- *Corresponding Author:
- T.S.S.P.N.S.Sanath Kumar
Siddha Central Reseqarch Institute, Arumbakkam, Chennai-600106, India
E-mail: drsanathkumar@ymail.com
| Date of Submission | 08 October 2010 |
| Date of Revision | 19 July 2011 |
| Date of Acceptance | 18 August 2011 |
| Indian J Pharm Sci, 2011, 73 (4): 470-473 |
Abstract
The aim of present work is to study the antibacterial activity of polyphenols isolated from the ethyl acetate soluble of methanol extract of stem bark of Garcinia indica against Staphylococcus aureus, Salmonella typhi and Escherichia coli by paper disc method. The results showed good antibacterial activity against S. aureus at higher concentrations, moderate at lower concentrations, against S. typhi moderate at higher concentrations but no activity against E. coli even at higher concentration for flavononylflavone. With proauthocyanin S. Aureus, S. Typhi and E. coli showed good antibacterial activity at higher concentration only.
Keywords
Antibacterial activity, biflavonoid, flavononylflavone, Garcinia indica, proauthocyanidin
Garcinia has more than 200 listed polygamodioecious trees and shrubs distributed widely in nature, of which 30 are identified in India. Garcinia indica (choiss) belongs to Clusiaceae (earlier Guttiferae) family is a slowly growing polygamodioecious tree [1]. It is distributed through out topical Asia, Africa and Polynesia [2]. In India it is found in the topical humid evergreen rain forest of Western Ghats of South India as well as in the North Eastern states of India [3]. It is popularly known as kokum in Hindi, amsol in Marati and punarpulli in Malayalam in India [1]. It is now included under the list of endangered species of medicinal plants of South India [4].
The root is astringent [5]. Fruit fat is demulcent and emollient [6]. It is a remedy for dysentery and diarehia, tumors, heart complaints, stomach acidity and liver disorders [7]. Fruit rind extracts have been shown antifungal and antioxidant properties [5]. Garcinol, the compound isolated from fruit rind exerts antiinflammatory effects and is a neuroprotectant[8]. (-) Hydroxycitric acid from leaves and fruit rind is antiobesity and anti cholesterol drug [9]. Seed oil rich of long chain fatty acid methyl esters can be used as environmental friendly non toxic biodiesel [10] a substitution for conventional diesel. The fruits are also used to prepare red beverage which has bilious action [11]. The fruit rind is widely used traditionally in Srilanka and southern parts of India because of pleasant flavour and sour taste for culinary purposes.
D-leucine from leaves [12], (-) hydroxyl citric acid from leaves and fruit rind [13], fatty acids and glycerides from seeds [14], anthocyanin glycosides from fruits [15], garcinol, isogarcinol and cambaginol from fruit rinds [16], phenolic compounds like xanthones, biflavanoids from heartwood [17] and stem bark [18] and fatty acids from seed oil [19] were so far isolated from this plant.
In this communication we report isolation, characterisation and antibacterial activity of two poly phenols – a proauthocyanidin (I) and a biflavanoid (III) from the ethyl acetate soluble fraction of methanol extract of stem bark of G. indica and preparation of their derivatives.
Stem bark of G. indica (500 g) was procured from south canara of Karnataka state, India during summer. The plant was identified and voucher specimen was deposited with Department of Botany, Osmania University, Hyderabad, India. The collected plant material subjected to shade drying and reduced to shavings. These shavings were coarsely powdered and first defatted with petroleum ether and then extracted with methanol under cold percolation method. The methanol extract on concentration provided a dark coloured semisolid (9 g). This semisolid mass was successively extracted with chloroform and ethyl acetate. The ethyl acetate soluble were washed with distilled water to remove water soluble compounds and dried over sodium sulphate. After solvent removal a brown coloured solid (5 g) was obtained. It was dissolved in ethyl acetate (50 ml) and fractionally precipitated with petroleum ether (15 ml) for 25 times. The solid fractions 5 to 15 were combined and the process of fractional precipitation was repeated. The solid obtained from the last 10 fractions were mixed and purified on safodox LH 20 using ethanoldichloromethane (1:1 v/v) to get proanthocyanidin I, mp is 230° (300 mg).
The alkali solubles of the solid fractions 18 to 25 was chromatographed over silica gel (200 mesh) and eluted with benzene, benzene - ethyl acetate 8:2, 7;3, 1:1 (v/v) and ethyl acetate successively. Benzene - ethyl acetate (7:3) fractions gave biflavonoid III, 340° (100 mg).
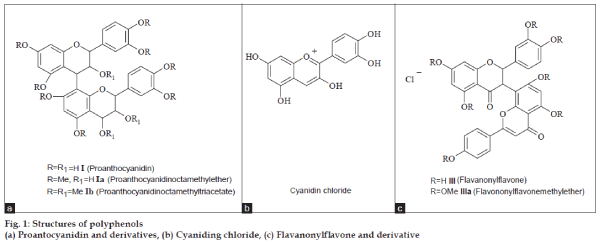
The proauthocyanidin (I) (fig. 1a) gave positive ferric chloride test suggests the presence of phenolic –OH. On treatment with dimethyl sulphate and potassium carbonate in acetone yielded octamethyl ether (Ia) suggesting eight phenolic –OH were present in the molecule. This ether further on treatment with acetic anhydride in pyridine yielded triacetate of ether (Ib). This suggests that three more –OH’s in the form of alcoholic nature were also present in the molecule. The high resolution mass spectra of I, Ia and Ib suggested that I is dimeric proauthocyanidin. Based on biogenetic considerations the linkage of dimer has intramolecular flavonyl linkage between C-4 and C-8. On hydrolysis of this proauthocyanidin (I) with methanolic hydrochloric acid cyanidin chloride (II) (fig. 1b) only obtained. No stereo chemical studies were carried out for these compounds.
The biflavanoid (III) (fig. 1c) gave positive phenolic and positive flavonoid tests with ferric chloride and Mg+HCl reagents respectively. This implies that the flavonoid is a phenolic hydroxy compound. On treatment with dimethyl sulphate and potassium carbonate in acetone a hepta methyl ether (IIIa) was formed. This methyl ether further treatment with acetic anhydride in pyridine did not yielded any acetyl derivative. This suggests that no alcoholic hydroxys were present in the molecule. based on 1H, 13C NMR and high resolution mass spectra the compound has been confirmed as I-5,II-5,I-7,II-7,I-3/,I-4/,II- 4/-heptahydroxy[I-3,II-8]flavononylflavone (III), a compound earlier reported from Garcinia nervosa by Babu et al. [20]. This is the first report from this plant and second report front Garcinia species.
The antibacterial studies were carried out by paper disc method which is easy, better and comparatively fast when compared with tube dilution method or phenol coefficient method or slide cell technique [21]. Ciprofloxacin was used as standard anatibacterial agent (1 mg/ml). The activity was evaluated by using 24 h cultures of Staphylococcus aureus, Salmonella typhi and Escherichia coli and the nutrient broth Labelmco was prepared from beef extract as culture medium. The bacteria strains used in this experiment were cultured in the Microbiology Department, Osmania University, Hyderabad.
Culture medium prepared by taking Labelmco, sodium chloride and peptene in double distilled water. The solution was filtered and the pH was adjusted to 6.8- 7.0. The medium was sterilised in autoclave at 121° at 15 Lbs pressure for 15 min. The test samples of 10 mg were dissolved in 10 ml of acetone to get 1000 μg/ml dilution. Different dilutions like 400 μg/m l, 200 μg/ ml, 100 μg/ml and 50 μg/ml were prepared from this.
Paper disc of 4 mm dia were dipped in test solutions of different dilutions and standard solution. After drying the disc it was placed on culture medium in petridishes and seeded with 1 ml of experimental bacteria culture of S. aureus, S. typhi and E. coli and incubated at 37±1° for 24 h. The petridishes were checked for growth inhibition zone after 24 h. The crude and flavononylflavone showed good activity against S.aureus even at lower concentrations while partial with proauthocyanidin. E. coli showed partial activity with crude and proauthocyanidin at higher concentrations no activity with flavononylflavone even at higher concentrations. Crude, flavononylflavone and proauthocyanidin showed medium activity only at higher concentrations with S. typhi. All the derivatives showed no activity with bacteria even at higher concentrations. Table 1 showed the anti bacterial activity of the compounds.
| Bacteria | Crude | Comp I | Comp III | CF10 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | A | B | C | D | A | B | C | D | E | |||
| Staphylococcus aureus | 10 | 13 | 19 | 30 | 12 | 19 | - | - | 10 | 13 | 18 | - | 9 | ||
| Salmonella typhi | 10 | 18 | - | - | 18 | - | - | - | 19 | - | - | - | 6 | ||
| Escherichia coli | 17 | 19 | - | - | 17 | - | - | - | - | - | - | - | 8 | ||
Values are mean inhibition zone in mm, A=400 µg, B=200 µg, C=100 µg, D=50 µg, E=10 µg; CF10=Ciflofloxacin 10 µg
Table 1: Anti bacterial activity of crude extract and poly phenols of Garcinia indica
Acknowledgements
The authors are thankful to Dr. Prabhakar, Department of Botany, Osmania University, Hyderabad for identifying the plant material, Dr. Gopal Reddy, Department of Microbiology, Osmania University, Hyderabad for providing antibacterial strains and Central Instrumental Centre, Ohio State University , USA for 1H, 13C NMR and high resolution mass spectra. One of the authors (CL) is thankful to UGC, New Delhi, India for a teacher fellowship.
References
- Anonymous, The Wealth of India, Raw materials, Vol-4, New Delhi, India: Council of Scientific and Industrial Research; 1956. p. 101-3.
- Chandran MDS. Nature watch. The Kokum tree. Resonance 1996;1:86-9.
- Bhat DJ, Kamat N, Shirodhkar A. Compendium and proceedings of 2nd National Seminar on Kokum (Garciniaindica Choisy), 2005.
- Rajashakaran PE, Ganeshan S. Conservation of medicinal plant biodiversity ? an India perspective. J Med Arom Plant Sci 2002;24:132-47.
- Selvi AJ, Joseph GS, Jayapraksha GK. Inhibition of growth and aflatoxin production in Aspegillus of growth and aflatoxin production in Aspegillus flavis by Garcinia indicae xtract and its antioxidant activity. Food Microbiol 2003;20:455-60.
- Chopra RN, Nayear SL, Chopra IC. Glossary of Indian Medicinal plants, New Delhi, India: Council for Scientific and Industrial Research; 1952. p. 122.
- Bhaskaran S, Nehta S. Stabilised anthocyanin extract from Garcinia indica US patent 2006/0230983/A.
- Liao CH, Ho CT, Lin JK. Effects of garcinol on free radical generation and NO production in embryonic rat cortical neurons and astrocytes. Biochem Biophys Res Commun 2005;329:1306-14.
- Heymsfield SB, Allison DB, Vasselli JR, Pietrobelli A, Greenfield D, Nuney C. Garcinia composia (Hydroxycitric acid) as a potential anti obesity agent a randomized controlled trail. JAMA 1998;280:1596-600.
- Hosmani KM, Heriemath VB, Keri RR. Renewable energy sources from Micheliachampala and Garciniaindicaseed oil. A rich source of oil. Biomass Bioenerg 2009;33:267-70.
- Kritikar KR, Basu BD. Indian Medicinal Plants. In: Blatter E, Caius JF, Mhaskar KS, editors. Deharadun, India: International Book Distributior; 1995. p. 262-3.
- Badami RC, Razdan MK. Isolation and Identification of L- leucine as DNP. L ?leucine hydrochloride in the leaves of Garciniaindica. J Indian ChemSoc 1972;49:583.
- Jayaprakasha GK, Sakariah KK. Determination of organic acids in leaves and rinds of Garciniaindica (Derr) by L.C. J Pharm Biomed 2002;28:379-84.
- Kantha AR. A chemical technique for determining the configuration of natural fats, operation of specific restricted random distribution rule B in Garciniaindica and Vateriaindica seed fats. Indian J Chem 1964;2:199-203.
- Nayak CA, Srinivas P, Rastogo NK. Charaterization of anthocyanins from Garciniaindica Choisy. Food Chem 2010;118:719-24.
- Krishnamurthy N, Leuis YS, Ravindranath B. On the structures of garcinol, isogarcinol and camboginol. Tetrahedron Lett 1981;72:793-6.
- Coiterill PJ, Scheinnamn F, Puranik GS. Phenolic compounds from heartwood of Garciniaindica. Phytochemstry 1977;16:148-9.
- Lakshmi C, Akshayakumar K, Dennis TJ. Polyprenoylated benzophenobes from Garciniaindica. J IndChemSoc 2002;79:968-9.
- Singhai M, Benerjee AK. Investigation on Garciniaindica seed oil. Asian J Chem 1994;3:720-1.
- Babu V, Ali SM, Sultana S, Ilyas M. A biflavonoid from Garcinianervosa. Phytochemistry 1988;27:3332-5.
- Bigger E. Hand book of bacteriology. London: Biller Indall and Lox; 1943. p. 35.