- *Corresponding Author:
- S. S. Khan
School of Pharmacy, Dhanalakshmi Srinivasan University, Trichy, Tamil Nadu 621112, India
E-mail: khansardar25@gmail.com
| Date of Received | 30 September 2022 |
| Date of Revision | 27 May 2024 |
| Date of Acceptance | 30 September 2024 |
| Indian J Pharm Sci 2024;86(5):1671-1677 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The present study assesses the antibacterial activities of whole plant extracts of Ocimum americanum Linn. tested against pathogenic microorganisms. Preparation of different extracts viz., chloroform, petroleum ether, ethyl acetate and ethanol through cold maceration extraction method. Various extracts were investigated against strains of Streptococci, Staphylococci, Escherichia coli and Salmonella typhi by agar well diffusion method. Phytochemicals and functional groups were observed by gas chromatographymass spectrometry. Chloroform extract of Ocimum americanum showed significant antibacterial activity against all tested pathogens in the agar well diffusion method in which Salmonella typhi (18.42±0.85) mm was observed high zone of inhibition, whereas the lowest inhibition was observed in ethyl acetate extract against Streptococci (8.32±0.20) mm. Phytochemical studies reveal that the whole plant contains alkaloids, terpenoids, fixed oils and flavonoids as phytoconstituents. The gas chromatography-mass spectrometry method screened one bioactive phytochemical in the chloroform extract and two bioactive phytochemicals in the petroleum ether extract of Ocimum americanum. The recognition of phytochemical compounds is based on the peak area, retention time molecular weight, molecular formula, mass spectrometric fragment ions and pharmacological actions. Therefore, chloroform and ethyl acetate extracts of Ocimum americanum could act as an antibacterial agent and further studies are recommended for the isolation of compounds and toxicological studies.
Keywords
Ocimum americanum, gas chromatography-mass spectrometry, antibacterial activity, bioactive compounds, well plate method, phytochemical screening
Medicinal plants are the only source for the healing of diseases. In ancient studies, plentiful herbs and plants have been recognized as remedial plants because of their potency to cure illness[1]. Secondary metabolites of plants have recently been referred to as phytochemicals, naturally occurring and biologically active plant compounds with potential disease-inhibiting capabilities[2]. The study of phytochemicals is considered a separate discipline called phytochemistry, defined as a branch of science, somewhere in concerning natural product organic chemistry and biochemistry concerned with organic substances accumulated by plants and deals with the chemical structure of these substances, their biosynthesis, turnover and metabolism, their natural distribution and their biological function. Due to the development of science and technology such as chromatographic and spectroscopic techniques, it is simple to isolate almost all plant components and their depiction is very imperative to improve the effectiveness, diminishing the dose and onset of action[3].
For antibacterial activity, medicinal plant extracts have been considered potential sources of bioactive compounds for many years. Ocimum americanum (O. americanum) belonging to the family Lamiaceae is an annual herb but can be grown as a short-lived perennial herb that grows up to 20-30 cm tall with some specimens up to 100 cm, grown especially in cooler climates. It is commonly called American basil, elumichai thulasi. It is usually found on roadsides, teak forests, and in open waste places, preferring sunny, wind-sheltered spots. It is found at elevations from sea level, usually to 500 m but occasionally to 2000 m. The aromatic leaves are used as a flavouring agent in a wide range of foods, whilst a cooling drink can be made from the seeds. O. americanum is native to a wide range of Tropical Africa, Indian subcontinent, China, Myanmar, Malaysia, Thailand, Vietnam and Indonesia[4]. The main chemical constituents of O. americanum are volatile oils like methyl heptanone, methyl nonyl ketone, d-camphor, citral, ocimene, methyl chavicol, linalool and flavonoids[5].
There are no previous studies involving this bacterial group in a single study using chloroform and ethyl acetate extracts of O. americanum and the antibacterial efficiency. The main aim of this study is to test antibacterial activity against selective pathogenic organisms by taking the whole plant extracts of O. americanum. The study is designed to prepare cold maceration extracts (chloroform, petroleum ether, ethyl acetate, and ethanol) from plant family Lamiaceae. This study primarily targets to evaluate antimicrobial efficacy of these extracts against , Staphylococci, Escherichia coli (E. coli) and Salmonella typhi (S. typhi) by the agar well diffusion method. Furthermore, the study is oriented to reveal and examine the phytochemical constituent along with functional groups that are present in extracts through Gas Chromatography-Mass Spectrometry (GC-MS). Consequently, this work has the objective to seek advantages in identifying bioactive subjects from O. americanum and verifying potential antibacterial agents in ethyl acetate extracts. Finally, it suggests more studies aiming to isolate active compounds and toxicological analysis based on the results obtained. The present study is aiming to summarize the antibacterial effectiveness of O. americanum and it will be useful for developing new antimicrobial agents from this plant.
Materials and Methods
Collection of plant material:
The plant of O. americanum was collected during the month of January at Tirunelveli, Tamilnadu. It was authenticated by Mr. Chelladurai, Research Officer- Botany (Scientist-C), Central Council for Research in Ayurveda and Siddha, Government of India. The collected material was dried under shade for 15 d and then it was blended into coarse powder by a mechanical grinder. The powdered drug was passed through sieve No. 10 to get uniform particle size.
Preparation of plant extracts:
The extraction of O. americanum was done by Cold maceration with methanol, chloroform, ethyl acetate and petroleum ether. About 300 g of coarse powder was cold macerated in methanol for 3 d with occasional shaking. After completion of the extraction, the extract was filtered and concentrated at 45° on a water bath, till it acquires 3/4th of concentration. A dark green residue was obtained. The concentrated extract was mixed with a small quantity of water and the aqueous ethanolic extract was fractioned successively. Further extraction can be done successively using petroleum ether, chloroform and ethyl acetate[6].
Microbial strains:
Clinical strains used for antibacterial studies include Streptococci, Staphylococci, E. coli, and S. typhi. They are sub-cultured by nutrient agar and stored at 4°. Active cultures for experiments were prepared by transferring 1 loop full of cells from the stock of nutrient agar which are incubated without agitation for 24 h at 37°[7].
Antibacterial activity:
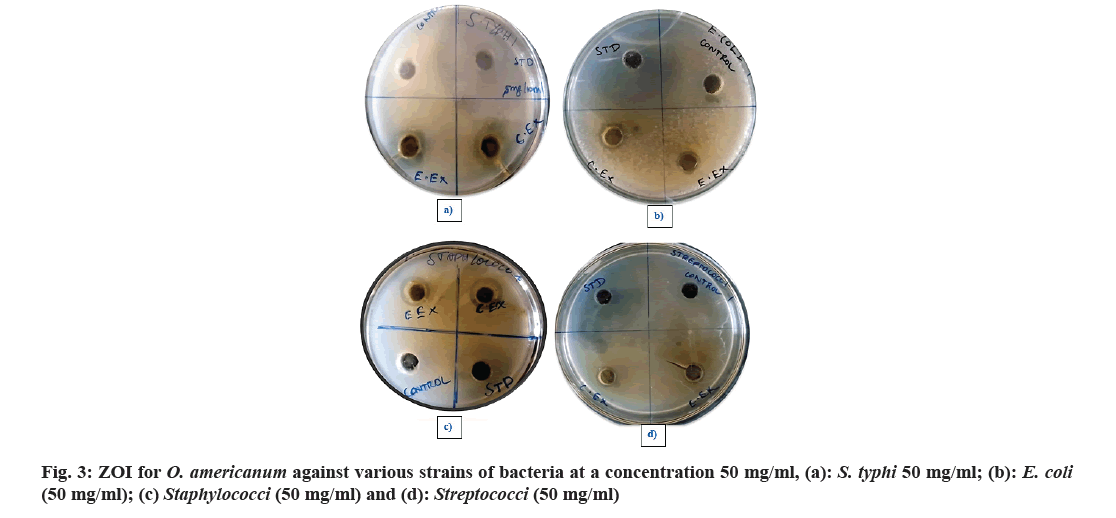
All bacterial strains were grown to the Nutrient Agar Medium (NAM), slant at 25° for 24 h. The antibacterial activity of O. americanum extracts such as ethyl acetate and chloroform was carried out using the agar well diffusion method. The agar well diffusion method was done by preparing wells of 8 mm that were punched in the respective medium using a sterile cork borer. The wells were dispensed with respective plant extracts (18-20) ml. All the plates were incubated for 24 h at 37°. Ciprofloxacin 10 mg is used as a standard drug, dissolved in ethanol to get a concentration of 10 μg/ml. The measurement of the Zone of Inhibition (ZOI) was taken from around the well (Table 1 and Table 2)[8].
| Microorganisms and concentrations (mg/ml) | ZOI (Mm) | |||
|---|---|---|---|---|
| Standard (Ciprofloxacin) | Control | Chloroform extract | Ethyl acetate extract | |
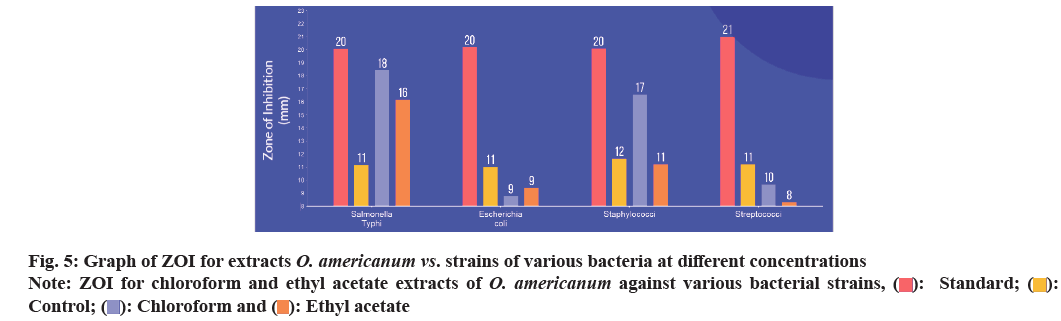
| S. typhi (50 mg/ml) | 20.08±0.25 | 11.15±0.21 | 18.42±0.85 | 16.16±0.00 |
| E. coli (50 mg/ml) | 20.19±0.79 | 11.02±0.55 | 8.77±0.33 | 9.41±0.45 |
| Staphylococci (50 mg/ml) | 20.10±1.05 | 11.66±0.01 | 16.57±0.25 | 14.71±0.55 |
| Streptococci (50 mg/ml) | 21.00±0.01 | 11.22±0.74 | 9.67±0.66 | 8.32±0.20 |
Note: Values are represented in terms of the mean of three observations (n=3)
Table 1: ZOI For Extracts of Ocimum americanum Against Various Microorganisms in 50 Mg/Ml
| Microorganisms and concentrations (mg/ml) | ZOI (Mm) | |||
|---|---|---|---|---|
| Standard (Ciprofloxacin) | Control | Chloroform | Ethyl acetate | |
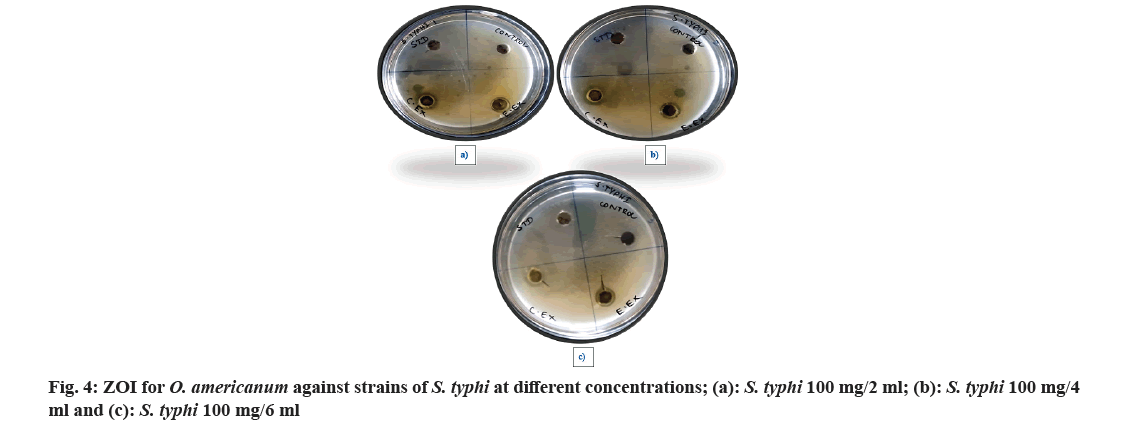
| S. typhi (100 mg/ 2ml) | 25.55±0.25 | 3.00±0.10 | 15.25±0.00 | 12.00±0.20 |
| S. typhi (100 mg/4 ml) | 24.85±0.10 | 4.10±0.25 | 12.90±0.50 | 10.60±0.30 |
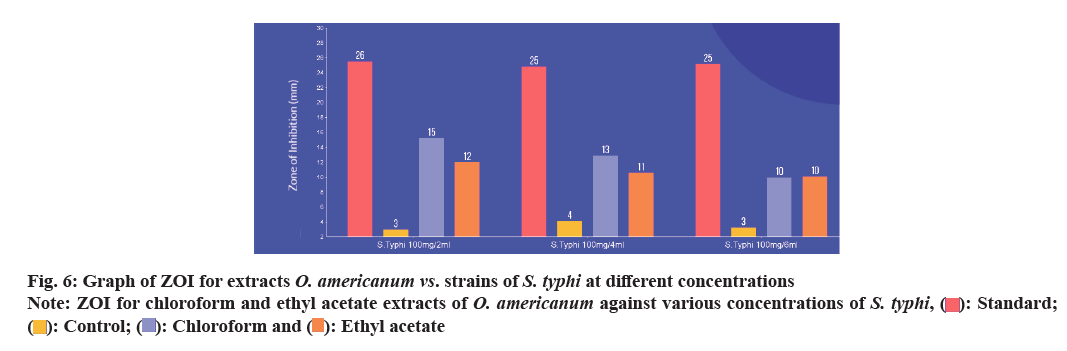
| S. typhi (100 mg/6 ml) | 25.20±0.00 | 3.25±0.80 | 10.00±0.10 | 10.10±0.10 |
Note: Values are represented in terms of the mean of three observations (n=3)
Table 2: ZOI of Ocimum americanum Extracts Against S. typhi in Different Concentrations
Gas Chromatography-Mass Spectrometry (GCMS):
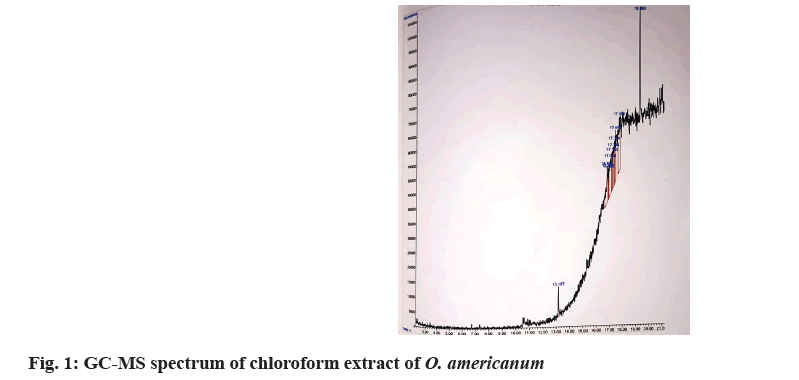
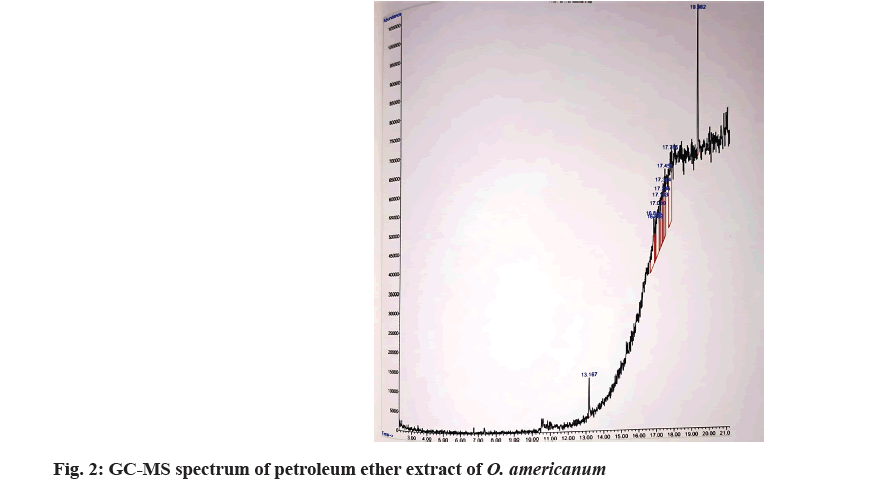
The bioactive components of O. americanum have been evaluated using GC-MS by taking the extracts of chloroform and petroleum ether. The extract contains both polar and nonpolar components of the plant material. 2 μl of the sample of the solutions was employed in GC-MS for analysis of different compounds. GC-MS analysis was performed using Agilent Technologies Inc. GC-MS comprising an AOC-20i auto-sampler and a GC-MS. For detection, DB-5 GC column was used with an electron ionization system operating in electron impact mode with ionization energy of 70 eV. Helium gas (99.999 %) was used as a carrier gas at a constant flow rate of 1 ml/min and an injection volume of 2 μl was employed (a split ratio of 10:1). The injector temperature was maintained at 250°, the ion-source temperature was 200° and the oven temperature was programmed from 100° (isothermal for 2 min) with an increase of 10°/min to 200°, then 5°/min to 270°, ending with a 9 min isothermal at 270°. Mass spectra were taken at 70 eV at a scanning interval of 0.5 s from 45 to 450 Da. The solvent delay was 0 to 2 min and the total running time was 26 min for petroleum ether and 20 min for chloroform extract. The relative percentage amount of each component was calculated by comparing its average peak area to the total area. Chemical compounds were identified by the National Institute of Standards and Technology (NIST 08) library match (Table 3)[9].
| Retention time (Rt) | Phytochemical compound | Molecular formula | Molecular weight (g/mol) | Peak area % | Biological activity |
|---|---|---|---|---|---|
| 16.525 | Valium | C16H13ClN2O | 284.74 | 48.91 | Anxiolytic and Antiepileptic |
| 13.169 | Phytol | C20H40O | 296.53 | 63.57 | Antioxidant |
| 19.362 | 5,9-Undecadien 2-one,6,10-dimethyl (Z) | C13H22O | 194.3132 | 62.20 | Flavouring agent |
Table 3: Phytochemical Compounds Identified in Chloroform and Pet. Ether Extracts of Ocimum americanum
Results and Discussion
The crude extract of O. americanum, which was obtained through cold maceration was dark greenish and it yielded 23.5 g. This amount represents 11.75 % (w/w) of the original 300 g of the coarse plant powder used for extraction. After being obtained by extraction, the extract was subjected to an elaborate drying method to ensure that residual solvents and moisture were eliminated from the extract to guarantee stability during subsequent analysis.
Phytochemical screening was used to investigate the presence and concentrations of alkaloids, terpenoids, flavonoids and fixed oils in the extracts of chloroform, petroleum ether, ethyl acetate and ethanol. The goal was to gain information regarding potentially useful medicinal properties and bioactive components of the extract. Phyto-chemical analysis of crude extract indicated that extract possessed alkaloids, terpenoids, fixed oils and flavonoids, while saponins glycosides and proteins were absent in crude extract as shown in Table 4[10].
| S no | Active constituents | Extracts | |||
|---|---|---|---|---|---|
| Petroleum ether | Ethanol | Chloroform | Ethyl acetate | ||
| 1 | Alkaloids | + | + | + | + |
| 2 | Carbohydrates | - | - | - | - |
| 3 | Steroids | + | - | + | - |
| 4 | Flavonoids | - | + | - | - |
| 5 | Terpenes | + | + | + | + |
| 6 | Protein and amino acids | - | - | - | - |
| 7 | Fixed oils | + | + | + | + |
| 8 | Glycosides | - | - | - | - |
| 9 | Phenolic compounds | - | - | - | - |
| 10 | Gums and mucilage | - | - | - | - |
Note: (+): Presence of active constituents and (-): Absence of active constituents
Table 4: Phytochemical Screening of Ocimum americanum
GC-MS method screened one bioactive phytochemical in the chloroform extract (fig. 1) and two bioactive phytochemicals (fig. 2) in the petroleum ether extract of O. americanum. The relative percentage amount of each component was calculated by comparing its average peak area to the total area. Chemical compounds were identified by the NIST 08 library match. Valium (48.91 %) is identified in the mass spectra of whole plant extract of petroleum ether at 16.525 min, which predominantly acts as an anxiolytic and exerts antiepileptic effects. The mass spectra of whole plant extract of chloroform show 2 compounds namely, phytol and 5,9-undecadien 2-one,6,10-dimethyl, (Z). Phytol (63.57 %) which is found at 13.169 mins acts as an antioxidant. Another flavouring agent namely, 5,9-undecadien 2-one,6,10-dimethyl, (Z) (62.20 %) is eluted at 19.362 min. The GC-MS spectrum of O. americanum and the structures of found components are shown in fig. 3 and fig. 4[11,12].
The antibacterial activities of O. americanum (chloroform and ethyl acetate) crude extract against the microorganisms such as Streptococci, Staphylococci, E. coli, and S. typhi were examined in the concentration range of 50 mg/ml and their potency were assessed by the presence or absence of inhibition zones with some standard antibiotic ciprofloxacin as shown in Table 1. The maximum ZOI was obtained for S. typhi in the chloroform extract 18.42±0.85 mm and 16.16±0.00 mm for ethyl acetate extract as shown in fig. 5. The lowest inhibition for ethyl acetate extract of Streptococci with 8.32±0.20 mm due to the presence of terpenoids in chloroform and ethyl acetate extracts of whole plant of O. americanum. Further, the chloroform and ethyl acetate extracts were tested against S. typhi in various concentrations such as 100 mg/2 ml, 100 mg/4 ml, and 100 mg/6 ml with some standard antibiotics shown in Table 2 to inhibit bacteria effectively. The concentration of S. typhi (100 mg/2 ml) showed a promising and greater extent of inhibition of bacteria compared to other concentrations with ZOI of 15.25±0.00 for chloroform extract as shown in fig. 6. The overall antibacterial activity and higher extent of ZOI is due to phytol in chloroform extracts. Further studies should be made on the whole plant of O. americanum for its pharmacological activity.
From the results of the present study, we have concluded that chloroform extract of the whole plant of O. americanum has proved potentially effective in antibacterial activity against pathogenic organisms. Various bioactive compounds were studied through GC-MS analysis. Therefore, ethyl acetate and chloroform extract whole plant of O. americanum possess promising bioactive compounds that are liable for antibacterial activity. Hence, extensive research is needed for compound isolation, and pharmacological, toxicological and clinical trials of the effective compound.
Acknowledgements:
The authors wish to thank to the Department of Pharmaceutical Chemistry, Sankaralingam Bhuvaneswari College of Pharmacy, Sivakasi for providing immense support to carry out this research work and authors are thankful to School of Pharmacy, DSU, Trichy for compiling the manuscript. We would also like to thank Ayya Nadar Janaki Ammal College Sivakasi, Tamil Nadu for helping in the GC-MS analysis of the compound.
Conflict of interest:
The authors declared no conflict of interests.
References
- Khan SS, Mangayarkarasi V. Preliminary phytochemical and antibacterial activity of roots of Jatropha glandulifera. World J Pharm Pharm Sci 2022;12(1):1620-7.
- Mangayarkarasi V. Preliminary phytochemical, antibacterial and pharmacological screening of the whole plant of Merremia umbellata. World J Biol Pharm Health Sci 2023;13(2):163-72.
- Nadkarni AK. Dr. KM Nadkarni's Indian materia medica: With Ayurvedic, Unani-tibbi, Siddha, allopathic, homeopathic, naturopathic & home remedies, appendices & indexes. Popular Prakashan; 2007.
- Chopra RN. Glossary of Indian Medicinal Plants; 1956.
- Almatroodi SA, Alsahli MA, Almatroudi A, Rahmani AH. Ocimum sanctum: Role in diseases management through modulating various biological activity. Pharmacogn J 2020;12(5):1198-205.
- Hasan MR, Alotaibi BS, Althafar ZM, Mujamammi AH, Jameela J. An update on the therapeutic anticancer potential of Ocimum sanctum L.:“Elixir of life”. Molecules 2023;28(3):1193.
[Crossref] [Google Scholar] [PubMed]
- Mandal AK, Poudel M, Neupane NP, Verma A. Phytochemistry, pharmacology, and applications of Ocimum sanctum (Tulsi). In: Edible Plants in Health and Diseases: Volume II: Phytochemical and Pharmacological Properties 2022;135-74.
- Dakshayani L, Merchant N, Smitha S, Surendra G, Reddy TG, Mamatha D, et al. Holy basil: A potential herbal source for therapeutic applications. Curr Trends Biotechnol Pharm 2021;15(1):87-100.
- Harsha M, Kumar KM, Kagathur S, Amberkar VS. Effect of Ocimum sanctum extract on leukemic cell lines: A preliminary: In vitro study. J Oral Maxillofac Pathol 2020;24(1):93-8.
[Crossref] [Google Scholar] [PubMed]
- Babu KN, Hemalatha R, Satyanarayana U, Shujauddin M, Himaja N, Bhaskarachary K, et al. Phytochemicals, polyphenols, prebiotic effect of Ocimum sanctum, Zingiber officinale, Piper nigrum extracts. J Herbal Med 2018;13:42-51.
- Roy S. Phytochemical screening and antimicrobial efficacy of Ocimum sanctum (Tulsi) and Swertia chirayita (Chirota) against Escherichia coli and Salmonella spp. isolated from poultry and their molecular study (Doctoral dissertation, Chattogram Veterinary & Animal Sciences University); 2020.
- Kumar A. A systemic review of Tulsi (Ocimum tenuiflorum or Ocimum sanctum): Phytoconstituents, ethnobotanical and pharmacological profile. Res J Pharmacogn Phytochem 2023;15(2):179-88.
 ): Standard; (
): Standard; ( ):
Control; (
):
Control; ( ): Chloroform and (
): Chloroform and ( ): Ethyl acetate
): Ethyl acetate