- *Corresponding Author:
- S. P. Deshmukh
P. G. Department of chemistry, Shri shivaji college, Akola - 444 001, India
E-mail: spd_dattatraya@rediffmail.com
| Date of Submission | 25 October 2005 |
| Date of Revision | 14 July 2006 |
| Date of Acceptance | 10 April 2007 |
| Indian J Pharm Sci, 2007, 69 (2): 295-298 |
Abstract
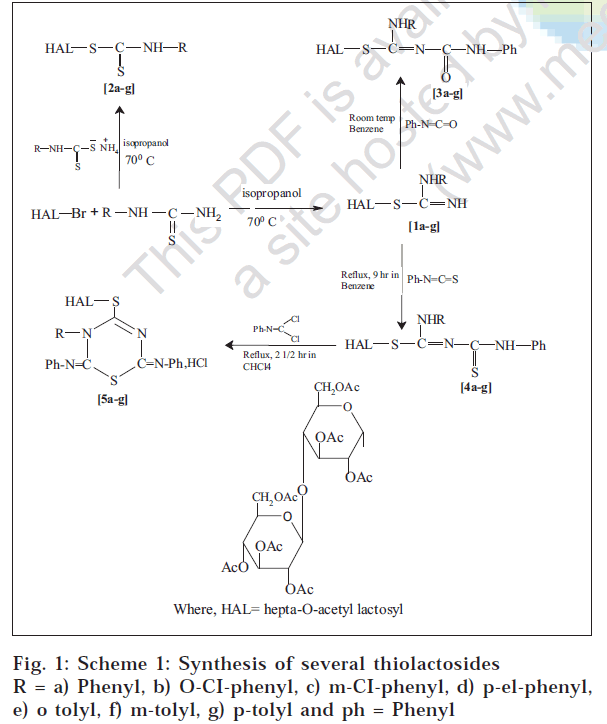
A series of novel thiolactosides like S -hepta- O -acetyllactosyl-1-arylisothiocarbamides (1a-g) and hepta- O -acetyl lactosyl arydithiocarbamates (2a-g) were prepared by the interaction of hepta- O -acetyl lactosyl bromide with arylthiocarbamides and ammonium aryldithiocarbamates respectively. Similarly (hepta- O -acetyl lactosyl)-1,5-disubstituted-2-isothiobiurets (3a-g) 1,5-disubstituted-2,4-isodithiobiurets (4a-g) and-1,2,4-thiadiazolines (5a-g) were synthesized by the interaction of (1a-g) with phenyl isocyanate, phenyl isothiocyonate and S -chloro- N -phenyl isothiocarbamoyl chloride respectively. The compounds 3-Aryl-2,6-diphenylimino 4-S-hepta- O -acetyl lactosyl-2,3dihydro-1,3,5-thiadiazines hydrochlorides (6a-g) were prepared by the interaction of (4a-g) with phenyl isocyanodichloride. In the present investigation activities of these thiolactosides against pathogenic bacteria and fungi such as E. coli, S. aureus, P. vulgaris, Salmonella typhi, Candida guilliermondii and A. niger are discussed.
Thiolactosides are those compounds in which lactosyl group or its derivatives are attached to the sulphur of the sulphur containing compounds. This class of compounds has several applications in industries, medicinal chemistry and in many other ways [1,2]. Literature survey revealed that the heterocyclic derivatives of sugars possess antibacterial and antitumor activity [3]. Benzothiazole derivatives found to exhibit anticancer, antiHIV and antimalerial activity [4-8]. With this end in view, we recently reported the synthesis of several thiolactosides [9-12] Scheme-1. In the present investigation, activities of these thiolactosides against pathogenic bacteria and fungi such as E. coli, S. aureus, P. vulgaris, Salmonella typhi, Candida guilliermondii and A. niger are reported.
Melting points were determined on an electrothermal melting point apparatus and were uncorrected. The structures of the synthesized compounds were elucidated on the basis of elemental analysis and IR [13-16], 1H NMR [14-19] and Mass [20-22] spectral studies (Table-1). IR spectra were recorded in KBr on a FT IR PerkinElmer (4000 450 cm-1) spectrophotometer. 1HNMR spectra are run on Brucker DRX 300 instrument operating at 300 MHz using CDCl3 solution with TMS as internal standard and mass spectra on Jeol SX 102 FAB instrument.
| Comp | Mol. Formula | IR(KBr) cm-1 | 1HNMR (ppm) | Mass (m/z) |
|---|---|---|---|---|
| 1a | C33H42O17N2S | 3350, 2970,1751, 1635, 1439, 1229, 1050, 758 |
d7. 6-6.8 (m, 5H, Ar), d 5.6-5.3 (2H, s, HN) d 5.3 -3.4 (m, 14H, lactose), δ 2.1-1.9 (m, 21H, 7 OAc) |
(M++1)771, 619, 313, 169,127, 109 |
| 1b | C33H41O17N2SCl | 3348,2965,1751,1636 1437,1229,1051,759 |
δ 7.5-7.0 (m, 5H, Ar), δ 6.5-6.1 (2H, s, NH), δ 5.4-3.8 (m,14H, lactose), δ 2.1-1.9 (m, 21H, 7OAc) |
(M++1) 805, 619, 331, 229, 169, 127,109 |
| 1e | C34H44O17N2S | 3458, 2969, 1751, 1642, 1437, 1228, 1050, 755 | δ 7.2-6.9 (m, 14H, Ar), δ 5.3-5.2 (2H, s, NH), δ 5.2-3.7(m,14H, lactose), δ 2.2-1.9 (m, 21H, 7OAc) | (M++1) 785, 619, 331,229,169,127, 109 |
| 2a | C33H41O17NS2 | 3408,2964,1753, 1443, 1229, 1172, 1052, 760 | δ 7.9-7.1(m, 5H, Ar), δ 6.8-6.6 (H, s, NH), δ 5.4-3.6 (m, 14H, lactose), δ 2.1-1.9 (m, 21H, 7OAc) |
(M+)787,619,331, 229,169,127, 109 |
| 2d | C33H40O17NS2Cl | 3426,2972,1754,1448, 1228,1173,1053,771 |
δ 8.0-7.1 (m, 4H, Ar), δ 6.5 (H, s, NH), δ 5.5-3.4 (m, 14H, lactose), δ 2.2-1.9 (m, 21H, 7OAc) |
(M+) 21,620,331, 229,169,127,109 |
| 2f | C34H43O17NS2 | 3374,2944,1752,1435, 1229,1171,1050,738 |
δ 7.3-7.0 (m, 4H, Ar), δ 6.6-6.4 (H, s, NH), δ 5.4-3.7 (m, 14H , lactose), δ 2.1-1.8(m, 21H, 7OAc) |
(M++1) 801, 619, 331, 229,169, 127,109 |
| 3a | C40H47O18N3S | 3414,3000,752,1601, 1441,1227,1053,756 |
δ 7.8-7.0 (m, 10H, Ar), δ 5.8-5.2 (2H, s, NH), δ 4.8-3.4 (m, 14H,lactose), δ 2.1-1.8 (m, 21H, 7OAc) |
(M++1) 890, 619, 331, 229, 169, 127, 109 |
| 3b | C40H46O18N3SCl | 3465,3000,1752,1633, 1443,1228,1052,756 |
δ 7.8-6.8 (m, 9H, Ar), δ 5.5-5.3 (2H, s, NH), δ 5.3-3.4 (m, 4H, lactose), δ 2.2-1.8 (m, 21H, 7OAc) |
(M++1) 924, 619, 331, 229, 169, 127,109 |
| 3e | C41H49O18N3S | 3350,3000,1754,1630, 1444,1226,1049,750 | δ 7.8-6.8 (m, 9H, Ar), δ 5.5-5.3 (2H, s, NH), δ 5.3-3.4 (m,14H,lactose), δ 2.2-1.9 (m,21H,7OAc) |
(M++2) 905, 619, 331,229, 169, 127,109 |
| 4a | C34H44O17N2S | 3458,2969,1751,1642, 1437,1228,1050,755 | δ 7.5-6.9 (m,10H, Ar), δ 4.7-4.4 (2H, s, NH), δ 4.3-3.0 (m,14H, lactose), δ 2.2-1.9 (m, 21H, 7OAc) |
(M++1) 905, 619, 331,229, 169, 127,109 |
| 4b | C34H44O17N2S | 3458,2969,1751,1642,1437, 1228,1050, 755 |
δ 7.5-7.2 (m, 9H, Ar), δ 5.1-4.9 (2H, d, NH), δ 4.9-2.4 (m,14H, lactose), δ 2.2-1.9 (m,21H,7OAc) |
(M++1) 939, 619, 331, 229, 169, 127,109 |
| 4e | C34H44O17N2S | 3458,2969,1751,1642,1437,1228,1050,755 | δ 7.2-6.9 (m, 9H, Ar), δ 5.3-5.2 (2H, d, NH), δ 5.2-3.7 (m, 14H, lactose), δ 2.2-1.9 (m, 21H, 7OAc) |
(M++1) 919, 619, 331, 229, 169, 127,109 |
| 5a | C47H51O17N4S2Cl | 2982, 1750, 1597, 1493, 1231, 1054, 757 | δ 7.6-7.2 (m, 9H, Ar), δ 5.3-3.8 (m, 14H, lactose), δ 2.1-1.9 (m, 21H, 7OAc) | (M+) 1042, 619, 331, 229, 169, 127,109 |
| 5d | C47H50O17N4S2Cl2 | 2974, 1749, 1598, 1542, 1231, 1054, 757 | δ 7.3-6.9 (m, 9H, Ar), δ 5.3-3.8 (m, 14H, lactose), δ 2.1-1.9 (m, 21H, 7OAc) | (M+) 1076, 619, 331,229, 169, 127, 109 |
| 5g | C48H53O17N4S2Cl | 2928, 1749, 1596, 1512, 1230, 1052, 757 | δ 7.6-6.9 (m, 9H, Ar), δ 5.3-3.8 (m, 14H, lactose), δ 2.1-1.9 (m, 21H, 7OAc) | (M+) 1056, 619, 331, 229, 169, 127, 109 |
R = a) Phenyl, b) o-Cl-phenyl, c) m-Cl-phenyl, d) p-Cl-phenyl, e) o-tolyl, f)p-tolyl and ph=phenyl
Table 1: Characterisation data of thiolactosides (1-5) (A-G)
Solutions of hepta-O-acetyl lactosyl bromide and arylthiocarbamides in isopropyl alcohol were kept at room temperature for 18 h. It was mixed with distilled water and basified with aqueous ammonia to yield a sticky mass. The sticky mass was purified with ethanol-water furnished a granular solids of S-hepta-O-acetyl lactosyl-1-arylisothiocarbamides (1a -g) [9].
Solutions of hepta-O-acetyl lactosyl bromide and ammonium arydithiocarbamates in isopropyl alcohol were kept at room temperature for 18 h. Upon adding distilled water, a sticky mass was separated. The sticky mass was purified with ethanol-water to give hepta-O-acetyl lactosyl arydithiocarbamates [10] (2a-g).
An equimolar (0.0025 mol) mixture of S-hepta-O-acetyl lactosyl-1-arylisothiocarbamides (1a-g) and phenyl isocyanate in dry benzene was kept at room temperature for 24 h. The benzene was distilled off. The sticky mass thus obtained was triturated several times with petroleum ether to obtain S-hepta-O-acetyl lactosyl-1-aryl-5-phenyl-2-isothiobiurets [11] in the form of granular solids (3a-g).
Condensation of S-hepta-O-acetyl lactosyl-1-arylisothiocarbamides (1a-g) with phenyl isothiocyanate in benzene was carried out for 9 h. The benzene was distilled off. The sticky mass obtained when triturated several times with petroleum ether furnished S-hepta-O-acetyl lactosyl-1-aryl-5-phenyl-2,4-isodithiobiurets [11] as granular solids (4a-g).
Condensation of an equimolar (0.0025 mol) mixture of S-hepta-O-acetyl lactosyl-1,5-disubstituted-2,4-isodithiobiurets (4a-g) and phenyl isocyanodichloride in chloroform was carried out for 2.5 h. The excess of chloroform was distilled off. The sticky mass obtained was triturated with petroleum ether to separate 3-aryl-2,6-diphenylimino-4-S-hepta-O-acetyl lactosyl-2,3-dihydro-1,3,5-thiadiazine hydrochlorides [12] as granular solids (5a-g).
All the compounds have been screened for both antibacterial and antifungal activity using cup plate agar diffusion method [23,24] by measuring the inhibition zone in mm. the compounds were taken at a concentration of 1 mg/ml using dimethyl formamide (DMF) as solvent. Amikacin (100 μg/ml) was used as a standard for antibacterial activity and fluconazole (100 μg/ml) as a standard for antifungal activity. The compounds were screened for antibacterial activity against Escherichia coli, Staphylococcus aureus, Proteus vulgaris, and Salmonella typhi in nutrient agar medium and for antifungal activity against Candida guilliermondii and Microsporum in potato dextrose agar medium. These sterilized agar media were poured in to Petri dishes and allowed to solidify. On the surface of the media microbial suspensions were spread with the help of sterlized triangular loop. A stainless steel cylinder of 8 mm diameter (pre-sterlized) was used to bore the cavities. In to these wells were added 0.1 ml portions of the test compounds in solvent. The drug solution was allowed to diffuse for about an hour into the medium. The plates were incubated at 37o for 24 h and 30o for 48 h for antibacterial and antifungal activities, respectively. The zone of inhibition observed around the cups after respective incubation was measured. The results are presented in Table 2.
| Compound No. | MP0 | Antibacterial** | Antifungal** | ||||
|---|---|---|---|---|---|---|---|
| E. c | S. a | P. v | S. t | C. g | A. n | ||
| 1a | 121 | 17 | 14 | 18 | 19 | 21 | 18 |
| 1b | 140-42 | 18 | 15 | 17 | 22 | 18 | 21 |
| 1c | 120 | 18 | 17 | 15 | 20 | 18 | 17 |
| 1d | 126 | 17 | 15 | 15 | 20 | 20 | 19 |
| 1e | 134-35 | 19 | 14 | 14 | 18 | 18 | 22 |
| 1f | 127 | 20 | 14 | 15 | 18 | 20 | 19 |
| 1g | 145 | 22 | 15 | 16 | 20 | 18 | 20 |
| 2a | 115-18 | 16 | 13 | 16 | 13 | 22 | 18 |
| 2c | 85-87 | 18 | 20 | 18 | 22 | 19 | 19 |
| 2d | 145-47 | 16 | 18 | 14 | 21 | 21 | 21 |
| 2e | 122-23 | 15 | 13 | 14 | 19 | 19 | 21 |
| 2f | 109-10 | 18 | 15 | 17 | 21 | 20 | 19 |
| 2g | 136-38 | 17 | 14 | 15 | 22 | 16 | 20 |
| 3a | 163-65 | 13 | 16 | 16 | 17 | 18 | 20 |
| 3b | 148-49 | - | 15 | 19 | 19 | 19 | 25 |
| 3c | 136-37 | 15 | 18 | 20 | 18 | 20 | 22 |
| 3d | 165-67 | 17 | 17 | 19 | 17 | 20 | 20 |
| 3e | 152-55 | 16 | 16 | 22 | 16 | 20 | 22 |
| 3f | 143-44 | 17 | 15 | 19 | | 22 | 22 |
| 3g | 160-62 | 18 | 14 | 15 | 18 | 18 | 18 |
| 4a | 142-45 | 17 | 15 | 16 | 18 | 21 | 18 |
| 4b | 153-54 | 16 | 15 | 19 | 17 | 20 | 20 |
| 4c | 130-32 | 18 | 16 | 20 | 16 | 22 | 20 |
| 4d | 135 | 19 | 14 | 21 | 17 | 20 | 19 |
| 4e | 123-25 | 17 | 13 | 20 | 20 | 19 | 17 |
| 4f | 164-65 | 17 | - | 17 | 19 | 19 | 16 |
| 4g | 160-62 | 21 | - | 18 | 18 | 19 | 24 |
| 5a | 175-77 | 18 | 16 | 18 | 19 | 21 | 24 |
| 5b | 150-51 | 16 | 14 | 18 | 17 | 20 | 18 |
| 5c | 170-72 | 16 | 13 | 20 | 18 | 21 | 20 |
| 5d | 161-62 | 16 | 14 | 18 | 19 | 20 | 23 |
| 5e | 158-60 | 17 | 15 | 17 | 20 | 24 | 20 |
| 5f | 163-64 | 16 | 15 | 18 | 19 | 20 | 18 |
| 5g | 182-84 | 18 | 15 | 17 | 20 | 20 | 20 |
| Amikacin | -- | 19 | 23 | 21 | 24 | - | - |
| Fluconazole | - | - | - | | 25 | 26 | |
| DMF | - | - | - | - | - | - | |
*including the well diameter of 8 mm. **zone of inhibition in mm (15.or less) resistant, (16-20 mm) moderate and (more than 20 mm) sensitive. E. c (E. coli), S. a (S. aureus), P. v (P. vulgaris) S. t (S. typhi), C. g (Candida guilliermondii), A. n (A. niger)
Table 2: Antimicrobial activities of thiolactosides (1-5) (a-g)
It has been observed that some of these compounds exhibited interesting microbial activities. 1b, 2c, 2d, 2f and 2g exhibited most significant activity against Salmonella. 1g and 4g inhibited E. coli while 3e, 4d inhibited S. aureus and P. vulgaris, respectively. All other compounds exhibited low to moderate activity (Table 2).
The results of antifungal activity are also tabulated in Table 1. 2a, 3f, 4c, and 5e are effective towards Candida guilliermondii while other exhibited moderate to low activity. 1e, 3b, 3e, 3f, 4g, 5a and 5d are effective against Microsporum while others exhibited moderate to low activity (Table 2).
Thus, the novel thiolactosides synthesized, exhibits comparable antibacterial and antifungal activities against the organisms tested. The method adopted in this investigation is simple, efficient, inexpensive, and is useful in synthesizing pharmacologically important molecules..
Acknowledgements
The authors thank Dr. S. G. Bhadange, Principal, Shri Shivaji College, Akola and Dr. P. R. Rajput, Principal, Shankarlal Khandelwal College, Akola for providing laboratory facilities.
References
- Clamp, J.R., Haugh, L., Hickson, J.L. and Whistler, R.L., Adv. Carbohydr. Chem. Biochem . , 1961, 16, 159.
- Baria, C.S., Antinio, T.P. and Santoyo-Gonzalez, F., Synlett, 1999, 12, 1891: Chem. Abst., 132 (15), 194573b (2000).
- Suhadolnik, R.J., a) Nucleoside Antibiotic, Wiley-Interscience, NY, 1970. b) Nucleosides as Biological Probes, Wiley-Interscience, NY, 1979.
- Tale, P.V. and Deshmukh, S.P., Heteroatom Chem. , 2006, 17(4), 306
- Webb, J.R., Mitsuya, H. and Broder, S., J. Med. Chem. , 1988, 31, 1475.
- Reitz, A.B., Tuman, R.W., Marchione, C.S., Jordan, A.D., Bowden, C.R. and Maryanoff, B.E.; J. Med. Chem. , 1989, 32 , 2110.
- Parrot-Lopez, H., Galons, H., Coleman, A.W., Mahuteau, J. and Miocque, M., TetarahedronLett., 1992, 33, 209.
- Feunts, J., Wenceslao M., Ortiz, C., Roina, J. and Welsh C., Tetrahedron, 1992, 48, 6413.
- Mangte, D.V. and Deshmukh, S.P., J. Indian Chem. Soc. , 2005, 82, 1025.
- Mangte, D.V. and Deshmukh, S.P., Int. J. Chem. Sci . , 2004, 2(2), 159.
- Mangte, D.V. and Deshmukh, S.P., Indian J. Chem. , 2006, 45B, 1285.
- Mangte, D.V. and Deshmukh, S.P., J. Indian Chem. Soc. , 2006, 83, 517.
- Segal, L., O'connor, R.T. and Eggerton, F.V., J. Chem. Soc., 1960, 82, 2807.
- Varma, R., Kulkarni, S.Y., Jose, C.I. and Pansare, V.S., Carbohydr. Res., 1984, 133, 25.
- Zhiqun, D., Fanqui, Q., Chengtai, W. and Wei, L., J. Chem. Res. ( S ), 2001, 106.
- Vergas-Bernguel, A., Ortega-Caballero, F., Santoyo-Gonzalez, F., Garcia-Lopez, J.J., Gimenez-Martinez, J.J., Garcia-Fuentes, L. and Ortiz-Salmeron, E., Chem. Eur. J., 2002, 8, 812.
- Isac-Garcia, J., Calvo-Flores, F.G., Hernandez-Mateo, F. and Santoyo-Gonzalez, F., Eur. J. Org. Chem. , 2001,383.
- Jimenez-Blanco, J.L., Barria, C.S., Benito, J.M., Mellet, C.O., Fuentes, J., Santoyo-Gonzalez, F. and Garcia-Fernandez, J.M., Synthesis , 1999, 11, 1911.
- Meng, X.B., Yang, L.D., Li, H., Li, Q., Cheng, T.M., Cai, M.S. and Li, Z.J., Carbohydr. Res. , 2002, 337, 977.
- Lonngren, J. and Svensson, S., Adv. Carbohydr. Chem. Biochem. , 1974, 39, 98.
- Budzikiewiez, H., Djerassi, C. and Williams, D.H., Structural Elucidation of Natural Products by Mass Spectroscopy, Part II, 2nd Edn., Interscience Publisher, Inc., NY, 1949.
- Hassan, H.H.A.M. and El-Husseiny, A.H.F., Polish J. Chem. , 2001,75, 809.
- Kawangh, F., Analytical Microbiology, Academic Press, New York, 1963.
- British Pharmacopaeia- II, Biological Assay and Tests, The Stationary Office Ltd., London, 1998, A-205.