to be maximum as well as comparable to Pterocarpus marsupium (372.16±.426) followed by Withania coagulans fruit (286.46±0.73) and in last Picrorhiza kurroa rhizomes (230.42±.156). The comparative analysis of all the different extracts is given there in fig. 1.
In addition to scavenging free radicals, flavonoids also chelate iron and copper, and block enzymes that produce free radicals. All known ROS may be neutralized by flavonoids. The flavonoidal content was again found to be maximum in Pterocarpus marsupium i.e. 184.36±6.37 RE/1 g followed by Withania coagulans (116.35±4.68), Azadirachta indica (35.68±4.28) and in last Picrorhiza kurroa (16.23±7.52) mg RE/g DPE. The comparative analysis of all the different extracts is given there in fig. 1.
If an electron or hydrogen radium is introduced into the compound, it becomes a stable, diamagnetic molecule. Because of the interaction between antioxidant molecules and radical advancement, the radical is scavenged by hydrogen donation when its absorbance decreases due to the activity of antioxidants. Ascorbic acid was shown to have a comparable antioxidant activity to the polyherbal extract at the different levels of concentration that were evaluated. All concentrations examined showed a dosage dependent increase in antioxidant activity. To determine the antioxidant activity Ascorbic acid was employed as a standard medication in combination with the DPPH technique. When compounds with reduction potential are exposed to potassium ferricyanide (Fe3+), potassium ferrocyanide (Fe2+) is formed, that in turn interacts with ferric chloride to create ferric–ferrous complexes which exhibit a maximum absorbance at 700 nm. Increasing sample and standard concentrations enhances the reduction power of the hydro alcoholic extracts and standards. The results so obtained are mentioned in Table 1.
As carbohydrates are broken down into glucose, they may be broken down by α-amylase and α-glucosidase enzymes. The enzyme α-amylase hydrolyses starch, which is broken down into glucose before absorption. A decrease in postprandial rise in glucose can be attained by inhibiting α-amylase, an enzyme that breaks down disaccharides in the small intestine to form glucose (α-glucosidase). In addition to boosting insulin secretion and glucose uptake by cells, plant metabolites can also decrease glucose synthesis and absorption. Inhibition of glucose absorption is one of the most significant methods used in the treatment of diabetes. Polysaccharides are hydrolyzed into little absorbable pieces by digestive enzymes that are inhibited. This prevents postprandial high blood glucose. α-amylase is regarded one of the most essential digestive enzymes due to its function in the breakdown of polysaccharides. It is present in saliva and pancreatic juice. α-glucosidase, another enzyme in digestion is found in the mucosal brush border of the small intestine. Processing and breakdown of complicated carbs into simple, absorbable sugars is its main function. An effective way to reduce excessive postprandial blood glucose levels is to block this enzyme[48].
In vitro experiments were used to determine the effect of polyherbal methanolic extracts on α-amylase and α-glucosidase enzymes[39,40]. IC50 values of acarbose, polyherbal extract for inhibition of α-amylase and of α-glucosidase are given in Table 2. The IC50 values of the drug were found to be 58.81 in case of α-amylase inhibition and 64.81 in case of α-glucosidase inhibition for the polyherbal extract. The composition of polyherbal granules has been mentioned in Table 3.
Granules are huge, multi-particle objects created by the process of granulation, which causes the initial powder particles to stick together. Key characteristics including particle form, particle size distribution, powder flowability, crystal stability, etc. are often determined through granulation. Depending on their intended application, pharmaceutical granules are usually between 0.05 and 1.0 mm in size. In wet granulation, a liquid typically a binder solution is added to help a powder blend get wet and clump together. The moist material is then dried and screened to create the required size of granules. Polyherbal capsules were prepared by making the polyherbal granules. The polyherbal granules were prepared in six different compositions via varying the amount of excipients. The bulk density, tapped density, Carr’s index, Hausner’s ratio and Angle of repose were checked for all the six compositions of polyherbal granules given in Table 4. It was found that Polyherbal Formulation (PHF)1 showed all the desired characters of the granules. So, the PHF1 was selected and was filled into the 0 sized capsules.
| Parameters | PHF 1 | PHF 2 | PHF 3 | PHF 4 | PHF 5 | PHF 6 |
|---|---|---|---|---|---|---|
| Bulk density (g/ml) | 0.42 | 0.52 | 0.58 | 0.62 | 0.67 | 0.75 |
| Tapped Density(g/ml) | 0.57 | 0.61 | 0.67 | 0.71 | 0.76 | 0.84 |
| Carr’s index (%) | 11.76 | 14.71 | 20.35 | 21.91 | 23.96 | 25.74 |
| Hausner’s ratio | 1.15 | 1.26 | 1.3 | 1.34 | 1.36 | 1.4 |
| Angle of repose (°) | 22.91 | 27.01 | 29.49 | 32.64 | 36.04 | 39.83 |
Note: PHF: Polyherbal Formulation
Table 4: Evaluation Parameters for Polyherbal Granules
The FTIR study of methanolic extracts of all the four drugs as well as polyherbal granules was done. Fig. 2 shows the FTIR spectra of the extracts and granules, respectively. Specific functional groupings of chemicals are shown by the frequency regions or bands in each sample. Each sample has a peak in the spectral range between 3400-3200 cm-1 and 2800-3000 cm-1, indicating OH groups (like water, alcohols, and phenols) and methylene CH symmetric/asymmetric vibrations 1600 and 1800 cm-1, alkenes respectively[49]. In Pterocarpus marsupium, Picrorhiza kurroa, Withania coagulans, and polyherbal granules, the particular peaks at 1603 cm-1, 1640 cm-1, 1711 cm-1, 1668 cm-1, and 1722 cm-1 reflect the carbonyl group of conjugated alkenes, alkenes, cyclohexanone, aliphatic ketone and disubstituted alkene. Azadirachta indica has a band at 2926 cm-1, 1544 cm-1, and 1247 cm-1 due to the doublet absorption of C-H stretching vibrations. The component Pterocarpus marsupium represented the presence of water (3310 cm-1), alkane (1603 cm- 1), nitro (1511 cm-1), methyl (1444 cm-1) and alkyl aryl ether (1242 cm-1) along with primary alcohols at 1073 cm-1 in it. Picrorhiza kurroa showed the presence of water (3330 cm-1), alkane (2940 cm- 1), alkene (1640 cm-1), and aromatic esters (1281 cm-1) in it. Withania coagulans contained alcohol (3352 cm-1), alkane (2924 cm-1), cyclohexanone (1711 cm-1), methyl (1456 cm-1) and amine (1023 cm-1) groups in it.
The results obtained from the different spectra show the presence of tannins, polyphenols and flavonoids in the extracts. The polyherbal granules showed the minimal change in wavenumber suggesting the inert behavior of the excipients added. The polyherbal capsules having blue colored cap and white body with average weight of 517 mg, disintegration time of 10.32 min, moisture content 4.05 %. The results are given below in Table 5.
| Parameters | Observations |
|---|---|
| Organoleptic characters | Capsules are of blue coloured cap and white body filled with brown coloured powder |
| Size | 0 size |
| Taste | Bitter |
| Odour | Characteristic |
| Average weight (g) | 517 |
| Weight variation (g) | 479-545 |
| Disintegration time (min) | 10.32 |
| Moisture content | 4.05 % |
| Drug content | Within IP limits |
Table 5: Evaluation Parameters of Polyherbal Capsules
The dissolution study of capsules was done in which PHF1 reached percentage Cumulative drug release CDR of 95.77 in 30 min as given in Table 6. Out of the 6 different types of capsule composition, PHF1 capsules showed best results in dissolution study, which may be attributed to the variation in pre-gelatinized starch which is acting as binding agent. The stability parameters analyzed for 30 min, 1, 3 and 6 h of storage at accelerated conditions of temperature, light and humidity have been tabulated in Table 7-Table 9.
| Time | PHF1 | PHF2 | PHF3 | PHF4 | PHF5 | PHF6 |
|---|---|---|---|---|---|---|
| 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 5 | 36.645 | 34.49 | 30.486 | 27.049 | 22.368 | 19.418 |
| 10 | 58.365 | 53.951 | 49.946 | 46.505 | 41.822 | 38.873 |
| 15 | 71.156 | 69.001 | 64.997 | 61.56 | 58.153 | 53.112 |
| 20 | 83.223 | 79.282 | 75.331 | 76.558 | 71.872 | 68.922 |
| 25 | 89.98 | 87.038 | 84.645 | 81.18 | 76.514 | 73.561 |
| 30 | 95.772 | 93.617 | 89.613 | 86.176 | 81.495 | 76.083 |
Note: Abs: Absorbance and CDR: Cumulative Drug Release
Table 6: Dissolution Study of Polyherbal Antidiabetic Capsules
| Light Source | Sunlight | Fluorescence | Tube light | UV light | Infra-Red Light | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exposure time (h) | ½ | 1 | 3 | 6 | ½ | 1 | 3 | 6 | ½ | 1 | 3 | 6 | ½ | 1 | 3 | 6 | ½ | 1 | |
| Polyherbal capsule | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
Note: (+) Degradation (-) No change
Table 7: Effect of Different Types of Lights on Polyherbal Capsules
| Storage condition | Testing condition (°) | Time duration (h) | Results | |||
|---|---|---|---|---|---|---|
| ½ | 1 | 3 | 6 | |||
| Ambient | 30 | - | - | - | - | No degradation in 6 h |
| Warm (30-40°) | 35 | - | - | - | - | No degradation in 6 h |
| Accelerated | 50 | - | - | - | - | No degradation in 6 h |
| Accelerated | 55 | - | - | - | + | Degraded after 4 h |
| Accelerated | 60 | - | - | + | + | Degraded after 2 h |
Note: (+) Degradation (-) No change
Table 8: Stability Test of Polyherbal Capsule at Different Temperature
| Temperature (°) | Humidity | |||
|---|---|---|---|---|
| 30 % | 50 % | 70 % | 90 % | |
| 30 | - | - | - | - |
| 35 | - | - | + | + |
| 55 | - | - | + | ++ |
| 65 | - | - | ++ | +++ |
Note: (+) Degradation and (-) No change
Table 9: Stability Study at Different Humidity with Respect to Different Temperature
Standardization, which assures quality, safety, and repeatability, is the most essential element of any formulation. A bioprospecting project covers the whole process of bioprospecting, from raw material gathering through the production of a final product. As part of the present investigation, an herbal combination standardized for polyherbs was prepared in a gelatin capsule and tested. Four components, belonging to distinct families, different morphological plant sections and diverse phyto-constituents, make up this polyherbal preparation.
All four medications were found to be of good grade by the pharmacopoeial standards. 500 mg of multi-herbal capsules broke down in 10.32 min under in vitro conditions. In 30 min, all six pills were 95.77 % dissolved in PHF1 which might be attributed to absence of pregelatinized starch when compared to other five compositions. This in vitro release pattern of drug from its capsule shell to predict how it will release in vivo. In light of phyto-pharmacological research, polyherbal capsule was found to be reasonably stable under accelerated conditions. The effectiveness of polyherbal combinations of selected plants against diabetes was examined through in vitro method. A poly-herbal mixture of plants shown potent anti-diabetic and antioxidant properties in an in vitro research. More research using more focused methods is required to identify the components responsible for the activity and the process by which it happens.
Acknowledgements:
The author is grateful to Director of ASBASJSM college of Pharmacy, Bela, Ropar, Punjab for generous conveniences for effective conduction of the research work.
Conflict of interests:
The authors declared no conflict of interests.
References
- Craig ME, Hattersley A, Donaghue KC. Definition, epidemiology and classification of diabetes in children and adolescents. Pediatr Diabetes 2009;10(Suppl 12):3-12.
[Crossref] [Google Scholar] [PubMed]
- Bommer C, Sagalova V, Heesemann E, Manne-Goehler J, Atun R, Bärnighausen T, et al. Global economic burden of diabetes in adults: Projections from 2015 to 2030. Diabetes care 2018;41(5):963-70.
[Crossref] [Google Scholar] [PubMed]
- Nagja T, Kumar V, Sanjeev A. Anti-diabetic activity of a polyherbal formulation in Streptozotocin induced Type 2 Diabetic rats. J Nat Remedies 2016;16(4):148-52.
- Ahmad S, Maqbool A, Srivastava A, Gogol S. Biological detail and therapeutic effect of Azadirachta indica (neem tree) products-a review. Evid Based Med Healthcare 2019;6(22):1607-12.
- Lakshmi T, Krishnan V, Rajendran R, Madhusudhanan N. Azadirachta indica: A herbal panacea in dentistry–An update. Pharmacogn Rev 2015;9(17):41-4.
[Crossref] [Google Scholar] [PubMed]
- Saleem S, Muhammad G, Hussain MA, Bukhari SN. A comprehensive review of phytochemical profile, bioactives for pharmaceuticals, and pharmacological attributes of Azadirachta indica. Phytother Res 2018;32(7):1241-72.
[Crossref] [Google Scholar] [PubMed]
- Agrawal S, Popli DB, Sircar K, Chowdhry A. A review of the anticancer activity of Azadirachta indica (Neem) in oral cancer. J Oral Biol Craniofac Res 2020;10(2):206-9.
[Crossref] [Google Scholar] [PubMed]
- Braga TM, Rocha L, Chung TY, Oliveira RF, Pinho C, Oliveira AI, Morgado J, Cruz A. Azadirachta indica A. juss. in vivo toxicity-an updated review. Molecules 2021;26(2):252.
[Crossref] [Google Scholar] [PubMed]
- Patil SM, Shirahatti PS, VB CK, Ramu R, Prasad N. Azadirachta indica A. Juss (neem) as a contraceptive: An evidence-based review on its pharmacological efficiency. Phytomedicine 2021;88:153596.
[Crossref] [Google Scholar] [PubMed]
- Londonkar RL, Hugar AL. Physicochemical, phytochemical profiling and antimicrobial activity of Pterocarpus marsupium. Int J Pharm Sci Res 2017;8(5):2177.
- Singh P, Bajpai V, Gupta A, Gaikwad AN, Maurya R, Kumar B. Identification and quantification of secondary metabolites of Pterocarpus marsupium by LC–MS techniques and its in-vitro lipid lowering activity. Ind Crop Prod 2019;127:26-35.
- Mishra A, Srivastava R, Srivastava SP, Gautam S, Tamrakar AK, Maurya R, et al. Antidiabetic activity of heart wood of Pterocarpus marsupium Roxb. and analysis of phytoconstituents. Indian J Exp Biol 2013;51(5):363-74.
[Google Scholar] [PubMed]
- Majeed M, Majeed S, Jain R, Mundkur L, Rajalakshmi HR, Lad PS, et al. An open-label single-arm, monocentric study assessing the efficacy and safety of natural pterostilbene (Pterocarpus marsupium) for skin brightening and antiaging effects. Clin Cosmet Investig Dermatol 2020:105-16.
[Crossref] [Google Scholar] [PubMed]
- L Hugar A, P Kanjikar A, L Londonkar R. A novel potential reproductive effects of Pterocarpus marsupium methanolic extract on testosterone propionate induced polycystic ovary syndrome in female albino rats. Endocr Metab Immune Disord Drug Targets 2017;17(4):317-23.
[Crossref] [Google Scholar] [PubMed]
- Deguchi T, Tamai A, Asahara K, Miyamoto K, Miyamoto A, Nomura M, et al. Anti-tyrosinase and anti-oxidative activities by Asana: The heartwood of Pterocarpus marsupium. Nat Prod Commun 2019;14(10):1934578X19883727.
- Shaju N, Gautam M, Khayum A, Venkatesh G. Prevention and healing of calcium signaling mediated neuronal damage on successive administration of flavonoid enriched Pterocarpus marsupium roxb in peripheral neuropathy model. Curr Bioactive Compounds 2020;16(9):1346-55.
- Dabur R. Identification of molecular pathways affected by treatment with heartwood water extract of Pterocarpus marsupium in MCF 7 cancer cell line. J Herbal Med 2017;9:42-52.
- Thani PR. A comprehensive review on Picrorhiza kurroa Royle ex Benth. J Pharmacogn Phytochem 2021;10(3):307-13.
- Khandekar S, Pachpor A, Maurya S, Pansare T, Kumar Maurya S. Role of Katuka (Picrorhiza Kurroa Royle Ex Benth.) in obesity WSR to ayurvedic and modern aspect: A review. Int J Herb Med 2019;7(6):31-5.
- Misar S. Hepatoprotective and hypolipidemic effect of kutaki (Picrorhizakurroa royle ex benth.): A review. IJRAR 2019;6(1):782-8.
- Maurya R, Singh R, Deepak M, Handa SS, Yadav PP, Mishra PK. Constituents of Pterocarpus marsupium: An ayurvedic crude drug. Phytochemistry 2004;65(7):915-20.
[Crossref] [Google Scholar] [PubMed]
- Mehta S, Sharma AK, Singh RK. Advances in ethnobotany, synthetic phytochemistry and pharmacology of endangered herb Picrorhiza kurroa (Kutki): A comprehensive review (2010-2020). Mini Rev Med Chem 2021;21(19):2976-95.
[Crossref] [Google Scholar] [PubMed]
- Gianfaldoni S, Wollina U, Tirant M, Tchernev G, Lotti J, Satolli F, et al. Herbal compounds for the treatment of vitiligo: A review. Open access Maced J Med Sci 2018;6(1):203-7.
[Crossref] [Google Scholar] [PubMed]
- Jain D, Chaudhary P, Kotnala A, Hossain R, Bisht K, Hossain MN. Hepatoprotective activity of medicinal plants: A mini review. J Med Plants 2020;8(5):183-8.
- Kumar S, Patial V, Soni S, Sharma S, Pratap K, Kumar D, et al. Picrorhiza kurroa enhances ß-Cell mass proliferation and insulin secretion in streptozotocin evoked ß-Cell damage in rats. Front Pharmacol 2017;8:537.
[Crossref] [Google Scholar] [PubMed]
- Ahmad R, Fatima A, Srivastava AN, Khan MA. Evaluation of apoptotic activity of Withania coagulans methanolic extract against human breast cancer and Vero cell lines. J Ayurveda Integr Med 2017;8(3):177-83.
[Crossref] [Google Scholar] [PubMed]
- Qureshi SA, Jahan M, Lateef T, Ahmed D, Rais S, Azmi MB. Presence of gallic acid and rutin improve the hepatoprotective strength of Withania coagulans. Pak J Pharm Sci 2019;32(1):301-8.
- Rehman S, Keefover-Ring K, Haq IU, Dilshad E, Khan MI, Akhtar N, et al. Drier climatic conditions increase withanolide content of Withania coagulans enhancing its inhibitory potential against human prostate cancer cells. Appl Biochem Biotechnol 2019;188:460-80.
[Crossref] [Google Scholar] [PubMed]
- Maher S, Choudhary MI, Saleem F, Rasheed S, Waheed I, Halim SA, et al. Isolation of antidiabetic withanolides from Withania coagulans Dunal and their in vitro and in silico validation. Biology 2020;9(8):197.
[Crossref] [Google Scholar] [PubMed]
- Maqsood M, Qureshi R, Ikram M, Ahmad MS, Jabeen B, Asi MR, et al. In vitro anticancer activities of Withania coagulans against HeLa, MCF-7, RD, RG2, and INS-1 cancer cells and phytochemical analysis. Integr Med Res 2018;7(2):184-91.
[Crossref] [Google Scholar] [PubMed]
- Salehi M, Aghamaali MR, Sajedi RH, Asghari SM, Jorjani E. Purification and characterization of a milk-clotting aspartic protease from Withania coagulans fruit. Int J Biol Macromol 2017;98:847-54.
[Crossref] [Google Scholar] [PubMed]
- Phull AR, Hassan M, Abbas Q, Raza H, Haq IU, Seo SY, et al. In vitro, in silico elucidation of antiurease activity, kinetic mechanism and COX-2 inhibitory efficacy of Coagulansin A of Withania coagulans. Chem Biodivers 2018;15(1):e1700427.
[Crossref] [Google Scholar] [PubMed]
- Sharma S, Joshi A, Hemalatha S. Protective effect of Withania coagulans fruit extract on cisplatin-induced nephrotoxicity in rats. Pharmacogn Res 2017;9(4):354.
[Crossref] [Google Scholar] [PubMed]
- Sharma A, Cannoo DS. Comparative evaluation of extraction solvents/techniques for antioxidant potential and phytochemical composition from roots of Nepeta leucophylla and quantification of polyphenolic constituents by RP-HPLC-DAD. J Food Measure 2016;10:658-69.
- Chanda S, Dave R. In vitro models for antioxidant activity evaluation and some medicinal plants possessing antioxidant properties: An overview. Afr J Microbiol Res 2009;3(13):981-96.
- Kifle ZD, Yesuf JS, Atnafie SA. Evaluation of in vitro and in vivo anti-diabetic, anti-hyperlipidemic and anti-oxidant activity of flower crude extract and solvent fractions of Hagenia abyssinica (rosaceae). J Exp Pharmacol 2020:151-67.
[Crossref] [Google Scholar] [PubMed]
- Rege AA, Chowdhary AS. Evaluation of alpha-amylase and alpha-glucosidase inhibitory activities of Rhizophora mucronata. Int J Pharm Sci Res 2014;5(6):2261.
- Aslani A, Eatesam P. Design, formulation and physicochemical evaluation of acetaminophen effervescent tablets. 2013;2(2):140–9.
- Kaur D. Formulation and evaluation of hard gelatin capsules containing Bacopa monnieri. Int J Pharm Edu Res 2020;1(02):33-7.
- Lachman L, Lieberman HA, Kanig JL. The theory and practice of industrial pharmacy. Varghese Publishing House; 2009.
- Quality control methods for herbal materials. World Health Organization, Geneva, Switzerland. 2011; 187.
- Kaur N, Sharma S. Formulation, evaluation, and stability testing of polyherbal antidiabetic capsules. Int J Pharm Invest 2023;13(1)1-8.
- Senthilvel CK, Karuppaiyan K, Pothumani A, Vedharethinam A, Jose AW, Muthu Mohamed JM, et al. Development of atorvastatin calcium biloaded capsules for oral administration of hypercholesterolemia. Evid Based Complement Alternat Med 2022;2022:4995508.
[Crossref] [Google Scholar] [PubMed]
- Bhatt D, Jethva K, Patel S, Zaveri M. Development and standardization of Polyherbal formulation for the management of breast cancer. Development 2017;2(3):24-8.
- Mechchate H, Es-Safi I, Louba A, Alqahtani AS, Nasr FA, Noman OM, et al. In vitro alpha-amylase and alpha-glucosidase inhibitory activity and in vivo antidiabetic activity of Withania frutescens L. Foliar extract. Molecules 2021;26(2):293.
[Crossref] [Google Scholar] [PubMed]
- Petit T, Puskar L. FTIR spectroscopy of nanodiamonds: Methods and interpretation. Diamond Related Mater 2018;89:52-66.
- Noorjahan CM, Shahina SJ, Deepika T, Rafiq S. Green synthesis and characterization of zinc oxide nanoparticles from Neem (Azadirachta indicia). Int J Sci Eng Technol Res 2015;4(30):5751-3.
- Riaz Z, Ali MN, Qureshi Z, Mohsin M. In vitro investigation and evaluation of novel drug based on polyherbal extract against Type 2 Diabetes. J Diabetes Res 2020;2020:1-9.
- Sigma-Aldrich. IR Spectrum Table. Sigma-Aldrich. 2019;1.
 ): Azadirachta indica; (
): Azadirachta indica; ( ): Picrorhiza Kurroa; (
): Picrorhiza Kurroa; ( ): Pterocarpus marsupium and (
): Pterocarpus marsupium and ( ): Withania coagulans
): Withania coagulans