- *Corresponding Author:
- K. K. Mali
Department of Pharmaceutics, YSPM’s Yashoda Technical Campus, Faculty of Pharmacy, Wadhe, Satara-415 011, India
E-mail: malikailas@gmail.com
| Date of Submission | 06 July 2016 |
| Date of Revision | 04 January 2017 |
| Date of Acceptance | 18 April 2017 |
| Indian J Pharm Sci 2017;79(3): 463-468 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The objective of the present study was to investigate the antidiabetic effect of garcinol on streptozotocin-induced diabetic rats. Acute toxicity of garcinol was studied according to the guidelines of Organisation for Economic Co-operation and Development. Oral glucose tolerance test was used as a preliminary screening method. Diabetes was induced in rats by intraperitoneal injection of streptozotocin (60 mg/kg). After induction of diabetes, rats were treated with garcinol (25, 50 and 100 mg/kg) and metformin (100 mg/kg) for 28 days. Blood samples were collected for estimating biochemical parameters. Administration of garcinol caused significant reduction in elevated levels of blood glucose, glycosyated hemoglobin and lipids in diabetic rats. Histopathological study revealed regeneration of pancreatic β cells in the garcinol-treated groups. These results indicated that garcinol showed significant antidiabetic and lipid-lowering effects in streptozotocin-induced diabetes rats.
Keywords
Garcinol, antidiabetic activity, streptozotocin
Diabetes mellitus (DM) is a group of metabolic diseases characterized by hyperglycaemia and disturbances in carbohydrate, fat and protein metabolism resulting from defects in insulin secretion, insulin action or both [1]. The number of patients with DM is predicted to increase globally to 200 million within the next few years [2].
Various oral hypoglycaemic agents such as sulfonylureas, biguanides, meglitinides, thiazolidinediones, glucosidase inhibitors and peptide analogues were used alone or in combination to treat diabetes. Some like biguanides and sulfonylureas have the potential to cause serious side effects but these agents are required to be taken throughout life [3]. Extracts of some medicinal plants have shown promising antidiabetic effects due to the presence of antioxidant and free radical scavenging constituents like polyphenols. These polyphenols might also help in regeneration of β-cells previously damaged due to the oxidation caused by high blood glucose level. Polyphenols might also protect pancreatic islets against the cytotoxic effects of streptozotocin (STZ) [4]. Thus, the natural antioxidants might have the potential to be used as effective alternatives to the oral hypoglycaemic agents in the treatment of diabetes.
Garcinol is a polyisoprenylated benzophenone derivative isolated from Garcinia indica (Kokum). Garcinol is structurally similar to a well-known antioxidant curcumin, which contained both phenolic hydroxyl groups and a β-diketone moiety [5]. Literature revealed that garcinol exhibited antioxidant, antiinflammatory, anticancer and hepatoprotective activities [6-9]. Garcinol was also reported to exhibit radical scavenging activity and suppressed protein glycation in vitro [5]. Till date, the antidiabetic activity of garcinol has not been tested in vivo. Hence, the present investigation was undertaken to investigate the effect of garcinol in STZ-induced diabetic rats and its acute toxicity.
Garcinol was obtained from Sami Labs Pvt. Ltd, Bangalore, India. STZ was procured from Chemvenio, LLC, USA. All other chemicals and kits used in the study were of analytical grade. Female Wistar rats (150-200 g) procured from Shri Venkateshwara Enterprises, Bengaluru, were used to assess antidiabetic activity [10]. Female Swiss albino mice (20-25 g) procured from Smt. Kashibai Navale College of Pharmacy, Pune were used for the acute toxicity study. Animals were housed in the animal house, Satara College of Pharmacy, Satara, at a temperature of 25±1° and 45±5% relative humidity with a 12:12 h day:night cycle. The animals were fed with standard laboratory diet and allowed drinking water ad libitum. Studies were carried out in the Pharmacology Department, Satara College of Pharmacy. The experimental protocol was approved by the Institutional Animal Ethics Committee (SCOP/IAEC/022/11-12).
Oral acute toxicity study of garcinol was carried out according to the Organization for Economic Co-operation and Development (OECD)-guidelines 423 [11]. Animals were divided in to six groups of three animals each. All animals were fasted overnight with access to water ad libitum. Single dose of garcinol (50, 100, 300, 500, 1000 and 2000 mg/kg) was given orally to each animal. The animals were observed individually during the first 30 min after dosing, periodically during the first 24 h and daily thereafter for 14 d.
Oral glucose tolerance test (OGTT) was performed in overnight fasted (18 h) normal rats. Rats were divided into six groups of six animals each. Normal control and diabetic control received 0.1% w/v carboxymethylcellulose (CMC) and standard treatment group received glibenclamide (2.5 mg/kg). Treatment groups I, II and III have received garcinol 10, 20 and 30 mg/kg, respectively. Glucose (3 g/kg) was administered orally 30 min after the administration of garcinol to treatment group. Blood was withdrawn from the retro orbital sinus under mild ether anaesthesia at 0, 30, 60 and 120 min of glucose administration and glucose levels were estimated using glucose oxidaseperoxidase method [12].
Rats weighing 150-200 g were selected and fasted for 16 h prior to experiments and allowed an excess ad libitum. Experimental diabetes was induced by single intraperitoneal injection of freshly prepared STZ (60 mg/kg) in 0.1 M citrate buffer (pH 4.5). Seven days after STZ administration blood glucose level of each rat was determined. Rats with blood glucose level above 200 mg/dl were considered diabetic and were included in the study [13].
Wistar rats were divided into six groups each consisting of six animals. CMC in distilled water (0.1% w/v) was used as vehicle. Doses of garcinol at 25, 50 and 100 mg/kg were suspended in the vehicle and administered to treatment groups orally once daily for 28 d. Group I served as control, group II as diabetic control, group III as standard treatment (metformin 100 mg/kg); group IV, V and VI as treatment groups and received garcinol at 25, 50 and 100 mg/kg, respectively. Body weight and blood glucose levels were estimated on 0, 7, 14, 21 and 28 d. Glycosylated haemoglobin and lipid profile were estimated on d 28. Blood glucose levels were estimated by glucose oxidase-peroxidase method.
Colorimetric method based on the phenol-sulphuric acid reaction of carbohydrates for the determination of sugars bound to haemoglobin was used. Glycosylated haemoglobin (HbA1c) was estimated by the method of Nayak and Pattabiraman [14]. Total cholesterol (TC), triglyceride (TG), high density lipoprotein (HDL) was estimated according to the procedure of the kits supplied by Merck, Mumbai, India. Low density lipoprotein (LDL) and very low density lipoprotein (VLDL) was calculated by using Friedewald's formula (FF) [15].
On d 28 of treatment, rats were anesthetised by intraperitoneal sodium pentobarbital (150 mg/kg) and euthanasia was confirmed by cervical dislocation. Pancreas was dissected and immediately kept in 10% formalin. The fixed tissues or organs were then dehydrated and embedded in paraffin wax before being sectioned to approximately 5 μm thickness. The sectioned tissues were stained with haematoxylin and eosin (H and E) followed by histopathological examinations [16]. Results were expressed as mean±SEM. Statistical analysis was carried out by one way analysis of variance (ANOVA) followed by Dunnet’s multiple comparison test using GraphPad Prism. P<0.05 was considered as the minimal level of statistical significance.
In the acute toxicity study, significant signs of toxicity such as respiratory embarrassment, sleep, lethargy and mortality were found at a dose 1000 and 2000 mg/kg. Two deaths were observed at a dose of 2000 mg/kg. According to the OECD 423 guidelines, dose of garcinol was reduced to 300 mg/kg. Repeated administration of 300 mg/kg of garcinol did not show any signs of toxicity or mortality. Hence a dose between 300-2000 mg/kg was selected, and when the study was repeated with 1000 mg/kg, garcinol showed death of one animal. The LD50 value of garcinol was thus taken as 1000 mg/kg.
Blood glucose levels increased rapidly above the fasting value and then subsequently decreased at 30 min after glucose administration. No significant difference between the effects of 10 and 30 mg garcinol/kg could be observed. Garcinol at 30 mg/kg showed maximum fall of 18.2% in blood glucose level at 60 min after glucose administration.
Administration of STZ (60 mg/kg) to groups of animals produced severe loss in body weight as compared to normal control. Oral administration of garcinol showed significant (P<0.05) increase in body weight on 14, 21 and 28 d after initiation of treatment. Administration of STZ (60 mg/kg) to diabetic control group showed increase in blood glucose level as compared to normal control. In all, garcinol-treated groups, blood glucose levels were found to be significantly (P<0.05) decreased on 14, 21 and 28 d (Table 1). Garcinol at 100 mg/kg on d 28 showed maximum reduction in blood glucose level (19.3%). HbA1c levels were found to be increased in diabetic animals compared to normal rats. After administration of garcinol at dose 25, 50 and 100 mg/kg, marked reduction in elevated HbA1c levels could be observed as compared to diabetic control group (Table 2).
| Experimental groups | Blood glucose levels (mg/dl) | ||||
|---|---|---|---|---|---|
| 0 day | 7th day | 14th day | 21st day | 28th day | |
| Normal control | 96.3±8.3 | 111±11 | 104±5.5 | 103±4.7 | 99.2±3.5 |
| Diabetic control | 213±7.4 | 231±6.3 | 251±6.6 | 261±6.8 | 270±7.2 |
| Standard treatment | 211±6.5 | 200±5.4 | 202±5.0 | 201±4.5 | 182±4.4 |
| Treatment group I | 208±4.5 | 221±2.9 | 229±5.6a | 235±3.8a | 242±7.8 |
| Treatment group II | 202±9.2 | 217±4.5 | 221±6.1 | 227±6.6 | 231±4.6 |
| Treatment group III | 213±7.3 | 212±7.4 | 219±4.2 | 224±5.6 | 218±6.0 |
Table 1: Effect of Garcinol on Blood Glucose Levels
| Groups | Dose | Day 28th |
|---|---|---|
| NC | 0.1% CMC | 5.40±0.13 |
| DC | 0.1% CMC | 5.80±0.03 |
| ST | 100 mg/kg metformin | 5.55±0.02 |
| TG I | 25 mg/kg garcinol | 5.60±0.02 |
| TG II | 50 mg/kg garcinol | 5.55±0.04 |
| TG III | 100 mg/kg garcinol | 5.52±0.07 |
Table 2: Effect of Garcinol on Glycoslyted Haemoglobin (HBA1C)
Administration of STZ (60 mg/kg) resulted in the levels of TC, TG, LDL and VLDL to get elevated significantly and HDL levels were significantly decreased as compared to controls. Administration of garcinol at doses of 25, 50 and 100 mg/kg, showed significant (P<0.05) reduction in TC, TG, LDL and VLDL levels and an increase in HDL levels as compared to diabetic control group (Table 3).
| Groups | Dose | TC (U/l) |
TG (mg/dl) |
HDL (mg/dl) |
LDL (mg/dl) |
VLDL (mg/dl) |
|---|---|---|---|---|---|---|
| NC | 0.1% CMC | 60.6±1.71* | 31.2±1.86* | 12.5±0.30* | 54.2±1.94* | 6.23±0.37*a |
| DC | 0.1% CMC | 70.5±1.41† | 40.1±1.84† | 11.1±0.11† | 67.5±1.38† | 8.63±0.36† |
| SC | 100 mg/kg metformin |
61.2±2.94* | 32.4±1.95* | 13.0±0.20* | 57.6±2.08* | 6.82±0.25*a |
| TG I | 25 mg/kg garcinol |
63.7±0.95*a | 33.7±1.26*a | 12.8±0.13*a | 58.5±1.29* | 6.87±0.20*a |
| TG II | 50 mg/kg garcinol |
64.3±1.34 | 33.0±0.86* | 13.0±0.46* | 57.8±2.15* | 6.38±0.27* |
| TG III | 100 mg/kg garcinol |
63.8±1.62*a | 32.3±0.31* | 12.8±0.11* | 57.5±1.55* | 6.45±0.06* |
Table 3: Effect of Garcinol on Lipid Profile
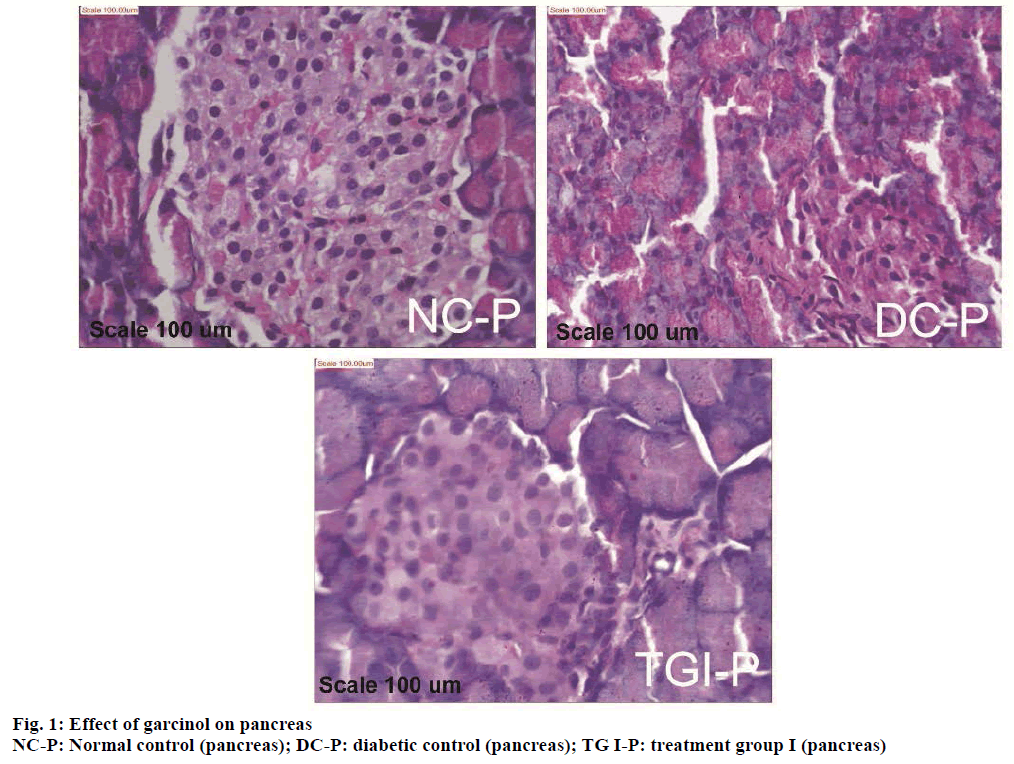
Histopathological study of the pancreas showed normal acini, and normal cellularity in the islets of Langerhans of control pancreas (NC-P, Figure 1). Diabetic control group showed extensive damage to islets of Langerhans and decreased number of islet cells (DC-P, Figure 1). Diabetic animals treated with (25 mg/kg) of garcinol (TG I-P, Figure 1) showed normal acini cells, presence of islet cells and few foci of regeneration of pancreatic β cells.
In this study, diabetes was induced in the rats using 60 mg/kg dose of STZ, a glucosamine derivative of nitrosourea, which selectively destroys pancreatic islets of β-cells and causes development of hyperglycaemia and glycosuria, as seen in type 1 diabetic patients. Metformin hydrochloride, a biguanide provides an effective treatment for patients with diabetes, which promotes glucose uptake by GLUT4 transporters and reduces glucose production in liver. Other than its glucose-lowering properties, metformin is supposed to have an antioxidant property. It is capable of restoring liver antioxidant enzymes, superoxide dismutase (SOD), and catalase (CAT) in STZ-induced diabetic rats [17].
The increase in blood glucose level might be due to shortage of insulin as a result of destruction of pancreas interceded by STZ action. STZ boosts ATP dephosphorylation, which in turn generates superoxide anions, hydrogen peroxide, and hydroxyl radicals. This leads to an elevation in the intracellular peroxides in pancreatic islets, which may induce damage due to the reactive oxygen species (ROS) [18]. Under hyperglycaemic conditions, antioxidants are supposed to regenerate damaged extracellular matrix proteins and promote cell growth [19]. Thus, antioxidants might help in the management of DM.
In acute toxicity study, garcinol was not safe above 1000 mg/kg possibly due to its toxic effect on the organs of the animals. When garcinol was administered to glucose-loaded animals, reduction in blood glucose levels was observed after 60 min. This may be due to prevention of damage to the β-cells of islets of langerhans resulting from the antioxidant effect of garcinol [4]. The phenolic hydroxyl groups coupled with the β-diketone moiety in garcinol may, via formation of resonance stabilized intermediates, help to prevent the free radical species from propagating and limit the further oxidative-stress-related damage [20]. Besides, closing of K+ATP (adenosine triphosphate) channel and release of insulin from the undamaged β-cells may also be responsible for this decrease in glucose level [21,22].
STZ-induced diabetes is characterized by a severe loss in body weight [23]. Oral administration of garcinol showed significant improvement in body weight on 21st and 28th d. The increase in body weight could be due to amelioration of glycaemic control and structural proteins synthesis [24]. Administration of STZ (60 mg/kg) to diabetic control group showed increase in blood glucose level as compared to normal control. All garcinol treated groups exhibited significant reduction in blood glucose level, which may be attributed to the regeneration of β-cells due to antioxidant effect of garcinol as discussed above, leading to more insulin release.
Generally, in uncontrolled or poorly controlled diabetes, there is an increased glycosylation of a number of proteins, including HbA1c [25]. The level of HbA1c is a reliable index of glycemic control in DM, which reflects the average blood concentration. In the present study, HbA1c levels were found to be increased in diabetic animals compared to normal rats. There is an evidence that glycation may itself induces the generation of oxygen-derived free radicals in diabetic conditions. Treatment with garcinol showed decrease in the glycosylated haemoglobin, comparable to animals, which received standard drug metformin [26]. Garcinol also showed significant reduction in TC, TG, LDL and VLDL as compared to diabetic control group. The levels of HDL increased significantly as compared to diabetic control group. This may be due to antihyperlipidemic properties of garcinol [25] associated with AMPK activation via LKB1, increasing glucose uptake and GLUt4 translocation to the plasma membrane [24].
STZ acted as a diabetogenic through destruction of the β cells of the islets of Langerhans [21]. Histopathological investigation supports presence of islets cell and few foci of regeneration of pancreatic β-cells, in garcinol treated animals. This suggests that garcinol assists in generation of insulin producing β-cells.
On the basis of results, garcinol showed significant antidiabetic and lipid lowering effect on STZ-induced diabetic rats due to its antioxidant activity. The present study indicated the usefulness of garcinol for further development as a therapeutic agent for treatment of diabetes. Moreover, further study is required to illustrate the mechanism of action of garcinol at the cellular and molecular level.
Acknowledgements
The authors thank Dr. Suresh Kumar, Sami Labs Ltd., Bengaluru for the garcinol gift sample.
Conflict of interest
All authors declare no conflict of interests.
Financial support
Nil.
References
- Mali KK, Dias RJ, Havaldar VD, Mahajan NS. Hypoglycemic activity of Caralluma adscendensin alloxan induced diabetic rats. Int J Chem Sci 2009;7:517-22.
- Belhekar SN, Chaudhari PD, Saryawanshi JS, Mali KK, Pandhare RB. Antidiabetic and antihyperlipidemic effects of Thespesia populneafruit pulp extracts on alloxan-induced diabetic rats. Indian J Pharm Sci 2013;75:217-21.
- Mohammed SA, Yaqub AG, Nicholas AO, Arastus W, Muhammad M, Abdullahi S. Review on diabetes, synthetic drugs and glycemic effects of medicinal plants. J Med Plants Res 2013;7:2628-37.
- Kamalakkannan N, Prince PS. Antihyperglycaemic and antioxidant effect of rutin, a polyphenolic flavonoid, in streptozotocin‐induced diabetic wistar rats. Basic Clin Pharmacol Toxicol 2006;98:97-103.
- Sang S, Cheng X, Bai X, Stark RE, Rosen RT. Chemical studies on antioxidant mechanism of garcinol: analysis of radical reaction products of garcinol and their antitumor activities. Tetrahedron 2001:57;9931-8.
- Yamaguchi F, Ariga T, Yoshimura Y, Nakazawa H. Antioxidative and antiglycation activity of garcinol from Garcinia indicafruit rind. J Agri Food Chem 2000;48:180-85.
- Yamaguchi F, Saito M, Ariga T, Yoshimura Y, Nakazawa H. Free radical scavenging activity and antiulcer activity of garcinol from Garcinia indicafruit Rind. J Agri Food Chem 2000;48:2320-5.
- Ahmad A, Sarkar SH, Aboukameel A, Ali S, Biersack B, Seibt S, et al. Anticancer action of garcinol in vitroand in vivo is in part mediated through inhibition of STAT-3 signaling. Carcinogenesis 2012;33:2450-6.
- Jing Y, Ai Q, Lin L, Dai J, Jia M, Zhou D, et al. Protective effects of garcinol in mice with lipopolysaccharide/D-galactosamine-induced apoptotic liver injury. Int Immunopharmacol 2014;19:373-80.
- Kumar AY, Nandakumar K, Handral M, Talwar S, Dhayabaran D. Hypoglycaemic and anti-diabetic activity of stem bark extracts Erythrina indicain normal and alloxan-induced diabetic rats. Saudi Pharm J 2011;19:35-42.
- http://www.oecd-ilibrary.org/docserver/download/9742301e.pdf?expires=1503319141&id=id&accname=guest&checksum=4D0D94ABE5EC05F41F704242A59F0DFA.
- Shirwaikar A, Rajendran K, Barik R. Effect of aqueous bark extract of Garuga pinnata Roxb. in streptozotocin–nicotinamide induced type-II diabetes mellitus. J Ethnopharmacol 2006;107:285-90.
- Vogel HG. Drug discovery and Evaluation: Pharmacological Assays. 2nd ed. Berlin: Springer-Verlag; 2002. p. 951.
- Nayak SS, Pattabiraman TN. A new colorimetric method for the estimation of glycosylated haemoglobin. Clin Chim Acta 1981;109;267-74.
- Friedewald, WT, Levy RT, Frederickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultra-centrifuge. Clin Chem 1972: 18;499-502.
- Vessal M, Hemmmmati M, Vasei M. Antidiabetic effects of quercetin in streptozotocin-induced diabetic rats. Comp Biochem Physiol C Toxicol Pharmacol 2003;135:357-64.
- Obi BC, Okoye TC, Okpashi VE, Igwe CN, Alumanah EO. Comparative Study of the Antioxidant Effects of Metformin, Glibenclamide, and Repaglinide in Alloxan-Induced Diabetic Rats. J Diabetes Res 2016;2016:1635361.
- Lenzen S, Drinkgern J, Tiedge M. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Rad Biol Med 1996;20:463-6.
- Smith PR, Thornalley PJ. Mechanism of the degradation of non‐enzymatically glycated proteins under physiological conditions. Eur J Biochem 1992;210:729-39.
- Saadat N, Gupta SV. Potential role of garcinol as an anticancer agent. J Oncol 2012;2012:647206.
- Pari L, Saravanan G. Antidiabetic effect of Cogentdb, a herbal drug in alloxan-induced diabetes mellitus. Comp Biochem Physiol C Toxicol Pharmacol 2002;131:19-25.
- Gupta R, Sharma AK, Dobhal MP, Sharma, MC, Gupta RS. Antidiabetic and antioxidant potential of β-sitosterol in Streptozotocin-induced experimental hyperglycemia. J Diabetes 2011:3:29-37.
- Al-Shamaony L, Al-Khazataji SM, Twaij HA. Hypoglycemic effect of Artemisia herb Alba II. Effect of valuable extract on some blood parameters in diabetic animals. J Ethnopharmacol 1994;43:167-71.
- Balamurugan R, Ignacimuthu S. Antidiabetic activity of γ-sitosterol isolated from Lippianodi floraL. in streptozotocin induced diabetic rats. Eur J Pharmacol 2011;5:3-9.
- Grover JK, Vats V, Rathi SS. Antihyperglycemic effect of Eugenia Jambolonaand Tinospora Cordifoliain experimental diabetes and their effect on key metabolic enzyme involved in carbohydrate metabolism. J Ethanopharmacol 2000;73:461-70.
- Roesler WJ, Khandelwal RL. Quantitation of glycogen synthase and phosphorylase protein in mouse liver: correlation between enzymatic protein and enzyme activity. Arch Biochem Biophys 1986;244:397-407.