- *Corresponding Author:
- Alby Alphons Baby
Department of Botany, Christian College, Chengannur, Kerala 689122, India
E-mail: albyalphonsbaby@gmail.com
| Date of Received | 30 September 2022 |
| Date of Revision | 27 May 2024 |
| Date of Acceptance | 25 September 2024 |
| Indian J Pharm Sci 2024;86(5):1663-1670 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The use of natural products against some prevalent ailments in human beings and household animals is a common phenomenon in both developed and developing countries. So, documentation of this indigenous system of knowledge is important because with the demise of the present traditional medical practitioners this treasure house of knowledge would become extinct. Oxidative stress disorders dangerously lead to malignant tumors, diabetes and other severe disease conditions. In the present study antioxidant property using 2,2-diphenyl-1-picrylhydrazyl free radical scavenging assay and in vitro cytotoxicity screening using Dalton’s lymphoma ascites cells were used to choose the bioactive solvent extract of Areca catechu for anti-cancer screening and phytochemical profiling. In the 2,2-diphenyl-1-picrylhydrazyl free radical scavenging assay aqueous extract was highly efficient with least half-maximal inhibitory concentration value (53 μg/l) and in in vitro cytotoxicity screening, maximum cytotoxicity (89 %) was attained at a concentration of 200 μg/l of ethanolic extract and minimum half-maximal inhibitory concentration value was showed by aqueous extract (38 μg/l). So the ethanolic and aqueous extracts were selected for the in vivo anti-cancer screening. The plant extract was found to be non-toxic in the dose of 250 mg/kg body weight of the mice. In vivo anti-cancer efficiency of the medicinal plant was screened via ascites tumour and solid tumour models. Treatment of Areca catechu ethanolic and aqueous extracts at different concentrations increased the survival rate of animals in ascites tumour and suppressed the development of solid tumour. The result was compared with the commercial drug cyclophosphamide. The aqueous extract was very efficient as an anti-tumour agent and caused 71.9 % of increase in life span in 100 mg/kg concentration. The percentage of life span increase in cyclophosphamide treated animals was 72.5. So, the plant extract was almost equally important to commercial drug in increasing the rate of survival of the affected animals. The volume of solid tumour in control group on 35th d was (4.550±0.622) mm while in case of aqueous root extract in 100 mg/kg concentration was (0.48±0.92) mm on the same day. The value shown by the standard drug (0.643±0.111) mm was more than that of the aqueous extract at 100 mg/kg. So, the aqueous extract of Areca catechu was subjected to high resolution liquid chromatograph mass spectrometric analysis. 14 compounds were identified through this analysis. That includes mitoxantrone, artemether, valporic acid, leupeptin etc., which proves the medicinal applications of Areca catechu root.
Keywords
Areca catechu, anti-oxidant property, in vitro cytotoxicity, anti-cancer property, phytochemical profiling
Human civilization begins in the forest as an integral part of the forest ecosystem. Humans acquired unique knowledge about various plants and animals around them by experiences and experimentation. They developed, maintained and preserved this knowledge over many generations. Through years of co-evolution and co-existence, the traditional communities are able to identify the useful and harmful elements around them. Traditional knowledge on health care is very important in giving clues regarding the various medicinally active plants, this is a very important field of research leading to the discovery of new bio-pharmaceuticals which are effective and less harmful to the mankind[1].
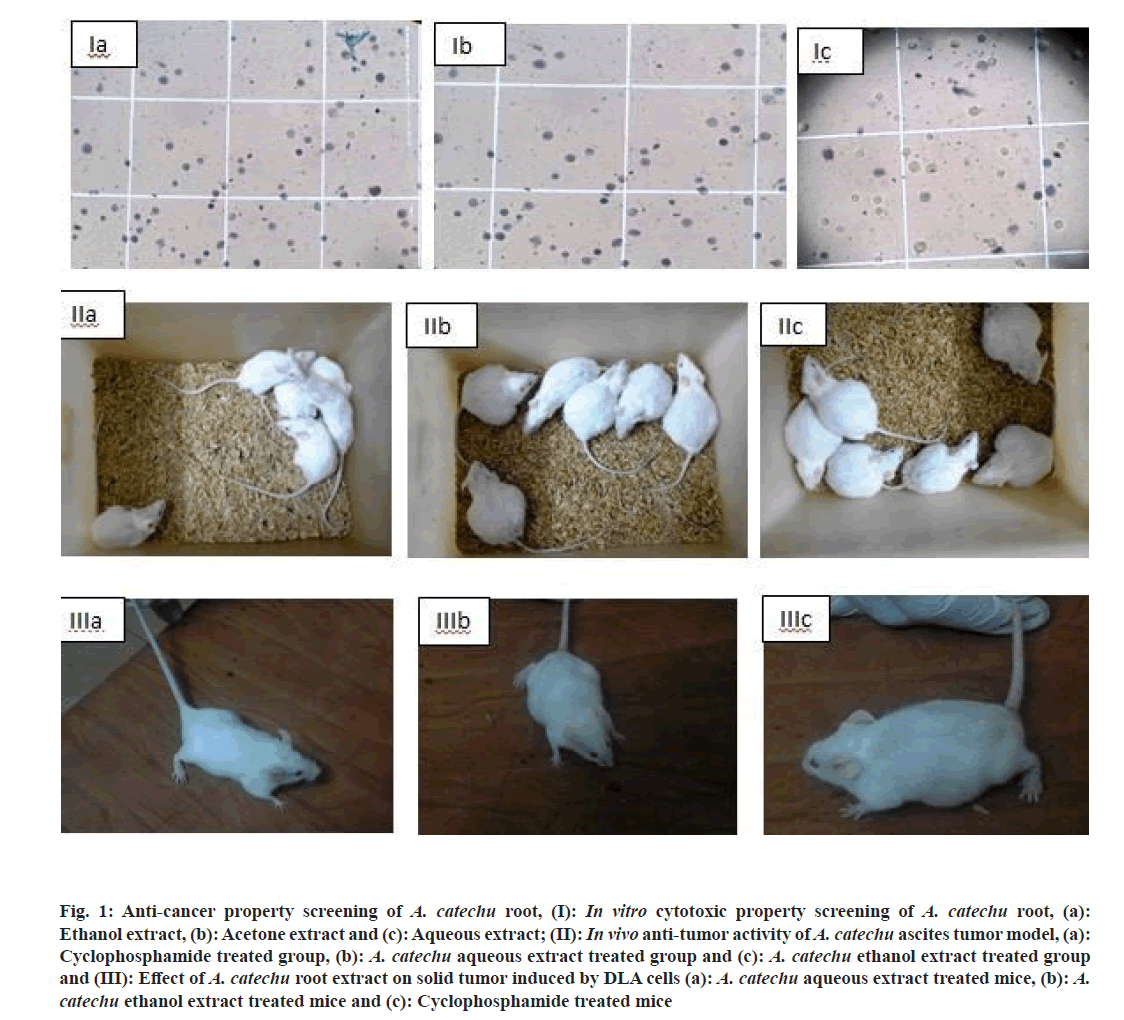
Betel nut (Areaca catechu (A. catechu)) from the family Arecaceae grows in India, Malaysia, Taiwan and many other Asian countries. Nut is the commercially useful part and is important in traditional medicine also. It contains alkaloids, tannins, polyphenols and sugars[2]. Betel nut have reported anthelmintic[3], wound healing[4], antidepressant[5], anti-Human Immunodeficiency Virus (HIV)[6] and anti-mycobacterial activities[7]. There are extensive studies on the medicinal properties and active principles in the nut and pericarp. In traditional medicines of Kerala, A. catechu root powder is applied for skin allergies, different worm infections including ring worm infection and adding as a component in health tonic preparations[8]. Our previous paper evaluated the anti-oxidant, antihelmintic and anti-microbial properties of A. catechu root crude extract[9]. The present study was carried out to examine the anti-cancerous property of A. catechu root and the identification of the active principles in it using Liquid Chromatography-Mass Spectrometric (LC-MS) analysis (fig. 1).
Fig. 1: Anti-cancer property screening of A. catechu root, (I): in vitro cytotoxic property screening of A. catechu root, (a): Ethanol extract, (b): Acetone extract and (c): Aqueous extract; (II): in vivo anti-tumor activity of A. catechu ascites tumor model, (a): Cyclophosphamide treated group, (b): A. catechu aqueous extract treated group and (c): A. catechu ethanol extract treated group and (III): Effect of A. catechu root extract on solid tumor induced by DLA cells (a): A. catechu aqueous extract treated mice, (b): A. catechu ethanol extract treated mice and (c): Cyclophosphamide treated mice
Materials and Methods
Plant material:
Fresh roots of A. catechu were collected from Mannamangalam Village of Thrissur district Kerala. The material was authenticated by Dr. Regi Raphael K and a voucher specimen is also deposited in the herbarium of Botany Department, St. Mary’s College, Thrissur, Kerala, India with voucher number SMC/ M/A-8.
Cell lines:
Daltons Lymphoma Ascites (DLA) cell-lines were procured from Amala Cancer Research Institute, Thrissur, Kerala, India. The mice were injected with a suspension of cells (1×106) intra peritoneally and the cells were aspirated from the peritoneal cavity on the 15th d.
Animals:
Swiss albino mice (non-pregnant females of 6-8 w age) were purchased from Small Animal Breeding Station (SABS), College of Veterinary and Animal Sciences, Mannuthi, Thrissur, Kerala. The animals were kept in well-aerated cages with controlled conditions of light and humidity for 14 d for acclimatization. The animals were fed with normal mouse chow (Sai Durga Food and feeds, Banglore, India) and water ad libitum. All experiments in the study were carried out with the prior approval of Institutional Animal Ethics Committee (IAEC) and were conducted as per the guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) constituted by the Animal Welfare Division, Government of India.
Plant extraction and fractionation:
Washed and cleaned materials were kept in hot air oven at 50° for 10 d. Dried material was ground into a coarse powder using an electric blender. Sequential extracts of varying polarity (petroleum ether, benzene, chloroform, acetone, ethylene and water) were prepared using column chromatography. The fractions collected were concentrated using rotary evaporator and percentage of yield in each solvent were calculated.
Bioactivity guided selection of the active solvent fraction:
Anti-oxidant assay (2,2-Diphenyl-1-Picrylhydrazyl (DPPH) free radical scavenging assay) of the serial extracts was performed to choose the most active solvent fraction for further studies. The procedure given by Braca et al.[10], was used for determining the anti-oxidant property. Test solutions in different concentrations (20, 40, 60, 80 and 100) μg/l and 6.34 μM solution of DPPH were prepared in methanol. 100 μl test solution, 100 μl DPPH and 800 μl methanol were taken in a test tube and mixed well. Optical density of the resulting solution was measured at 517 nm after incubation at dark for 20 min. Methanol (900 μl) with 100 μl 6.34 μM DPPH was taken as control and methanol as blank. The optical density was recorded and the potential of plant extracts to scavenge the DPPH free radicals were calculated using the formula.
Percentage of inhibition=A-B/A×100
Where, A is optical density of control and B is optical density of sample
Acute toxicity study:
Acute toxicity assay was performed in healthy adult non-pregnant female Swiss albino mice (25-28 g body weight). The mice were divided into two groups of three each and treated with 250 mg/kg drug intraperitoneally. The control group received 2 % carboxymethyl cellulose suspension at the same volume.
in vitro cytotoxicity screening:
Short term cytotoxic activity of A. catechu sequential extracts were assayed by determining the percentage viability of the DLA cells using trypan blue exclusion methods[11]. The cells were aspirated from the peritoneal cavity of tumour bearing mice. The collected cells were washed using Phosphate Buffered Saline (PBS) and checked for their viability. Different dilutions of the cells were made (10-1, 10-2, 10-3) and the number of cells in the 10-3 dilution was counted using haemocytometer and the cell number was adjusted to 1×107 cells/ml. This cell suspension was added to tubes containing various concentrations of test in 1 ml PBS and the tubes were incubated at 37° for 3 h. 100 μl of trypan blue was added after the incubation period and the percentage of viability were determined.
Anti-cancer effect of A. catechu on ascites tumour bearing animals:
Ascites tumour was induced by injecting DLA cells (1×106 cells/animal) in the peritoneal cavity of Swiss albino mice. 36 animals get divided into six groups, each group consist of 6 animals. Group I was maintained as negative control (not treated with any drug). Group II-V received 50 and 100 mg/kg body weight of aqueous and ethanolic extracts of A. catechu (which shows highest activity in in vitro cytotoxicity screening). Animals in the group VI received cyclophosphamide (10 mg/kg body weight). The drugs were given intraperitoneally after 24 h of tumour implantation as 5 doses on alternate days. The death of the animals due to tumour burden was noted every day and the Percentage of Increase in Lifespan (% ILS) was calculated using the below mentioned formula[12].
% ILS=(T-C/C)×100
Where, T is mean survival days of treated and C is mean survival days of control animals.
Anti-cancer effect of A. catechu on solid tumour bearing animals:
Animals were divided into 6 groups and each group carried 6 mice. Viable DLA cells aspirated from the peritoneal cavity of ascites tumor bearing mice in the concentration 1×106 cells in 0.1 ml PBS were transplanted into the right hind limb of mice. 50 mg/kg and 100 mg/ kg drugs (aqueous and ethanolic extracts of A. catechu root) were administered intraperitoneally after tumour transplantation and continued for 10 consecutive days. Control group received only DLA cell line and standard group were treated with cyclophosphamide in the concentration 10 mg/kg. The development of solid tumor in each group was determined by measuring the diameter of tumour in perpendicular planes using vernier calipers in for every 7 h. The tumour volume was calculated using the formula[12].
V=4/3πr12r2
Where, r1 is the minor radius and r2 is the major radius.
LC-MS analysis:
LC-MS system facilitates the analysis of samples, which have difficulty to analyze traditionally. Even though the technique Gas Chromatography-Mass Spectrometry (GC-MS) is powerful as an analytical agent, many compounds are impossible to analyze with GC-MS. LC-MS is suitable for the analysis of large, ionic, thermally unstable and non-volatile compounds. A mass spectrometer combined with a LC can detect masses characteristic of a compound or a class of compounds. The system can selectively detect compounds of interest in a complex matrix, thus making it easy to find out and identify.
Statistical analysis:
The numerical data obtained were statistically analyzed and expressed as mean±Standard Deviation (SD). The significant levels of comparison were analyzed using one way Analysis of Variance (ANOVA) and Duncan Multiple Range Test (DMRT). The differences between the groups were considered statistically significant at p<0.05.
Results and Discussion
Yield of the extract in different solvents were determined as follows; petroleum ether-3 %, benzene-2 %, chloroform-1.5 %, acetone-2.6 %, ethanol-6 % and distilled water-18.3 %. Evaluation of the extractive values of powdered drugs is beneficial for their evaluation especially in the cases where their constituents cannot estimate readily. Here the aqueous extract of A. catechu gave maximum yield.
There are several mechanisms which involved in the antioxidant activity like free radical mediated chain reaction termination, donation of hydrogen, hydrogen abstraction prevention, peroxide elimination and catalytic ion chelation. So, a single assay would not express the antioxidant potential of a plant extract. Here the only method, DPPH free radical scavenging activity was used to find out the biologically active solvent extract for phytochemical screening.
The basic information of the efficacy of compounds in A. catechu extracts to quench the free radicals can be determined using DPPH free radical scavenging assay. All the extracts screened showed scavenging of free radicals based on their concentration (Table 1). 50 % radical scavenging in least concentration was observed in aqueous extract (53 μg/l). So the aqueous extract of A. catechu was selected for anti-cancer studies and phytochemical profiling to detect the various active principles present in it.
| S. No. | Concentration (µg/l) | Percentage of inhibition | |||||
|---|---|---|---|---|---|---|---|
| Petroleum ether | Benzene | Chloroform | Acetone | Ethanol | Distilled water | ||
| 1 | 20 | 14.5±2.1 | 18.6±0.67 | 5.03±4.2 | 6.9±1.89 | 24.6±0.72 | 12.6±0.51 |
| 2 | 40 | 24.6±1.5 | 25.5±0.92 | 17±1.15 | 18.2±2 | 35.4±0.8 | 33±0.92 |
| 3 | 60 | 39.6±0.57 | 32.8±2.42 | 26.3±0.38 | 27.6±0.56 | 49.1±1.10 | 60.5±1.49 |
| 4 | 80 | 47.6±1.15 | 48±2.3 | 48.6±1.56 | 49.4±1.08 | 72.2±2.1 | 76.46±1.8 |
| 5 | 100 | 59.5±0.7 | 61.8±0.72 | 67.6±1.72 | 63±1 | 85.5±1.05 | 92.8±1.13 |
| IC50 | 84±0.84 | 83±1.59 | 82±0.98 | 81±1.05 | 61±1.15 | 53±0.87 | |
Note: Values expressed as mean±SD
Table 1: Dpph Free Radical Scavenging Property of A. catechu Root
In the toxicity test, dose of 250 mg/kg body weight of the mice did not cause mortality or any signs of toxicity or change in general behaviour during the 14 d of observation. So, it is confirmed that, the plant extract is not toxic to animals. Table 2 shows the results of the in vitro cytotoxicity screening of A. catechu root. Both polar and non-polar extracts of A. catechu found to be cytotoxic towards DLA cells. Maximum cytotoxicity (89 %) was attained at a concentration of 200 μg/l of ethanolic extract. Least half maximal Inhibitory Concentration (IC50) value was showed by aqueous extract (38 μg/l). So, ethanolic and aqueous extracts were selected for the in vivo anticancer screening.
| S. No. | Concentration (µg/l) | Percentage of inhibition | |||||
|---|---|---|---|---|---|---|---|
| Petroleum ether | Benzene | Chloroform | Acetone | Ethanol | Distilled water | ||
| 1 | 10 | 8±0.67 | 11±0.62 | 10±0.65 | 9±0.61 | 10±0.58 | 9±0.48 |
| 2 | 20 | 20±0.87 | 19±0.83 | 31±0.78 | 37±0.76 | 30±0.67 | 21±0.73 |
| 3 | 50 | 42±1.63 | 40±0.93 | 44±0.97 | 54±1.23 | 57±0.69 | 36±0.98 |
| 4 | 100 | 61±0.72 | 49±1.23 | 61±0.69 | 68±0.87 | 63±1.67 | 59±01.46 |
| 5 | 200 | 84±0.48 | 61±1.56 | 79±0.88 | 82±0.58 | 89±0.95 | 76±1.93 |
| IC50 value | 40±0.93 | 41±0.97 | 45±0.73 | 56±0.97 | 55±0.83 | 38±1.12 | |
Note: Values expressed as mean±SD
Table 2: In vitro Cytotoxic Property Screening of A. catechu Root
Animals of the control group survived only for a period of (15±2.09) d. Treatment of A. catechu ethanolic and aqueous extracts at different concentrations increased the survival rate of animals (Table 3). One way ANOVA was carried out for comparing number of days survived among different treatment groups. F-value was found to be significant at 0.01 level as the p<0.01. This shows that there exists significant difference in the number of days survived among different treatment groups. DMRT was carried out as post hoc analysis to find out which of the groups are homogeneous and which of them are significantly different. Results shows that number of days survived are not significantlydifferent; both aqueous and ethanolic plant extracts are equally significant in action to the commercial drug cyclophosphamide. And the number of days survived in these groups is significantly higher than the treatments in group 1, 2 and 4. Treatment groups 2 and 4 shows no significant difference in the number of days survived. Number of days survived is significantly lower in the first group compared to all other groups.
| S. No. | Treatment | Number of days survived | Percentage increase in life span |
|---|---|---|---|
| 1 | DLA cells alone | 15.0±2.09c | - |
| 2 | DLA+A.catechu ethanolic extract | 21.7±3.01b | 28.75 |
| (50 mg/kg body weight) | |||
| 3 | DLA+A.catechu ethanolic extract | 26.9±2.80a | 66.25 |
| (100 mg/kg body weight) | |||
| 4 | DLA+A.catechu aqueous extract | 23.8±2.14b | 42.5 |
| (50 mg/kg body weight) | |||
| 5 | DLA+A.catechu aqueous extract | 26.5±1.07a | 71.9 |
| (100 mg/kg body weight) | |||
| 6 | DLA+cyclophosphamide (10 mg/kg) | 26.7±2.07a | 71.5 |
| F | 22.58** | ||
| p | <0.001 |
Note: **Significant at 0.01 level and means having same letter as superscript are homogeneous
Table 3: in vivo Anti-Tumour Activity of A. catechu: Ascites Tumour Model
Development of solid tumor found to be reduced in the A. catechu root treated groups when compared to the control group from 21st d of observation. The volume of solid tumour in control group on 35th d was (4.550±0.622) mm while in case of aqueous root extract in 100 mg/kg was (0.48±0.92) mm on the same day. The value shown by the standard drug (0.643±0.111) was more than that of the aqueous extract at 100 mg/kg. The result was presented in the Table 4.
| Tumor volume in treated group | Day of determining tumour volume | |||||
|---|---|---|---|---|---|---|
| 1st | 7th | 14th | 21st | 28th | 35th | |
| Control | 0.440±0.0317 | 0.643±0.20 | 0.956±0.16 | 2.972±0.452 | 3.790±0.62 | 4.550±0.622 |
| Standard cyclophosphamide (10 mg/kg) | 0.442±0.092 | 0.635±0.87 | 0.982±1.45 | 1.742±0.172 | 1.122±0.227 | 0.643±0.111 |
| Ethanol extract (50 mg/kg) | 0.456±0.072 | 0.67±0.87 | 0.964±0.15 | 1.84±0.98 | 1.359±0.31 | 0.816±0.13 |
| Ethanol extract (100 mg/kg) | 0.426±0.03 | 0.643±0.201 | 0.953±0.86 | 1.615±0.12 | 1.18 ±1.79 | 0.71 ±0.69 |
| Aqueous extract (50 mg/kg) | 0.450±0.02 | 0.643±0.20 | 0.984±0.236 | 1.64±0.68 | 1.359±0.3 | 0.88± 0.85 |
| Aqueous extract (100 mg/kg) | 0.439±0.04 | 0.621±0.068 | 0.956±0.168 | 1.554±0.49 | 1.036±0.48 | 0.48 ±0.92 |
Note: Values expressed as mean of tumor volume in cm±S
Table 4: Effect of A. catechu Root Extract on Solid Tumour Induced by Dla Cells
From these results it is clear that the plant extracts are highly efficient as anti-tumour agents, the percentage of increase in life span is increasing with the increase in concentration of the plant extract in ascites tumour and solid tumor development is significantly reduced by the plant drug. Aqueous extract of A. catechu, which shows maximum anti-cancer property were given for High Resolution LC-MS (HR LC-MS). 59 compounds were detected through the analysis; from the list database formula difference in the range of -10 to +10 is considered as significant. 14 compounds are present in significant amount which is given in the Table 5.
| S. No. | RT | Mass | Name | Formula | DBDIFF (parts per million) | HITS database |
|---|---|---|---|---|---|---|
| 1 | 0.145 | 426.2983 | Leupeptin | C20H38N6O4 | -6.62 | 15 |
| 2 | 0.147 | 218.1145 | 3-Hydroxysebacic acid | C10H18O5 | 4.4 | 2 |
| 3 | 0.153 | 737.5105 | 1-heptadecanoyl-2-(9Z- tetradecenoyl)-sn-glycero-3- phosphoserine | C37H74N2O10P | -3.27 | 5 |
| 4 | 0.153 | 616.413 | 6'-Hydroxysiphonaxanthin | C40H56O5 | -0.35 | 4 |
| 5 | 0.154 | 196.1093 | 4-(2-hydroxypropoxy)-3,5-dimethyl-Phenol | C11H16O3 | 3.54 | 11 |
| 6 | 0.154 | 513.2771 | Sulfolithocholylglycine | C26H43NO7S | -2.03 | 3 |
| 7 | 0.155 | 567.2872 | Dihydrodeoxystreptomycin | C21H41N7O11 | -1.45 | 1 |
| 8 | 0.156 | 639.3083 | Protorifamycin I | C35H45NO10 | -6.2 | 1 |
| 9 | 0.168 | 296.1823 | Farnesylthioacetic acid | C17H28O2S | -4.31 | 10 |
| 10 | 0.17 | 466.3841 | Ergosterol Acetate | C32H50O2 | -6.53 | 3 |
| 11 | 0.177 | 320.1461 | Valproic acid glucuronide | C14H24O8 | 3.09 | 15 |
| 12 | 0.177 | 444.2033 | Mitoxantrone | C22H28N4O6 | -5.44 | 1 |
| 13 | 0.177 | 298.1769 | Artemether | C16H26O5 | 3.74 | 6 |
Note: RT: Retention Time
Table 5: Compounds Present In The A. catechu Root Aqueous Extract
Medicinal plants are nature’s gift to human beings to lead a healthy, disease free life. Most of these plants used today are believed to be much safer and proved as elixir in the treatment of various ailments. Plant derived compounds have played an important role in the development of several clinically useful anticancer agents[13]. Oxidative stress induced by an imbalance between production of reactive oxygen species and antioxidants are associated with pathogenic disease conditions like carcinogenesis[14]. So, radical scavenging activity is very important in the searching of natural sources of cancer drugs.
Cytotoxicity is one of the chemotherapeutic targets of antitumor drugs[15]. Most of the clinically proved antitumour agents possess significant cytotoxic activity in cell culture systems. The cytotoxic activity of A. catechu root extracts against DLA cell lines partially explains its significant anti-tumour activity. The drug shows toxicity towards the tumour cell line and not toxic to normal cells. The anti-cancer activity was evaluated using ascites tumour and solid tumour models. Both ethanolic and aqueous extracts of A. catechu effectively increased the life span of affected mice. Highest activity was observed in aqueous extract.
The result of the HR LC-MS of aqueous extract revealed the presence of valuable compounds with proved medicinal properties in the root of A. catechu. Mitoxantrone, which is available in the trade name Novantrone® is used in the treatment of certain cancers, mostly in metastatic breast cancers, non-Hodgkin’s lymphoma and acute myeloid leukemia. It is a type II topoisomerase inhibitor, it disrupts Deoxyribonucleic Acid (DNA) synthesis and repair in both healthy and cancer cells by intercalation between DNA bases[16].
Artemether is a medication used in the treatment of malaria. In the case of severe malaria, artemether is given in its injectable form[17]. Valproic acid is used as the medication in the treatments of epilepsy, migraine headache and bipolar disorders. Due to the broad spectrum action of valproate, it is used in the treatments of anticonvulsant activity, as a firstline treatment in tonic clonic seizures, mycoclonic seizures and absence seizures. It also used as second line treatment for infantile spasms and partial seizures[18].
Leupeptin, it is a well-known antioxidant and antiinflammatory agent widely used in the medical field[19]. The present study reveals that, this plant is a reservoir of several medicinally active compounds. So, there is no doubt that this plant is very promising as a traditional medicinal plant. Proteases are enzymes that play a crucial role in the regulation of various cellular processes, including cell growth, differentiation and apoptosis (programmed cell death). Leupeptin is a protease inhibitor that has been studied for its potential role in cancer therapy. Dysregulation of proteases has been implicated in cancer development and progression. Leupeptin inhibits several proteases, including serine, cysteine and thiol proteases, thereby affecting multiple pathways involved in cancer[20]. Potential mechanisms through which leupeptin may exert its anti-cancer effects are apoptosis induction, cell cycle arrest, angiogenesis inhibition and metastasis suppression[21].
In conclusion the results of the in vitro cytotoxicity screening and anti-tumour studies of A. catechu shows that, it can act as a source of active compounds for the preparation of anti-cancer drugs. The presence of various secondary metabolites like alkaloids, saponins, phenols, steroids and flavonoids provides some scientific evidence for the biological activities and also account for the pharmacological uses. LCMS analysis of the aqueous extract shows the presence of large number of compounds with proved medicinal uses. So this unravelled medicinal plant will be a prominent contributor of medicinal compounds in the near future.
Acknowledgements:
The authors are thankful to the Department of Botany, St. Mary’s College Thrissur and Amala Cancer Research Centre, Thrissur, Kerala, India for providing the laboratory facilities to carrying out the research.
Conflict of interest:
The authors declared no conflict of interests.
References
- Menon RVG. Validating traditional knowledge: Compedium of traditional knowledge, 26th Kerala Science Congress 2014;76-9.
- Staples GW, Bevacqua RF. Areca catechu (betel nut palm). Species profiles for Pacific Island agroforestry 2006;1(13):1-9.
- Norton SA. Betel: Consumption and consequences. J Am Acad Dermatol 1998;38(1):81-8.
[Crossref] [Google Scholar] [PubMed]
- Azeez S, Amudhan S, Adiga S, Rao N, Rao N. Wound healing profile of Areca catechu extracts on different wound models in wistar rats. Kuwait Med J 2007;109(1):128-33.
- Dar A, Khatoon S. Behavioral and biochemical studies of dichloromethane fraction from the Areca catechu nut. Pharmacol Biochem Behav 2000;65(1):1-6.
[Crossref] [Google Scholar] [PubMed]
- Vermani K, Garg S. Herbal medicines for sexually transmitted diseases and AIDS. J Ethnopharmacol 2002;80(1):49-66.
[Crossref] [Google Scholar] [PubMed]
- Gautam R, Saklani A, Jachak SM. Indian medicinal plants as a source of antimycobacterial agents. J Ethnopharmacol 2007;110(2):200-34.
[Crossref] [Google Scholar] [PubMed]
- Baby AA, Raphael KR. Pharmacognostic characteristics of an unexplored traditional medicine Areca catechu L. root. WJJPS 2014; 3(10):740-6.
- Baby AA, Raphael KR. Pharmacognostic characteristics of an unexplored traditional medicine Areca catechu L. root. IJPPS 2014;6(6):486-9.
- Braca A, Sortino C, Politi M, Morelli I, Mendez J. Antioxidant activity of flavonoids from Licania licaniaeflora. J Ethnopharmacol 2002;79(3):379-81.
[Crossref] [Google Scholar] [PubMed]
- Moldéus P, Högberg J, Orrenius S. Isolation and use of liver cells. Methods Enzymol 1978;52:60-71.
[Crossref] [Google Scholar] [PubMed]
- Kuttan R, Bhanumathy P, Nirmala K, George MC. Potential anticancer activity of turmeric (Curcuma longa). Cancer Lett 1985;29(2):197-202.
[Crossref] [Google Scholar] [PubMed]
- Smitha KR, Ansa PU, Babu TD, Raghavamenon AC. Cytotoxic and anti-tumor properties of an alkaloid positive fraction from Uvaria narum Wall seed oil. Amala Can Res Bulletin 2014;34:68-74.
- Niki E, Noguchi N. Evaluation of antioxidant capacity. What capacity is being measured by which method? IUMB Life 2000;50:323-9.
[Crossref] [Google Scholar] [PubMed]
- Suffness M, Pezzuto JM. Assays related to cancer drug discovery. Dey, PM, Harborne, JB Methods in Plant Biochemistry 1991;6:71-133.
- Parker C, Waters R, Leighton C, Hancock J, Sutton R, Moorman AV, et al. Effect of mitoxantrone on outcome of children with first relapse of acute lymphoblastic leukaemia (ALL R3): An open-label randomised trial. Lancet 2010;376(9757):2009-17.
[Crossref] [Google Scholar] [PubMed]
- Esu EB, Effa EE, Opie ON, Meremikwu MM. Artemether for severe malaria. Cochrane Database Syst Rev 2019(6);11(9):1-83.
[Crossref] [Google Scholar] [PubMed]
- Löscher W. Basic pharmacology of valproate: A review after 35 years of clinical use for the treatment of epilepsy. CNS Drugs 2002;16:669-94.
[Crossref] [Google Scholar] [PubMed]
- Perrin C, Vergely C, Zeller M, Rochette L. in vitro antioxidant properties of calpain inhibitors: Leupeptin and calpain inhibitor-1. Cell Mol Biol 2002;48:OL267-70.
[Google Scholar] [PubMed]
- Salminen AN. Effects of the protease inhibitor leupeptin on proteolytic activities and regeneration of mouse skeletal muscles after exercise injuries. Am J Pathol 1984;117(1):64-70.
[Google Scholar] [PubMed]
- Aoyagi T, Miyata S, Nanbo M, Kojima F, Matsuzaki M, Ishizuka M, et al. Biological activities of leupeptins. J Antibiot 1969;22(11):558-68.
[Crossref] [Google Scholar] [PubMed]